Published online Mar 25, 2024. doi: 10.5501/wjv.v13.i1.89934
Peer-review started: November 17, 2023
First decision: December 26, 2023
Revised: December 28, 2023
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: March 25, 2024
The aim of our minireview is to provide a brief overview of the diagnosis, clinical aspects, treatment options, management, and current literature available regarding herpes simplex keratitis (HSK). This type of corneal viral infection is caused by the herpes simplex virus (HSV), which can affect several tissues, including the cornea. One significant aspect of HSK is its potential to cause recurrent episodes of inflammation and damage to the cornea. After the initial infection, the HSV can establish a latent infection in the trigeminal ganglion, a nerve cluster near the eye. The virus may remain dormant for extended periods. Periodic reactivation of the virus can occur, leading to recurrent episodes of HSK. Factors triggering reactivation include stress, illness, immunosuppression, or trauma. Recurrent episodes can manifest in different clinical patterns, ranging from mild epithelial involvement to more severe stromal or endothelial disease. The severity and frequency of recurrences vary among individuals. Severe cases of HSK, especially those involving the stroma and leading to scarring, can result in vision impairment or even blindness in extreme cases. The cornea's clarity is crucial for good vision, and scarring can compromise this, potentially leading to visual impairment. The management of HSK involves not only treating acute episodes but also implementing long-term strategies to prevent recurrences and attempt repairs of corneal nerve endings via neurotization. Antiviral medications, such as oral Acyclovir or topical Ganciclovir, may be prescribed for prophylaxis. The immune response to the virus can contribute to corneal damage. Inflammation, caused by the body's attempt to control the infection, may inadvertently harm the corneal tissues. Clinicians should be informed about triggers and advised on measures to minimize the risk of reactivation. In summary, the recurrent nature of HSK underscores the importance of both acute and long-term management strategies to preserve corneal health and maintain optimal visual function.
Core Tip: Our minireview is based on herpes simplex keratitis. This type of corneal viral infection is caused by the herpes simplex virus (HSV), which can affect several tissues, including the cornea and deeper uvea. Its ability to remain dormant for extended periods and reactivate with serious morbid ocular presentation makes it an important pathogen to be reviewed. The body’s immune response to HSV is another potential cause of herpes simplex keratitis. Clinical management is short-term and long-term to prevent reactivation. Clinicians should be informed about triggers and advised on measures to minimize the risk of reactivation.
- Citation: Musa M, Enaholo E, Aluyi-Osa G, Atuanya GN, Spadea L, Salati C, Zeppieri M. Herpes simplex keratitis: A brief clinical overview. World J Virol 2024; 13(1): 89934
- URL: https://www.wjgnet.com/2220-3249/full/v13/i1/89934.htm
- DOI: https://dx.doi.org/10.5501/wjv.v13.i1.89934
The eye is the organ of sight responsible for collecting and converting visual stimuli for onward transmission to the brain. The cornea, specifically, was believed to have full 'ocular immune privilege'[1] but recent studies challenged this submission[2]. Infectious keratitis ranks fifth among the leading causes of severe visual impairment globally[3]. Microbial keratitis can be bacterial, fungal, or viral/protozoal[4,5]. Viruses are implicated in up to 4/5th of all conjunctivitis in humans[6]. Herpes simplex virus (HSV) is the most common ocular disease-causing viral agent[7-9]. Ocular HSV infection can affect various local tissues[10]. Corneal manifestations of HSV-1 are termed Herpes simplex keratitis (HSK). It often presents with dendritic ulceration and eventual corneal scarring[11].
Systemic conditions like diabetes are not known to exacerbate HSK[12]. Dendritic corneal epithelial lesions found commonly on eyes infected with HSV, may also be seen in diabetes-associated neurotrophic keratopathy[13,14]. HSK may manifest as only mild keratitis at one end of its spectrum but may potentially result in ulcerative keratitis and perforation at its end stage[15,16]. HSV intrastromal keratitis was more common than epithelial keratitis in large-group studies[17,18]. The herpes virus may infect an eye as a single infective organism or may occur in a polymicrobial form together with other types of microbes[19-21]. HSV causes diseases around the genitalia (herpes labialis) and the brain (herpes encephalitis)[22].
Published articles in English were sought by searching through the PubMed database and with Reference Citation Analysis (https://www.referencecitationanalysis.com) with search net of the past 5 years between 2018 and 2023. Articles without full texts and abstracts were not considered. Also, articles written in other languages, out of the scope of the topic, or deemed unclear in methodology were screened out. The PubMed search query was: “("keratitis, herpetic" [MeSH Terms] OR ("keratitis" [All Fields] AND "herpetic" [All Fields]) OR "herpetic keratitis" [All Fields] OR ("herpes" [All Fields] AND "simplex" [All Fields] AND "keratitis" [All Fields]) OR "herpes simplex keratitis" [All Fields]) AND (2018:2023 [pdat])”.
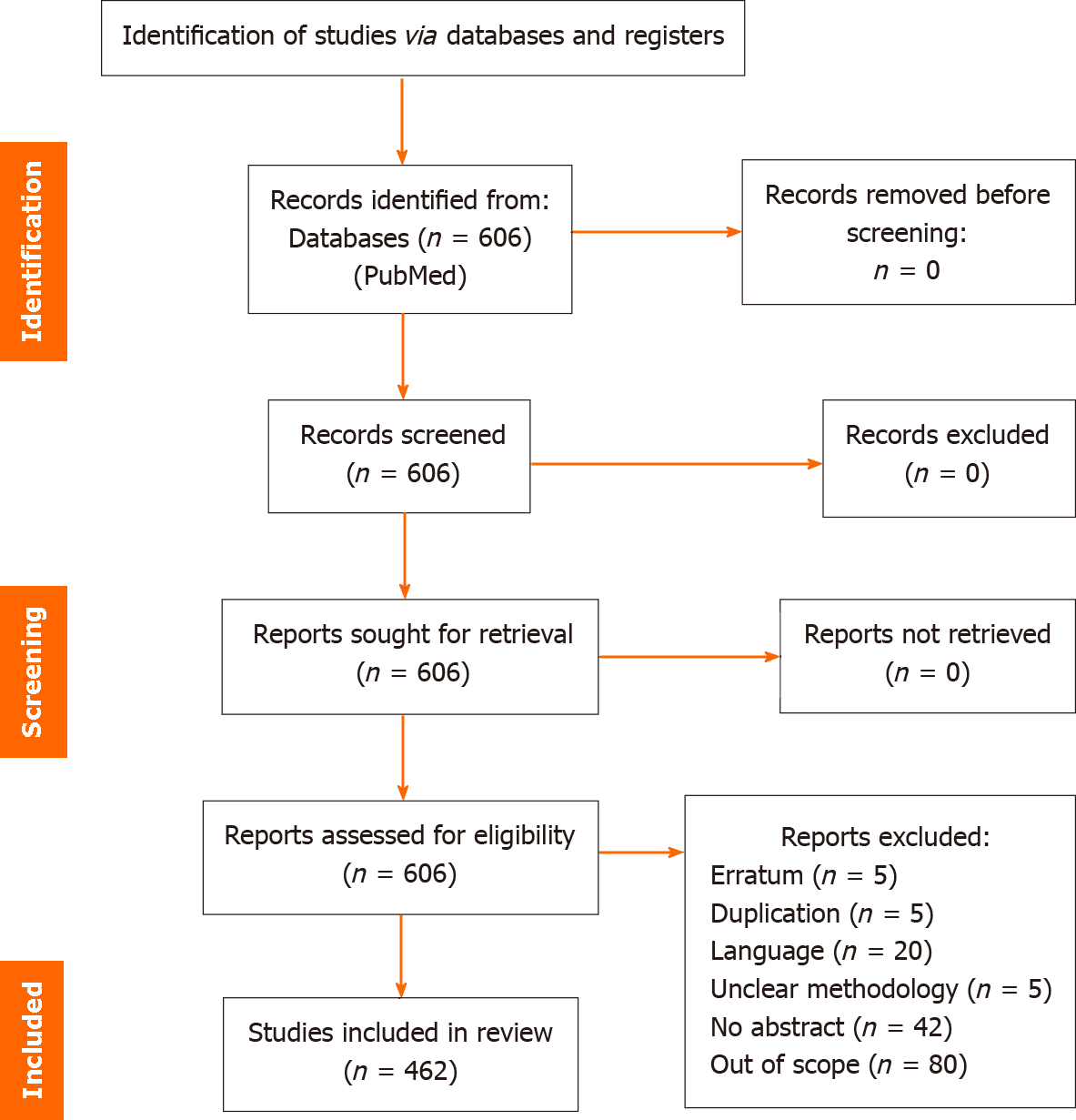
A total of 606 records were returned from the search string and preferred reporting items for systematic reviews and meta-analyses[23] table showing selection criteria is shown below in Figure 1.
The HSV causes HSK[24], It is among the most ubiquitous human pathogens on earth[25-27]; and a major blinding disease in developed countries[28,29]. It is the second most common cause of corneal scarring according to a large study[30], It has a seroprevalence in the range of 60%-90%[31]. It is also commonly seen in immunocompromised individuals[32]. Multiple strains of the virus present with differing symptomatology according to duration and virulence[33,34]. HSV can deceive toll-like receptors (TLR) which would ordinarily signal the innate immune system to attack the virus[35]. Cui et al[36] reported on quantitative proteomics of the infected corneal epithelial cells suggesting that P4HB, ACLY, HSP90AA1, and EIF4A3 proteins are involved in the relationships between hosts and viruses [36]. It is known that HSV-1 infection alters the host metabolism to suit its propagation in vitro[37]. Costimulatory molecules like CD80 and CD28 may also reduce the expression of HSV symptoms[38-40].
The virus typically affects humans more than other animals. After initial infection with HSV, hosts carry the infection for life[41-43]. Transmission generally is by direct contact of the skin or mucous membranes with lesions or secretions bearing virions[44,45]. HSV is generally divided into two which include HSV-1 and HSV-2[46,47]. HSV-1 is primarily the cause of infections around the mouth, face, and eyes[48]; while HSV-2 is majorly transmitted sexually and causes genital diseases. HSV-2 could also infect the eyes by contact or spread from lesions present in the genital area. This explains the presence of HSV-2 infection in the eyes of neonates, the route of infection being the mothers' birth canal. This underscores the importance of HSV testing in pregnant women especially those showing signs such as cold sores, and blisters[49]. Biological sex differences do not seem to induce changes in ocular HSV-1 disease expression, as evidenced by BALB/C and C57BL/6 murine models[50,51].
Yadav et al[52] documented 10 cases of HSV-2-linked blepharokeratoconjunctivitis over 16 years[52]. HSV-1 typically affects the mucocutaneous distribution of the trigeminal nerve. It usually does not present any symptoms but could sometimes manifest as a nonspecific upper respiratory tract infection. The virus then spreads from the infected epithelial cells to close-by sensory nerve endings and is carried along the nerve axon to the cell body of the trigeminal ganglion. At this level, the virus genome enters the nucleus of a neuron where it stays indefinitely in a dormant state[52,53]. Studies on non-human primates also revealed the persistence of HSV-1 in the ciliary ganglion and cornea during latency[54].
Harrison et al[55] hypothesized that stress stimulates viral gene expression and productive infection during reactivation from the latency stage[55]. Yan et al[56] also postulated that stress can reactivate a dormant HSV infection[56].
There are typically four subtypes of HSK which include: (1) epithelial keratitis; (2) immune stromal keratitis; (3) stromal necrotic keratitis; and (4) endotheliitis.
This classification depends on the layer of cornea affected; and whether the keratitis is caused by a reactivated or primary infection. The most common subtype is epithelial keratitis which appears as rough granular spots that form punctate lesions on the cornea; they usually coalesce quickly to create the typical dendritic lesions. These dendritic cells typically change in density wildly along the course of herpetic keratitis[57]. Keratic precipitates may also be observed[58]. Shah et al[59] reported that stromal keratitis (with no identifiable ulceration) was the most common presentation of HSK[59]. There were reports of both acute & subacute dendritic epithelial keratitis findings following incisional cataract surgeries; and transepithelial photorefractive keratectomy in one patient[60,61]. It is key to differentiate toxic epithelial keratitis following cataract surgery from HSK[62]. HSV geographic corneal epithelial defects increase predisposition to ocular infections via fastidious bacteria[63]; and Stenotrophomonas maltophilia[64].
An ocular manifestation of HSV specifically in the cornea is termed HSK: Which had a 2016 prevalence of about 1.7 million people worldwide[65]. It is the most prevalent infectious blindness-causing disease in the developed world[66,67], and is also prevalent in developing countries[68]. HSK commonly occurs monocularly, but can also present binocularly in certain conditions such as HIV and rheumatoid arthritis[69]. HSK has been known to affect all layers of the cornea and can be a cause of interstitial keratitis when the stroma is involved[70]. Whether inherent reports of unilateral posterior interstitial keratitis with hypoesthesia can be wholly attributed to HSK remains unproven[71]. Primary infection of any branch of the trigeminal nerve can cause an inactive infection of nerve cells in the trigeminal ganglion. Without prior ocular HSV infection, a person could still develop HSV keratitis due to the interneuronal spread of HSV within the trigeminal ganglion[72]. While a majority of patients initially present with corneal epithelial inflammation, about a third of them either present with or develop stromal keratitis[73].
Neutrophil and CD4+ mediated mechanisms are involved in the pathogenesis of herpetic stromal keratitis following viral spread along the corneal epithelium[74]. In mouse models of recurrent HSK, corneal sensory nerve retraction and replacement with aberrant sympathetic nerves potentiate pathologic processes with CD4+ T cells post-HSV-1 reactivation[75]. Damage at the level of sensory corneal nerves is termed neurotrophic keratopathy[76,77]. Substance-P (SP) production is largely depleted during the early stage of corneal nerve damage in HSK, followed by increased levels, possibly via a positive feedback loop during corneal disease manifestation; this surge of SP binds to neurokinin-1-receptor, upregulating the release of pro-inflammatory cytokines on the ocular surface[78]. Bell's facial nerve palsy reportedly occurred with herpetic stromal keratitis in the case of an immunocompetent individual[79].
Recurrence of HSV infection is widely agreed to be the result of virus reactivation in the trigeminal nerve ganglion; which travels along nerve axons to introduce its genome into the eye and initiate replication using the eye’s cellular processes to make new copies of the virus. US-11 protein encoded in HSV-1 may play a role in the pathogenesis of worsening keratitis by promoting the translation of viral amino acids following the reactivation of HSV[80]. There is evidence supporting the presence of latent virus in cornea tissue, this could be the cause of recurrent donor-derived HSV, however, this is still very controversial[81].
Evidence shows the reactivation of HSV among patients using latanoprost in managing glaucoma[82,83]. HSV reactivation has also been linked to local, systemic, and topical steroid medications including the use of intravitreal triamcinolone injection[84]. Narang et al[85] further reported a case of reactivation of HSK after bilateral botulinum toxin injection to manage epiphora[85]. Ishimaru et al[86] reported that the HSV-1 virus replicated in host tissue partly due to proteasomal degradation of the Ras-GRF2 factor, while also demonstrating that this can be reversed by the proteasome inhibitor MG132[86]. Differentials for HSK include Acanthamoeba, Mycobacterium[87], Nocardia[88], Microsporidia[89] and Arthrographis kalrae keratitis[90]. Dong et al[91], identified trauma to be the main cause of infectious keratitis in their study[91]. HSK may also complicate the course of acute retinal necrosis in 20% of sufferers[92-93].
Research has shown that endosomal and cytoplasmic Pattern Recognition Receptors and the cell surface recognize HSV and as such start a cascade of immune response which includes Interferons (IFN), Chemokine, and Cytokine production as well as the recruitment of other inflammatory cells to the location of the infection[94]. IFN-1 release in acute HSV keratitis limits dendritic lesion enlargement[95]. Tripartite motif 21 (TRIM21) proteins reportedly inhibit IFN-beta; increasing the release of more proinflammatory cytokines, e.g. interleukin (IL)-6, TNF-alpha[96]. Reactive oxygen species (ROS) release from neutrophils in HSK-infected eyes was hypothesized to be catalyzed by nicotinamide adenine dinucleotide phosphate oxidase 2 enzyme[97]. The protein Osteopontin may also mitigate the inflammatory process observed in ocular HSV inflammation at the risk of also upregulating viral replication[98].
HSV-1-encoded ICP-5 proteins are crucial for capsid reassembly processes during viral replication[99]. Novel studies suggest that the upregulation of tryptophan hydroxylase during viral replication implicates the derangement of serotonin neurotransmitter pathways[99]. Ubiquitination is a process of viral protein modification; its first step is the bonding of ubiquitin to ubiquitin-activating enzyme1; HSV-1 induces bonding of ubiquitin-activating-enzyme 1a isoform with Lys604: Ubiquitination at Lys604 functions as a rate-limiting step of HSV-1 replication[100].
HSK may be diagnosed by its clinical presentation on the slit-lamp biomicroscope[101]. Slit-lamp biomicroscopy provides better sensitivity than other low-resource alternatives[102]. It should be noted that slit-lamp findings in HSV infection are similar to endotheliitis secondary to other Herpesviridae: Cytomegalovirus and varicella zoster[103]. Visually, this diagnosis usually includes dendritic/geographical ulceration[104]. The common presenting symptoms: Photophobia, redness, itching, tearing, irritation, pain, discharge, and watery eyes usually subside after about 2 wk. Pain experienced by HSK sufferers has been likened to post-refractive surgery pain[105]. Deep Learning Artificial Intelligence has been identified as a positive aid in diagnosing HSK, especially in areas with less eye care-related manpower[106-108].
Ancillary Deep Learning methods integrated into clinical practice aided earlier diagnosis of HSK from its differentials[109]. Machine learning-based multinomial regression reduced the frequency of misdiagnosing HSV anterior uveitis from other uveitis etiologies[110]. Optical coherence tomography (OCT) is useful in monitoring patients' reactions to medication therapy[111,112]. Soliman et al[113] described the anterior segment-OCT findings of HSK as having sub-epithelial infiltration and specific stromal hyper-reflective patterns. Although these features are not unique to HSK they could help in diagnosing and monitoring HSK[113].
Spectral domain OCT has been used in vivo to provide a better understanding of the inflammatory and repair processes involved in HSK. Active HSK shows significant epithelial and stromal thickening while the inactive disease process shows a change in the structure at the site of stromal thinning due to the scarring[114]. Acanthamoeba keratitis (AK) and HSV-keratitis are mimics[115,116]. Subjective determination of corneal lesion depth via anterior segment OCT may distinguish between these pathogens at earlier stages[115]. AK tends to invade the corneal stroma aggressively, causing radial keratoneuritis, in contrast to herpetic keratitis[115].
Diagnosing HSV typically involves identifying the virus or its proteins, HSV-specific antibodies, or HSV genetic materials in the blood[117]. Culture staining is limited as most diagnostic dyes have poor sensitivity to non-bacterial pathogens[118]. As early as 1996, immunofluorescence and polymerase chain reaction were already being used to detect HSV in corneal tissue[119]. Hirota et al[120] have reported successful monitoring of HSV levels in tears by using polymerase chain reaction analyses[120].
The conventional strategies for the diagnosis of HSV include serological tests, viral culture, and molecular techniques[121]. Viral culture is done by needle aspiration or the use of a swab and then cultured for a few days before microscopic analysis is carried out to determine HSV cytopathic effects. Viral culturing requires great-quality specimen collection, proper handling, and transportation of the specimen.
Compared to Herpes Zoster ophthalmicus, older age, diabetes mellitus and history of surgery are poor prognostic correlates for HSK[122]. Neonatal herpetic stromal keratitis can be confirmed by polymerase chain reaction (PCR)[123]. Multiplex real-time PCR (RT-PCR) has been found to identify HSV DNA reliably and is ideal in the diagnosis of HSV keratitis in the microbiology laboratory[124]. HSV superinfection can be diagnosed using multiplex PCR[125].
Diagnosing HSK is paramount as other conditions could mimic the typical appearance pattern in the cornea, Chang et al[126], showed that antiglaucoma medications could cause pseudo dendritic keratitis which is typically in the center-lower cornea as horizontal linear lesions. Benzalkonium chloride has been implicated in most cases[126]. Haidar et. al published a case report on the subject of the misdiagnosis of a foreign body as HSK[127].
Research has found that Nocardia keratitis, AK, and intraepithelial neoplasia can be misdiagnosed clinically for HSV infection, correct diagnosis of HSV is paramount as treatment modalities are different for these conditions[128-132]. Atypical microsporidial and fungal keratitis may mimic expected HSV findings from the clinicians' perspective also[133,134]. Another problem occurs when other microbes superimpose on HSV to cause or exacerbate keratitis[135].
The tear HSV-slgA test has been identified as a technique to identify HSV infection[136]. Diagnosing HSV prenatally has proved difficult as ultrasound results are usually not specific to congenital HSV infection[137].
Quantitative RT-PCR was shown by Mohammadpour et al[138], to be an excellent technique for detecting HSK[138] while Tóth et al[139] showed that PCR could identify HSV in about every 2.8th patients with a clinical history of HSK[139].
Parekh et al[140] have advocated for the use of Shotgun sequencing to analyze samples for the presence of HSK[140]. PCR is not sufficiently reliable in the diagnosis of HSV-induced stromal keratitis and endotheliitis due to limitations associated with assessing diseased corneal tissue specimens; hence, viral etiologies in these cases are often inferred or presumed clinically[141]. Real-time PCR was useful in making differential diagnoses of diffuse HSV endotheliitiswith feathery infiltration from fungal keratitis[142].
Tear film analysis via quantitative microfluidic PCR yielded good sensitivity for the detection of Herpesviridae in instances of epithelial HSK[143]. Tear film protective analysis identified high concentrations of IL-1A, IL-12B, DEFB4A, and CAMP for eyes infected with HSV epithelial keratitis[144,145]. Polymerase chain reaction and viral culture sensitivity in the diagnosis of HSV is limited, with the sensitivity of viral culturing being 50% and PCR being between 55%-88% while the sensitivity even reduces further in the identification of recurrent HSV disease[146].
HSV-1 DNA, antigens, and Latency-associated Transcript (LAT) in the cornea can prove crucial to the diagnosis of atypical clinical presentations or post-infectious stages where it might be difficult to identify the cause of an innocuous cornea scar[147].
Detection of the HSV LAT gene by reverse transcriptase quantitative PCR is superior to conventional PCR and Immunohistochemistry[148].
Louise and Sotiria reported the use of cornea pachymetry and epithelial thickness maps to provide an objective assessment of stromal inflammation. They reported that pachymetry and corneal thickness maps helped to identify HSV stromal keratitis; differentiating it from less debilitating HSV keratitis, and even neurotrophic keratopathy. It also offered an objective measurement for stromal inflammation resolution[149]. Studies have shown[149] Amplivue to be a rapid, potentially office-based diagnostic test for detecting HSV-1 and 2 as compared to more expensive and time-consuming PCR testing. In-vivo confocal microscopy was successfully used to study microscopic changes in cornea structures of feline and canine models with HSK[150].
Metagenomic deep sequencing can help to identify specific nucleic acids in complex ocular samples and assign them to specific organisms, thereby aiding diagnosis[151].
Ferreira et al[152] examined records of 235 keratitis patients presenting to a tertiary center between 2007 and 2015. As part of their comparisons and conclusions, HSK negatively correlated with poor outcomes after management[152].
Clinical characteristics of HSV-induced anterior uveitis can mimic other viral and non-infectious uveitis most particularly at the onset of disease. PCR and Goldman-Witmer coefficient should be carried out on aqueous humor samples in suspected viral cases[153].
Danileviciene et al[154], identified the role of the C21orf91 gene in the development of HSK where they described the condition to be 2.9 times more likely in patients with the rs10446073 genotype being more common[154]. According to Borivoje et al[155], the CC IL28B gene has been identified to be present in individuals with recurrent HSK[155].
Cornea sensitivity of patients with HSK especially stromal keratitis or those who had suffered before is usually lower; they typically have lower Cornea hysteresis, and lower Corneal resistance factor (CRF). Even the contralateral eyes of patients with previous HSK infection have less CRF and cornea hysteresis[156].
Cornea esthesiometry and Laser scanning confocal microscopy have been shown to reveal a significant decrease in cornea sensitivity and sub-basal nerve fibers: Which recover after around 6 months but never return to normal anatomy[157].
Studies have shown that obese and overweight individuals are more likely to develop recurrent simplex keratitis[158]. The use of corneal impression membranes led to higher detection of HSK compared to swab techniques[159]. Storing HSV-1 inoculated polytetrafluorethylene impression membranes at +35 °C for three months led to a reduction of DNA recovery; storage at +4 °C, -20 °C and -70 °C for 10 d were optimal for HSV-1[160].
Computational bio-sequencing methodology identified HSK and other corneal virulent organisms in vitro[161]. Bioinformatics analyses suggest that UL24.5 is a possible determinant of pathogenesis[162] Miyazaki et al[163] in their study showed that RAGEs (receptors for Advanced glycation end products) is a sensor of HSV-1 infection, this is a route to possible diagnosis for HSV[163].
There are lots of anti-viral prescribing patterns currently, Cabrera-Aguas et al[164,165], described the need to standardize the indication and dosage of antiviral therapy in the management of HSK[164,165]. Ultimately, the decision to treat and treatment regimen selection is largely dependent on the individual clinician[166]. Lázaro-Rodríguez et al[167] reported isolated primary herpes-simplex virus neuroretinitis in an immunocompetent adult; thus, underpinning the need for starting antiviral therapy for individuals with macular stars who are not immunocompromised but seropositive for HSV IgM after ruling out other infectious causes, ionizing radiation, and arterial hypertension[167].
Antiviral resistance of HSK is generating concern, but the exact mechanisms of resistance have not been fully articulated[168]. Acyclovir is the most common drug used in the management of HSK[169], although eyedrops are now being proposed[170]. Resistance to acyclovir occurs due to extended use[171], and mutation in the viral thymidylate kinase and DNA polymerase which decreases enzyme affinity for its substrate[172-174]. Topical cyclosporine drops and prednisolone acetate drops are statistically similar in potency for stemming inflammation and preventing scar development[175].
HSK may predispose the eye to opportunistic infections. Vigilance must be applied when managing HSK patients with steroid-antibiotic eye drops as this is contraindicated[176]; and can result in epithelial defects and vascularization[177]. On the other hand, steroid-antiviral therapy performs better than fixed antiviral therapy[178].
In scenarios where the clinician is considering treatment-related side effects or conventional antiviral therapies not giving the required results, oral Valganciclovir could be used as an alternative for treatment and prophylaxis against HSK[179]. Watson et al[180] described a novel way of attacking the dormant LAT responsible for reactivating HSK after periods of latency using adeno-associated virus vectors to prevent reactivation[180]. A novel antiviral agent, SC93305, reportedly showed effectiveness against acyclovir-resistant strains of HSV-1 & HSV-2; SC93305 also reportedly did not interfere with host humoral immune responses[181]. Amentoflavone was found to inhibit resistant strains of HSV-1 including HSV-1/106, HSV-1/153, and HSV-1/blue by interfering with early-stage transcription of viral genes[182].
Novel delivery approaches such as prodrugs, nanocarriers, and peptides do cover against the systemic toxicity of oral antiviral prescription, as well as the rapid nasolacrimal clearance of topical antiviral therapy. The use of gel formulations and novel delivery approaches function tremendously to achieve desired outcomes[183].
The prophylactic use of antiviral agents such as acyclovir and valacyclovir is successful in treating HSK[184]. Cacicol® which is a topical eye biopolymer that contains poly-carboxymethyl glucose sulfate solution has been identified to have antiviral action on HSV and Varicella Zoster Virus (VZV)[185]. Due to emerging resistance to antiviral medication, there is a need to use other medications that target other viral proteins. This prompted Guan et al[186] to study the effect of stapled peptides on HSV-1 DNA synthesis and HSV-1 infection[186].
In the application of antiviral therapy for recalcitrant HSK, epithelial debridement, high-frequency dosing, and reduction of immunosuppression could help in achieving a better outcome[187].
The infection of the cornea by HSV secondary to an immune-inflammatory reaction by proinflammatory T cells is a significant cause of vision impairment[188].
There is a greater incidence of HSV infection in patients with atopy and the course of HSV keratitis in patients with severe atopic disease is usually more difficult to manage[189]. Patients with immune deficiencies or atopy usually present with bilateral HSK and it has been proven that long use of antiviral therapy can reduce the recurrence rate[190].
Presenting as an unwanted side effect, HSK has been found to spontaneously occur in those managed for MDA5-DM with rapidly progressive interstitial lung disease with tofacitinib at a dose of 20 mg per day[191].
Lappin et al[192] trialed immunotherapeutic management of HSV via topical administration of an ocular activating nanoparticle, feline models exhibiting re-occurrence of HSK were managed with this nanoparticle therapy and showed marked improvement as demonstrated by reduced viral shedding and ocular morbidity markers[192]. Davido et al[193] derived the KOS-NA mutant HSV mutant as a vaccine for the prevention of HSV-1[193,194]. Their data suggests that the mutated agent performed considerably well by preventing keratitis eruption in a mouse model.
Matundan et al[195], suggested that the ICP22 gene of HSV protected a murine model against corneal scarring[195]. Plasmid DNA administered with Interleukin 4, 10, 12, and 18 is reported to reduce inflammation and subsequent scarring in HSK[196]. Naidu et al[197] reported that the human HSV1 VC2 vaccine administered intramuscularly to mice mitigated the expression of HSK after subsequent infection with HSV-1 (McKrae) virus[197]. Similar results using the HSV1 VC2 vaccine in mice were shown to protect against HSV virus-linked immunopathogenesis[198].
Optineurin, a host protein, has been suggested as a possible inhibitor of the spread of HSV-1 while also mitigating neural damage[199]. This may be due to its selective autophagy regulatory properties. Hirose et al[200] investigated the role of TH17 responses in an HSV-1-infected murine model and concluded that interleukin-17 protects against ocular morbidity secondary to HSV-1[200].
Periocular corticosteroid injection resulted in hypopyon formation in a small sample of patients with HSV stromal keratitis and endotheliitis; the subsequent resolution was reportedly gained with topical antivirals, steroids, and systemic antivirals[201]. Upon infection with HSV-1, innate immune TLR-2 forms dimers with TLR-1, TLR-2, and TLR-6, cytokines, and IFN. TLR-2/2 Ligand activates the expression of specific antiviral genes[202]. Multiple microRNAs (miRNA) suspected to play significant roles concerning host immunity are upregulated in tear film samples of patients with HSV-induced epithelial keratitis[203]. Tenascin-C, an extracellular matrix glycoprotein increased in expression following injury, was discovered on the corneal epithelium of eyes with HSK keratitis[204].
Understanding the crucial role immune response plays in the development of herpes simplex stromal keratitis is necessary to control Stromal keratitis, especially macrophages, T cells, proinflammatory cells such as Th1 and Th17 CD4 T cells, and in some cases CD8 T cells in addition to memory CD19+ and CD27+ cells[205-209]. The transmission properties of HSV keratitis would be better managed if the role of the CD4+ TRM (Tissue Resident Memory T cells) and their induction by vaccines is well understood[210]. CXCR4-expressing cells may be key in the migration of neutrophils and the progression of lymphatics onto HSV-infected corneas[211]. During latent stages, viral proteins maintain low-level sporadic expression without full virion production, and ganglionic HSV-1 specific CD8+ T cell retention during latency serves protective functions[212]. Priming CD8+ T lymphocytes formed a basis for the hypothesis of future vaccination against HSV-1 reactivation[212]. Novel epitope peptide/CXCL-10 based prime/pull HSV vaccine elicited increased migration of HSV-specific CD8+ T-cell lymphocytes to the cornea and trigeminal ganglia of human leukocyte antigen (HLA) transgenic rabbits, thus protecting against ocular herpes virus infection[213].
Plasmacytoid dendritic cells are the main source of IFN-alpha within corneal stroma; higher density of plasmacytoid dendritic cells was associated with better in-vivo immunity against HSV-1 inoculation[214]. Higher peripheral blood levels of interleukin-1beta in patients with inactive/Latent HSK were correlated with increased levels of STAT1 and IRF3: Essential proteins for antiviral immune responses[215].
Interleukin-27 production by macrophages limited HSV-1 corneal shedding and consequent disease progression in mice[216]. Enhancement of P13K-Akt pathway signaling was hypothesized to cause increased susceptibility to HSV infection among test mice[217].
TRIM21 has been hypothesized to regulate type-1 IFNs' response to viral pathogens. The absence of TRIM21 proteins in knockout mice reportedly correlated with greater HSV-1 titers within the trigeminal ganglion during acute infection[218]. IFNalpha/beta could represent promising immune-mediated targets in HSV-1.
Antibody-dependent cellular cytotoxicity immune mechanisms provide protection from both cutaneous and ocular manifestations of HSV-1 and-2 infection[219]. Hence, novel vaccines eliciting the production of polyfunctional antibodies can offer protection to the immune-privileged eye[219].
The absence of Lymphotoxin-α was reported by Wang et al[220] to affect the expression of HSK in mice[220]. Dhanushkodi et al[221] also discovered IFN-γ-producing PLZFloRORγtlo as the most prevalent Invariant Natural Killer 1 cells in HSK-infected corneas[221]. At the level of the infected endothelial layer, IFN regulatory factor-7 has been shown to upregulate acquired immunity and mediate the major histocompatibility complex resulting from HSV infection[222].
HSK is known to lie latent for extended periods before being reactivated by any number of factors[223]. These factors may be as simple as ultraviolet radiation[35,126,129] to major ocular surgeries.
Tear fluid exosomes may be a site of HSV-1 Latency; involved in the spread of HSV-1 infection to human corneal epithelial cells (HCECs). Corneal grafts can also be a source of HSK infection if not properly screened[224]. With a global shortage of available viable cornea graft tissue, Li et al[225] demonstrated that porcine corneal tissue is a good option for human corneal tissue[225], Recurrent HSK has been implicated in Deep Anterior Lamellar Keratoplasty failure[226]. It is therefore important to differentiate between HSV-linked endotheliitis and actual graft rejection[227]. The use of tectonic grafts for non-healing keratitis was reported by Tourkmani et al[228], with around 50% postoperative recurrence of HSK lesions by Suzuki et al[229]. Being elderly, male, and having a large graft > 9 mm are all risk factors for developing epithelial defects after penetrating keratoplasty[230].
Researchers have reported cases of HSV relapse with ocular presentations after Botulinum Toxin injection[231-233]. Recurrence of HSK dendritic epithelial keratitis should be considered for following CXL in keratoconus patients with suspicious history[234,235]. It has been postulated that the ultraviolet radiation A light, damage to the epithelial/stroma trauma, or damage to the nerves of the cornea during the cornea cross-linking could result in recurrent HSV[236,237]. However, other authors have advocated for corneal cross-linking as a therapy for HSK[238].
Mohanty et al[239] recommended a two-week steroid regimen for the management of HSV-induced interstitial keratitis[239]. Topical steroids have been reported to reduce the risk of stromal progression of HSK by up to 60%[240]. Stromal keratitis commonly occurs following Varicella-Zoster viral infection in children[241]. Instituting steroids empirically in all clinically suspected HSK cases without prior culture may result in aggravation of microbial differentials and long-term topical agent reliance[242].
Research has shown that HSV keratitis could recur after ocular surgery such as strabismus[243], penetrating keratoplasty, cataract, corneal crosslinking, lamellar keratoplasty, photorefractive, and phototherapy[244-247]. Intraocular surgery, it seems, is also a risk factor for HSV reactivation with uveitic presentation[248]. Latent HSV in morphologically normal donor corneal grafts reactivated following keratoplasty[249].
There is substantive evidence for the prophylactic use of oral medications in penetrating keratoplasty who have had a previous history of HSK[250]. Herpetic keratitis lesions were found in a section of keratoconus eyes after collagen cross-linking procedures[251]. Small incision lenticule extraction has also been reported to precede a case of HSK manifestations[252]. Laser-assisted in-situ keratomileusis and photorefractive keratectomy triggered HSV reactivation which presented as endotheliitis in some patients post-corneal refractive surgery[253]. In another case reported by Basak and Basak[254] and Samak, oral Valacyclovir was used to resolve acyclovir-resistant endotheliitis resulting from deep membrane epithelial keratoplasty[254].
Nutritional deficiencies with subsequent hematologic abnormalities may trigger a recurrence of HSK[255]. Long-term use of systemic immunosuppressants for treating autoimmune disorders like systemic lupus erythematosus (SLE), lymphoproliferative diseases; and prophylaxis of organ transplant rejection, increases the likelihood of HSV recurrence/reactivation[256-259]. In certain individuals, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination triggers a cascade of autoimmune responses that can reactivate herpetic keratitis[260,261]. There were some reported incidences of reactivated herpetic keratitis following the reception of SARS-CoV-2 vaccines; patients' immune status before vaccination was not specified[262-266]; one patient had prior PKP surgery owing to HSK scarring[263]. Palpebral demodex infestation was associated with refractory and recurrent herpetic keratitis[267].
A retrospective study reviewing a large sample size from Canadian health databases reported no causal recurrence of HSK due to topical antiglaucoma medications[268]. Ozturk et al[269] reported a case of HSK in an adult male patient one week after receiving Ranibizumab. Behera et al[270] reported another case of reactivation of HSK in eyes and they investigated aster instilling bevacizumab[270]. A similar occurrence was also detailed by Al-Kaabi and Choremis[271]. Eyes with previous herpetic keratitis or uveitis were reported to manifest more recurrent episodes following phacoemulsification without longer periods of disease quiescence[272].
Kim et al[273] described the clinical features of HSK showing the most common subtype as epithelial keratitis (49.7%) followed by stromal keratitis (23.5%). They also show that epithelial keratitis had the highest likelihood of recurrence. The most common complication was cornea opacity. There was a 32.2% recurrence of HSK. The recurrence rate was seen to be less in the group that used prophylactic antiviral agents and the ascorbic acid treatment group[273]. There are bi-directional relationships between HSK and Atopic dermatitis[274].
Stromal involvement is usually a precursor for corneal scarring due to the infiltration of inflammation regulatory agents[275]. Rao et al[276] in their study showed the development of lacrimal gland inflammation in a mouse model of herpes stromal keratitis underpinning the inflammatory origin of herpes stromal keratitis[276]. Ocular infestation of the HSV is usually secondary to an infection in another part of the body[277]. Buccal to ocular transmission is a common transfer route. HSV keratitis can cause cornea scars which can make it difficult for intraoperative procedures such as cataract surgery and implantation of intraocular lenses[278]. That said, a follow-up of 37 HSV-infected eyes undergoing cataract surgery returned reasonably good outcomes even though the authors advised cataract surgeons to ensure the disease is latent before surgery. Scarring secondary to HSK is thought to result from the infiltration of inflammatory bodies and angiogenesis[279,280]. Corneal subbasal nerve density reduces markedly even after keratitis lesion scarring in eyes infected with HSV[281,282]. Subbasal nerve density losses, and consequent corneal hypoesthesia, are less in cases of herpetic epithelial keratitis[282]. Stromal inflammation in HSK is believed to be Chemokine (specifically ACKR2) mediated[283]. Hence these chemokines can determine the amount of ocular involvement in an individual.
Yoshida et al[284] reported on a case of HSV in a 64-year-old immunocompetent female who developed corneal scarring even after the resolution of HSK[284]. A similar presentation managed by Pisitpayat et al[285] did not progress to corneal scarring and had better outcomes[285]. HSV has also been associated with a risk of developing Neurotrophic Keratopathy[286-289]. In children and neonates who have suffered HSK episode(s), the possibility of amblyopia and strabismus in the latter years is present[290].
Herpetic keratitis is the leading cause of cornea ulcer and corneal perforation in the world, recurrence of the condition predisposes the individual to developing cornea ulcer and perforation. HSK has been found to cause neurotrophic keratitis[291-293]. HSK may also be a causative factor of corneal graft failure[294,295]. Using OCT, Ichikawa et al[296] were able to show an increase in cornea densitometry in HSV-affected eyes[296]. Retrograde inflammation from the cornea to the anterior chamber can result in viral anterior uveitis[297]. This is usually characterized by granulomatous precipitates with/without cornea scarring. HSV may also lead to uveitis. Posterior uveitis has been postulated to occur following long periods of post-keratitis latency[298].
Testing an HSK-compromised eye presents unique challenges for the patient and the clinician. Tananuvat et al[299] reported corneal perforation secondary to non-contact tonometry in two cases with thin corneas secondary to HSK and scarring[299]. Rebound tonometry may be more appropriate as it presents less stress to the corneal tissues.
HSK has been found to get worse in areas where air pollution is more frequent[300].
HSK has been associated with cornea denervation, although a certain degree of cornea nerve regeneration occurs a lot of the nerves do not come back to normal[301]. Sensory neuronal voltage-gated ion channels were associated with pain propagation in HSV-1 infection[302]. Further investigations are needed to probe a possible association between HSK and limbal stem cell deficiency as a sequel[303]. This may account for cases of poor corneal re-epithelialization[304]. Limbal stem cells' loss in herpetic keratitis was associated with density alterations of central basal epithelia, and the subbasal nerve plexus[305]. Moein et al[306], using a specific HSV-1 strain KOS-63 showed that the recurrence of HSK causes more denervation[306].
Chirapapaisan et al[307], demonstrated the reduction in corneal subbasal nerve density (CSND) using in vivo confocal microscopy denoting the reduction in CSND even in contralateral eyes that did not show any scar[307]. The location of the cornea scar has a role to play in the lower likelihood of cornea regeneration[308].
It has been discovered that METTL3 (Methyltransferase 3) promotes pathological angiogenesis through canonical Wnt and VEGF signaling[309]. Ultraviolet A light used to carry out corneal collagen cross-linking could cause or trigger reactivation of latent HSV in a patient without clinical symptoms[310]. HSK has been reported to predispose individuals to be affected by Burkholderia cepacia which usually affects people with cystic fibrosis or immunocompromised[311]. Montgomery et al[312] postulated that ocular glands could be affected by HSV infection or other bacterial infections of the cornea[312]. The mentioned underlying physiopathological mechanisms in HSK can give rise to corneal scarring, vision loss, intraocular pressure elevation, and glaucoma.
Stopping the reactivation of HSK is essential for the development of vaccine strategies against HSV-1[313]. The discovery of a vaccine for HSK has been plagued with concerns about their overall safety for the public, leading to non-licensure and eventually shutdown of these labs[314]. Carr et al[315] demonstrated that higher doses of their HSV-1 0∆NLS vaccine were able to prevent HSV-mediated disease[315].
It has been discovered that intrastromal injection of Bevacizumab could result in the regression of neovascularization in patients with neurotrophic keratitis secondary to HSV infection[316]. Topical therapeutic management is plagued with many factors including corneal epithelial toxicity to antiviral drops[317] and the development of tolerance[318]. The HEDS study recommended a guideline for oral antiviral drugs as a safer method of managing this disease process[319,320]. Intravenous acyclovir was reportedly therapeutic for herpetic stromal keratitis[321].
The use of Retinoic acid to stabilize regulatory T-cells which mediate inflammation and control the progression of stromal keratitis in an HSK model[322]. Wang et al[323] engineered a mouse with knocked-out signal peptide peptidase and demonstrated that these mice expressed reduced viral replication and reactivation as compared to control mice[323,324]. Sodium polyanethol sulfonate has also been described to reduce the replication of HSV in corneal epithelial cells[325].
Antiviral therapy is the mainstay in the management of HSK, The development of vaccines against the HSK virus has met a roadblock due to therapeutic effects in humans which are controversial, even though several vaccine candidates are effective in animal models that would require testing in humans[326]. One such vaccine was developed and reported by Hasan et al[327] using selected proteins from ViralZone, however, it remains to be tested on animal models[327]. The use of certain chemical compounds that can modulate the chromatin state of the viral genome resulting in the enhancement of antiviral immunity or suppression of infection and recurrence is another option; novel therapeutic techniques such as CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats) have significant potential to make changes in the latent viral DNA in sensory neurons and as such cure the neuronal location of the infection[328-333]. Wu et al[334] have also reported on AT-533, a heat shock protein-90 antagonist that expressed inhibitory effects on HSV keratitis.
The fermented extract of the Pomegranate fruit has been shown to alleviate inflammation and pain associated with HSK[335]. Similarly, Zannella et al[336] used a grape pomace extract to heal and prevent HSK lesions[336] successfully. Staying with herbal management options, Ribelato et al[337] reported that the Trichilia catigua extract shows promise as a therapeutic agent for the management of HSV/HSK[337]. Topical application of selected insulin formulations has also shown promise in accelerating re-epithelization in infective keratitis[338]. Extracts from the carnivorous purple pitcher plant have also been demonstrated to inhibit early viral transcription of HSV-1[339].
Maqsood et al[340] successfully used Omnilenz® to manage corneal epithelium defects successfully. They were able to adapt the contact lens to apply an amniotic membrane-derived cell matrix with 57% of recipients reporting complete resolution of lesions after treatment[340]. Varela-Garcia et al[341] also designed a special hydrogel contact lens to carry Acyclovir and Valacyclovir. They reported that the contact lenses so designed were able to carry Valacyclovir better than Acyclovir. They concluded that these special hydrogel contact lenses are a viable tool for extended delivery of antiviral therapy to infected eyes[341]. BostonSight developed the PROSE special contact lenses for clearing chronic corneal opacities. The Boston keratoprosthesis (KPro) is another example of an artificial cornea used in cases of HSK affecting corneal clarity[342]. Cressey et al[343] reported a case series where the PROSE was successfully used to clear corneal scarring in HSK patients[343]. In addition to contact lenses, ocular inserts are now being designed to provide continued delivery of the drug inside the eye[344]. Autologous bone marrow stem cells also show promise in managing immune-mediated corneal inflammation with little or no side effects[345,346].
Cryopreserved amniotic membrane has been used as adjuvant therapy[347]. Conversely, Insulin-Like Growth Factor Binding Protein-3 worsens HSK keratitis, hence its downregulation may mitigate HSK expression[348]. Treating human cornea explants that are infected with HSV with cold atmospheric plasma (CAP) has been shown to deactivate HSV-1 and lessen the severity[349]. Ointment-based matrix regenerating agent also offers some promise in the treatment of neurotrophic corneal ulcers secondary to HSK[350].
MiRNAs are novel discoveries that deepen our understanding of disease processes in the body[351]. Amniotic membrane transplantation is a good option for managing indolent epithelial keratitis and HSK in diabetic patients following cataract surgery[352]. Drops formulated from peripherally derived autologous blood may quicken the healing of neurotrophic HSV keratitis[353]. miRNAs affect infection in the eyes by regulating the human immune system[354]. An attenuation of miR-155/miR-183/96/182 mitigated the intensity of PA keratitis in feline models as reported by Xu and Hazlett[355]. miRNAs have also been used to deliver HSV-1-erasing lentiviral particles, blocking the reoccurrence of HSV in at least three disease models[356].
The TBK1 and IKKε inhibitor, bx795 has been reported to suppress HSV-1 both in-vivo and in-vitro[357] In a study carried out by Zhang et al[358], they identified Ras-related C3 botulinum toxin as a potential target for treating HSV-1-related diseases using NSC23766 and Ehop016[358]. Following subconjunctival injection in mice, a novel SHIP-1 activator, AQX1125, reduced CD4+ lymphocyte infiltration via modulation of P13K signaling[359-360].
Diphenyleneiodonium (DPI), an inhibitor of NADPH Oxidase 2, yielded a reduction in ROS release from neutrophils; thus, DPI was hypothesized to ameliorate herpetic stromal keratitis via its reduction effects[361].
Harringtonine, isolated from Cephalotaxus harringtonia, showed potential as a novel viral entry inhibitor to strains (HSV-1 blue & HSV-1 153) with resistance to acyclovir by targeting herpesvirus entry mediator (HVEM)[362]. Fujimoto et al[363] suggested the use of HVEM and nectin-1 products as therapeutic and preventive drugs against HSV-1 and HSV-2 infection particularly nectin-1 Lg as an eyedrop[363].
Subconjunctival injection of PKHB1 peptide in murine eyes infected with HSV-1 triggered the release of antigen-presenting cells, CD8+ lymphocytes, and other immunodeficiency cascades which were attributed to the alleviation of viral antigens[364]. One percent dispirotripiperazine gel proved efficacious for the resolution of HSV keratitis in preclinical rabbit models[365]. Small interfering (siRNA) delivered to HSV-infected in-vitro cells with an adenovirus type-5 vehicle showed potential for prophylaxis via inhibition of herpesvirus replication[366]. Inhibiting effects on 'disruptor of telomeric silencing 1-like' (Dot1 L) by siRNA and EPZ ameliorated corneal inflammation via non-release of ROS both in-vivo & in-vitro[367]. Dendritic cell-based DNA vaccination relieved manifestations of primary and recurrent HSK in murine experiments[368]. The rare sugar, i-picose was identified as a promising novel therapeutic target in murine HSV-1-related diseases, including HSK[369].
Pigment Epithelium Derived Factor has been shown in mice to reduce the severity of HSK[370]. An atypical presentation of presumed herpetic stromal keratitis was reportedly controlled following inoculation with Staphylococcus aureus lysates[371]. Ke et al[372] demonstrated the role of the FAK/PI3/Akt signaling pathway and MMP-2 and MMP-9 play in the development of HSK[372]. In treating stromal keratitis, it is important to add topical steroids to quell inflammation[373].
Topical Tacrolimus has been shown to improve visual acuity and reduce cornea inflammation, neovascularization, and cornea scarring, thus it is possible to inculcate it into the armamentarium of HSK management[374]. Transplantation of acellular porcine corneal stroma was a viable short-term substitute in a sample of Chinese patients with HSK keratitis[375].
Gene therapy (using an adenovirus type-2 vector) via meganuclease delivery to HSV-1 infected rabbit cornea transplantation models led to reduced HSV expression and attenuated immune responses[376]. Metabolic reprogramming via intraperitoneal metformin in mice infected with ocular HSV led to reduced expression of HSK lesions; with marked limitation of CNS complications induced by attempted metabolic therapy with 2-deoxy-d-glucose[377]. Lipid mediators are suggested to mediate the induction and mitigation of inflammatory processes. Zhang et al[378] have suggested that the Lipid mediator 11(12)-EET is potentially able to treat HSK[378].
The use of Von Willebrand factor has been identified as a good anchor to help in the delivery of therapeutics to prevent scarring and poor vision secondary to damaged cornea surface[379].
Dhanushkodi et al[380] discovered the use of engineered fibroblast growth factor-1 as a novel technique to reduce primary and recurrent HSK[380,381]. Shan et al[382] further suggested a crucial role oleanolic acid plays in the treatment of HSV keratitis, especially skin lesions HSV zosteriform model[382]. γδ T cells in the cornea help in early immune defense against several infections including HSV, exploring further ways to boost its response to HSV could prove crucial in managing the condition[383].
Transcriptomic data and bioinformatic analysis could possibly provide clues into the detailed molecular mechanism of HSK action and the potential therapeutic targets[384]. Targeting the IL-27 which is a pro-inflammatory cytokine controlled CD4+ Foxp3+ Tregs (regulatory T-cells) could aid in treating HSV stromal keratitis[385,386].
Minegaki et al[387] in their study showed that tandem pentapeptides repeat a derivative of Major royal jelly proteins found in royal jelly has an anti-inflammatory ability which is beneficial in reducing IL-6 and TNF-α which are stimulated in HSV infection of the cornea[387].
In their work Rao and Suvas stated the role of hypoxia in the development of HSK lesions, they investigated the expression of hypoxia-associated glycolytic genes in HSV-1 infected corneas laying a great foundation for more research into inflammatory hypoxia and hypoxia associated genes and the possibility of targeting hypoxia-inducible factor[388]. Wang et al[389] suggested that blocking the interaction between glycoprotein K and signal peptide peptidase may have a therapeutic effect in the management of HSV-1-associated eye disease[389].
Jiang et al[390] in their research suggested that BMS-265246(CDK) which is a CDK ½ inhibitor is effective and potent against HSV-1 especially as it interferes with multiple steps in the replication of HSV-1[390]. Sumbria et al[391] in their study suggested a dietary change to increase levels of short-chain fatty acid as a possible modality to be in place to reduce the impact of herpes recurrence in humans[391]. Majmudar et al[392] in their work showed strong evidence to support that SPGG (sulfated pentagalloylglucoside) is a viral entry inhibitor against HSV infection of the eyes[392].
Contact lenses have been implicated in infectious processes[393]. Subramaniam et al[394] detailed a case report of HSK in a contact lens wearer that completely resolved on oral antiviral therapy of 800 mg of Acyclovir five times daily alone[394]. However, it should be noted that Acanthamoeba is more likely to cause keratitis in contact lens wearers than HSV[395-397]. AK is occasionally misdiagnosed as HSV in, especially, very early stages[398,399]. Toshida and Sadamatsu also reported an incidence of HSK in a myopic individual wearing contact lenses for orthokeratology[400].
Live-attenuated vaccines may also show promise in preventing outbreaks of HSK. These vaccines introduce a weakened strain of the virus to the body, thereby allowing the body to develop natural defenses to the disease[401].
In childhood stromal keratitis is the most common cornea manifestation of HSV infection; it usually progresses with scarring, residual astigmatism, and amblyopia. The recurrence rate is higher in the pediatric population especially those with immunosuppression[402]. Autosomal recessive Tyrosinemia type II presents with pseudo-dendritic keratitis and palmoplantar hyperkeratosis in affected infants and young children[403]. It is a worthy differential in the pediatric population.
Identifying HSK and treatment in children is challenging as they are at high risk for developing visual morbidity and a more aggressive HSK course that results in the scarring of the cornea and possibly amblyopia[404]. HSV keratitis should be considered as a differential diagnosis in a pediatric patient with keratitis[405].
In the management of HSK in children, the use of oral acyclovir as prophylaxis is safe, and its efficacy is related to compliance with therapy[406]. The incidence of HSK in penetrating patients who had cornea refractive surgeries is higher than in the general population[407].
Pediatric patients who have undergone penetrating keratoplasty for HSK have been shown to experience graft rejection, this must be diagnosed to minimize permanent damage significantly[408,409]. Treatment of pediatric HSK usually involves acyclovir, which generally gives a good prognosis[410]. Suppressive oral therapy may however be needed in the future if a recrudescence occurs after initial topical therapy. Of worthy mention is the ability of the pediatric myelogenous leukemia drug 6-thioguanine to mitigate HSV both in vitro and in vivo[411].
The SARS-CoV-2[412] has been implicated in HSV Keratitis[413,414]. HSK has been reported to relapse post mRNA coronavirus disease 2019 vaccination[415]. Herpetic eye disease has been seen to result more from individuals who received the BNT162b2 vaccine than those who received the mRNA-1273 or Ad26.COV2.S vaccines[416].
An atypical type of HSV keratitis, Archipelago Keratitis has been identified in immunosuppressed persons[417,418]. Table 1 below summarizes further literature on HSV occurrence in immunocompromised individuals[419-434].
| Ref. | Predisposing condition | Summary |
| Gupta et al[419] | Necrotizing fascitis | HZO was the first sign of reactivation of varicella-zoster |
| Murgova and Balchev[420] | COVID-19 | COVID-19 vaccines caused a reactivation in HSV ocular diseases |
| Yildiz et al[421] | COVID-19 | COVID-19 vaccines caused a reactivation in HSV ocular diseases |
| Huang et al[422] | COVID-19 | HSK was the 3rd most common corneal complication after COVID-19 vaccination |
| Matharu et al[423] | Cancer | VZV and HSV cornea co-infection in a patient with systemic immunosuppression |
| Cohen et al[424] | COVID-19 | Herpetic cornea infection may develop post SARS-CoV-2 vaccinations |
| Yoshida et al [425] | RA | Individuals with RA have the tendency to develop HSK which is usually more severe due to their immunocompromised state |
| Fei et al[426] | COVID-19 | HSK could happen after vaccination with a possible preponderance |
| Al-Dwairi et al[427] | COVID-19 | Reactivation of HSK on cornea graft after taking SARS-CoV-2 mRNA vaccine |
| Majtanova et al[428] | COVID-19 | Five incidences of HSK after COVID-19 vaccination |
| Roberts et al[429] | COVID-19 | Negative result of COVID-19 in the tears in a patient with recurring HSV Keratitis |
| Kuziez et al[430] | COVID-19 | HSK was reviewed as an adverse effect occurring after COVID-19 |
| Mohammadzadeh et al[431] | COVID-19 | HSK-suspected reactivation resulted in corneal graft rejection |
| Ichhpujani et al[432] | COVID-19 | HSK was implicated in a review of associated complications reported after vaccination |
| Ono et al[433] | Human herpes virus | Reactivation of HHV-6B infection |
| Sinha et al[434] | Psoriasis | HSV keratitis after taking secukinumab for the treatment of psoriasis |
Descemet membrane endothelial keratoplasty has been identified as an effective option for treating cornea edema resulting from HSV-1-related endotheliitis[435-438]. Novel endothelium-free grafts with endothelial cell regenerative capability may improve outcomes for high-risk transplant cases secondary to chronic HSV endotheliitis[439]. Intensive antiviral prophylaxis could reduce the risk of graft failure and recurrence of the condition[440]. The use of topical steroids, antibiotics, and higher doses of oral acyclovir leads to better postoperative outcomes of deep anterior lamellar and penetrating keratoplasties for corneal scarring caused by HSK[441].
There are several surgical interventions for the management of HSK but it basically involves either the replacement of the infected tissue or support of the tissue to aid healing. Cornea neurotization is gaining more acceptance today[442-444]. Lin and Lai reported a novel technique where the supratrochlear nerve of the same side as the affected eye was tunneled to the cornea to re-innervate damaged trigeminal nerve fibers with good results[445]. Bourcier et al[446] also reported similar results using the Lateral Antebrachial Cutaneous Nerve via a minimally invasive cornea neurotization procedure[446].
Roberts et al[447], reported the usefulness of sutureless tectonic pul-through mini-DSAEK in the management of corneal perforations secondary to herpes simplex infection or other causes[447]. New Onset HSK after keratoplasty could be managed by antiviral medications or amniotic membrane transplantation[448].
A Bowman’s layer onlay graft is relatively easier as it does not resolve to deeper keratoplasty, it has the potential to reduce superficial cornea scarring and/or anterior cornea abnormalities[449]. Amniotic membrane transplantation reduces ocular opacity and scarring by inhibiting the secretion of inflammatory cytokines and fibroblast proliferation[450,451].
Lamas-Francis et al[452] reported the use of amniotic membrane transplantation for corneal ulceration secondary to infectious causes, they reported a success rate of 62.8% with 37.2% requiring additional surgery[452]. Similarly, Hayek et al[453] reported using a lyophilized amniotic membrane for the treatment of a 2 mm wide perforating cornea ulcer and didn’t need keratoplasty[453].
Corneal trauma during surgery poses special problems due to the possibility of recrudescence of latent HSV infections in carriers. Preventive medication before surgery has been suggested for HSV seropositive patients[454] Patients with co-morbid ocular conditions such as cataracts could undergo Penetrating Keratoplasty before the cataract surgery as it has been shown in the literature that this has fewer complications and higher graft survival rate[455].
Graft failure after Penetrating Keratoplasty is common in eyes with HSK, hence it is important that HSV-1 or VZV PCR testing is done on all explanted cornea[456]. High-dose antivirals with prolonged tapering steroid doses prior to performing mushroom keratoplasty on eyes with herpetic vascularized corneal scars resulted in lower rates of graft failure and immunologic rejection in a longitudinal study conducted by Yu et al[457]. There were also higher than normal rates of graft failure with the Boston type I KPro for eyes having prior corneal HSV infection[458]. Suzuki et al[228] considered lamellar graft patching a safe and effective option for managing corneal perforations secondary to HSK-associated neurotrophic keratopathy[228].
Tape splint Tarsorrhaphy has been identified as a useful inexpensive technique to treat Persistent corneal epithelial defects[459]. Hata-Mizuno et al[460] reported a case of conjunctival epithelial ingrowth after PKP in a patient with Herpetic corneal keratitis[460].
The quest for better diagnosis, prevention, and management of HSK was uppermost in the minds of sampled participants in a paper by Liu et al[461]. Novel corneal active storage mediums enable better study and research of ex-vivo disease patterns in herpetic keratitis[462]. In the future, target extraocular (maxillary) vaccination to inhibit ocular herpes simplex reactivation may improve the epidemiology of the disease[463].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li GG, China S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Stepp MA, Menko AS. Immune responses to injury and their links to eye disease. Transl Res. 2021;236:52-71. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Kwon MS, Carnt NA, Truong NR, Pattamatta U, White AJ, Samarawickrama C, Cunningham AL. Dendritic cells in the cornea during Herpes simplex viral infection and inflammation. Surv Ophthalmol. 2018;63:565-578. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Cabrera-Aguas M, Khoo P, Watson SL. Infectious keratitis: A review. Clin Exp Ophthalmol. 2022;50:543-562. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Enaholo ES, Musa MJ, Zeppieri M. Objective Refraction Technique: Retinoscopy. 2023 Oct 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] [Cited in This Article: ] |
| 5. | Pramanick P, Sengupta M, Banerjee M, Ghosh S, Mitra AN, Chakraborty M. Microbiological Profile in Patients Having Keratitis in a Tertiary Care Hospital in India. Cureus. 2022;14:e31653. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Muto T, Imaizumi S, Kamoi K. Viral Conjunctivitis. Viruses. 2023;15. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Patil S, Beck P, Nelson TB, Bran A, Roland W. Herpes Simplex Virus-2 Meningoencephalitis With Abducens Nerve Palsy With Literature Review. Cureus. 2021;13:e15523. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Arshad S, Petsoglou C, Lee T, Al-Tamimi A, Carnt NA. 20 years since the Herpetic Eye Disease Study: Lessons, developments and applications to clinical practice. Clin Exp Optom. 2021;104:396-405. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Shahnazaryan D, Khalil R, Wynne C, Jefferies CA, Ní Gabhann-Dromgoole J, Murphy CC. Herpes simplex virus 1 targets IRF7 via ICP0 to limit type I IFN induction. Sci Rep. 2020;10:22216. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Asanuma Y, Ishimaru H, Sato T, Yamamoto T, Aoyama Y. Herpes simplex virus-induced murine dry skin model through sweating disturbance. J Dermatol Sci. 2022;107:151-159. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Filiberti A, Gmyrek GB, Montgomery ML, Sallack R, Carr DJJ. Loss of Osteopontin Expression Reduces HSV-1-Induced Corneal Opacity. Invest Ophthalmol Vis Sci. 2020;61:24. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Kosker M, Hammersmith KM, Nagra PK, Nassef AH, Rapuano CJ. The Association between Diabetes and Herpes Simplex Eye Disease. Ocul Immunol Inflamm. 2018;26:125-129. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Bian Y, Ma KK, Hall NE, Elze T, Lorch A, Miller JW, Dana R, Yin J. Neurotrophic Keratopathy in the United States: An Intelligent Research in Sight Registry Analysis. Ophthalmology. 2022;129:1255-1262. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Giannaccare G, Borselli M. Herpes Simplex Dendritic Keratitis. N Engl J Med. 2023;389:e26. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Yun H, Yee MB, Lathrop KL, Kinchington PR, Hendricks RL, St Leger AJ. Production of the Cytokine VEGF-A by CD4(+) T and Myeloid Cells Disrupts the Corneal Nerve Landscape and Promotes Herpes Stromal Keratitis. Immunity. 2020;53:1050-1062.e5. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Chranioti A, Malamas A, Metallidis S, Mataftsi A, Chalvatzis N, Ziakas N. Bilateral Herpes Simplex Virus-related Peripheral Ulcerative Keratitis Leading to Corneal Perforation in a Patient with Primary Herpes Simplex Virus Infection. J Ophthalmic Vis Res. 2019;14:93-96. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Das AV, Satyashree G, Joseph J, Bagga B. Herpes simplex virus keratitis: electronic medical records driven big data analytics report from a tertiary eye institute of South India. Int Ophthalmol. 2023;43:4669-4676. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Guo LL, Zhang Y, Li N, Wang ZQ, Tian L, Deng SJ, Sun XG. [Clinical manifestations of 1 015 cases of herpes simplex virus keratitis]. Zhonghua Yan Ke Za Zhi. 2022;58:778-783. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Harbiyeli II, Oruz O, Erdem E, Cam B, Demirkazik M, Acikalin A, Kibar F, Ilkit M, Yarkin F, Yagmur M. Clinical aspects and prognosis of polymicrobial keratitis caused by different microbial combinations: a retrospective comparative case study. Int Ophthalmol. 2021;41:3849-3860. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Roels D, Coorevits L, Lagrou K. Tintelnotia destructans as an emerging opportunistic pathogen: First case of T. destructans superinfection in herpetic keratitis. Am J Ophthalmol Case Rep. 2020;19:100791. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Sun Y, Li W, Wang M, Xing Q, Sun X. Clinical diagnosis and treatment of rare painless keratitis caused by three pathogens: clinical practice and experiential discussion. J Int Med Res. 2020;48:300060519895671. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Wang Y, Li F, Wang Z, Song X, Ren Z, Wang X, Wang Y, Zheng K. Luteolin inhibits herpes simplex virus 1 infection by activating cyclic guanosine monophosphate-adenosine monophosphate synthase-mediated antiviral innate immunity. Phytomedicine. 2023;120:155020. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Sibley D, Larkin DFP. Update on Herpes simplex keratitis management. Eye (Lond). 2020;34:2219-2226. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Guo H, Koehler HS, Dix RD, Mocarski ES. Programmed Cell Death-Dependent Host Defense in Ocular Herpes Simplex Virus Infection. Front Microbiol. 2022;13:869064. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Park SJ, Riccio RE, Kopp SJ, Ifergan I, Miller SD, Longnecker R. Herpesvirus Entry Mediator Binding Partners Mediate Immunopathogenesis of Ocular Herpes Simplex Virus 1 Infection. mBio. 2020;11. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Kawaguchi Y. [Recent Advances in Basic Research on the Herpes Simplex Virus]. Uirusu. 2019;68:115-124. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Liu Z, Xia L. E3 Ligase RNF5 inhibits type I interferon response in herpes simplex virus keratitis through the STING/IRF3 signaling pathway. Front Microbiol. 2022;13:944101. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Bajracharya L, Bade AR, Gurung R, Dhakhwa K. Demography, Risk Factors, and Clinical and Microbiological Features of Microbial Keratitis at a Tertiary Eye Hospital in Nepal. Clin Ophthalmol. 2020;14:3219-3226. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Lasagni Vitar RM, Triolo G, Fonteyne P, Acuti Martellucci C, Manzoli L, Rama P, Ferrari G. Epidemiology of Corneal Neovascularization and Its Impact on Visual Acuity and Sensitivity: A 14-Year Retrospective Study. Front Med (Lausanne). 2021;8:733538. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Yadavalli T, Koganti R, Shukla D. Infection-Induced Porcine Ex Vivo Corneal Wound Model to Study the Efficacy of Herpes Simplex Virus-1 Entry and Replication Inhibitors. Methods Mol Biol. 2021;2193:183-196. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Saadouli D, Ammari L, Ben Mansour K, Yahyaoui Y, Aissa S, Mohamed Ali EA, Yahyaoui S, Tiouri H. Ocular manifestations of people living with HIV in Tunisia. South Afr J HIV Med. 2021;22:1193. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Koujah L, Allaham M, Patil CD, Ames JM, Suryawanshi RK, Yadavalli T, Agelidis A, Mun C, Surenkhuu B, Jain S, Shukla D. Entry receptor bias in evolutionarily distant HSV-1 clinical strains drives divergent ocular and nervous system pathologies. Ocul Surf. 2021;21:238-249. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Coulon PG, Dhanushkodi N, Prakash S, Srivastava R, Roy S, Alomari NI, Nguyen AM, Warsi WR, Ye C, Carlos-Cruz EA, Mai UT, Cruel AC, Ekmekciyan KM, Pearlman E, BenMohamed L. NLRP3, NLRP12, and IFI16 Inflammasomes Induction and Caspase-1 Activation Triggered by Virulent HSV-1 Strains Are Associated With Severe Corneal Inflammatory Herpetic Disease. Front Immunol. 2019;10:1631. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Jahanban-Esfahlan R, Seidi K, Majidinia M, Karimian A, Yousefi B, Nabavi SM, Astani A, Berindan-Neagoe I, Gulei D, Fallarino F, Gargaro M, Manni G, Pirro M, Xu S, Sadeghi M, Nabavi SF, Shirooie S. Toll-like receptors as novel therapeutic targets for herpes simplex virus infection. Rev Med Virol. 2019;29:e2048. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Cui YH, Liu Q, Xu ZY, Li JH, Hu ZX, Li MJ, Zheng WL, Li ZJ, Pan HW. Quantitative proteomic analysis of human corneal epithelial cells infected with HSV-1. Exp Eye Res. 2019;185:107664. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Patil CD, Suryawanshi RK, Kapoor D, Shukla D. Postinfection Metabolic Reprogramming of the Murine Trigeminal Ganglion Limits Herpes Simplex Virus-1 Replication. mBio. 2022;13:e0219422. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Matundan HH, Wang S, Jaggi U, Yu J, Ghiasi H. Suppression of CD80 Expression by ICP22 Affects Herpes Simplex Virus Type 1 Replication and CD8(+)IFN-γ(+) Infiltrates in the Eyes of Infected Mice but Not Latency Reactivation. J Virol. 2021;95:e0103621. [PubMed] [DOI] [Cited in This Article: ] |
| 39. | Tormanen K, Wang S, Ghiasi H. CD80 Plays a Critical Role in Increased Inflammatory Responses in Herpes Simplex Virus 1-Infected Mouse Corneas. J Virol. 2020;94. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Yin XT, Baugnon NK, Potter CA, Tai S, Keadle TL, Stuart PM. CD28 Costimulation Is Required for Development of Herpetic Stromal Keratitis but Does Not Prevent Establishment of Latency. J Virol. 2019;93. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Marcocci ME, Napoletani G, Protto V, Kolesova O, Piacentini R, Li Puma DD, Lomonte P, Grassi C, Palamara AT, De Chiara G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020;28:808-820. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Gege C, Bravo FJ, Uhlig N, Hagmaier T, Schmachtenberg R, Elis J, Burger-Kentischer A, Finkelmeier D, Hamprecht K, Grunwald T, Bernstein DI, Kleymann G. A helicase-primase drug candidate with sufficient target tissue exposure affects latent neural herpes simplex virus infections. Sci Transl Med. 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Koujah L, Suryawanshi RK, Shukla D. Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea. Cell Mol Life Sci. 2019;76:405-419. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | Labib BA, Chigbu DI. Clinical Management of Herpes Simplex Virus Keratitis. Diagnostics (Basel). 2022;12. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Caputo A, Marconi P. Vaccine Development for Herpes Simplex Viruses: A Commentary of Special Issue Editors. Vaccines (Basel). 2021;9. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Ahmad B, Patel BC. Herpes Simplex Keratitis. 2023 Apr 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] [Cited in This Article: ] |
| 47. | Meyer JJ. Rates of Herpes Simplex Virus Types 1 and 2 in Ocular and Peri-ocular Specimens. Ocul Immunol Inflamm. 2023;31:149-152. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Gupta D 4th, Daigavane S. A Clinical Case of Viral Keratitis. Cureus. 2022;14:e30311. [PubMed] [DOI] [Cited in This Article: ] |
| 49. | Alekseev O, Donegan WE, Donovan KR, Limonnik V, Azizkhan-Clifford J. HSV-1 Hijacks the Host DNA Damage Response in Corneal Epithelial Cells through ICP4-Mediated Activation of ATM. Invest Ophthalmol Vis Sci. 2020;61:39. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Kolb AW, Ferguson SA, Larsen IV, Brandt CR. Disease parameters following ocular herpes simplex virus type 1 infection are similar in male and female BALB/C mice. PLoS One. 2023;18:e0287194. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Riccio RE, Park SJ, Longnecker R, Kopp SJ. Characterization of Sex Differences in Ocular Herpes Simplex Virus 1 Infection and Herpes Stromal Keratitis Pathogenesis of Wild-Type and Herpesvirus Entry Mediator Knockout Mice. mSphere. 2019;4. [PubMed] [DOI] [Cited in This Article: ] |
| 52. | Yadav S, Commiskey P, Kowalski RP, Jhanji V. Herpes Simplex Virus 2 Blepharokeratoconjunctivitis. Curr Eye Res. 2022;47:361-364. [PubMed] [DOI] [Cited in This Article: ] |
| 53. | Zhu S, Viejo-Borbolla A. Pathogenesis and virulence of herpes simplex virus. Virulence. 2021;12:2670-2702. [PubMed] [DOI] [Cited in This Article: ] |
| 54. | Li L, Li Y, Li X, Xia Y, Wang E, Gong D, Chen G, Yang L, Zhang K, Zhao Z, Fraser NW, Fan Q, Li B, Zhang H, Cao X, Zhou J. HSV-1 infection and pathogenesis in the tree shrew eye following corneal inoculation. J Neurovirol. 2020;26:391-403. [PubMed] [DOI] [Cited in This Article: ] |
| 55. | Harrison KS, Zhu L, Thunuguntla P, Jones C. Antagonizing the Glucocorticoid Receptor Impairs Explant-Induced Reactivation in Mice Latently Infected with Herpes Simplex Virus 1. J Virol. 2019;93. [PubMed] [DOI] [Cited in This Article: ] |
| 56. | Yan C, Luo Z, Li W, Li X, Dallmann R, Kurihara H, Li YF, He RR. Disturbed Yin-Yang balance: stress increases the susceptibility to primary and recurrent infections of herpes simplex virus type 1. Acta Pharm Sin B. 2020;10:383-398. [PubMed] [DOI] [Cited in This Article: ] |
| 57. | Wang T, Dong M, Jiang Y, Wang S, Shi W. Role of Dendritic Cells and Inflammatory Cells in Herpetic Endotheliitis: Analysis Using In Vivo Confocal Microscopy. Cornea. 2018;37:748-754. [PubMed] [DOI] [Cited in This Article: ] |
| 58. | Shipton C, Hind J, Biagi J, Lyall D. Anterior segment optical coherence tomographic characterisation of keratic precipitates. Cont Lens Anterior Eye. 2020;43:465-468. [PubMed] [DOI] [Cited in This Article: ] |
| 59. | Shah A, Joshi P, Bhusal B, Subedi P. Clinical Pattern And Visual Impairment Associated With Herpes Simplex Keratitis. Clin Ophthalmol. 2019;13:2211-2215. [PubMed] [DOI] [Cited in This Article: ] |
| 60. | Barequet IS, Wasserzug Y. Herpes simplex keratitis after cataract surgery. Cornea. 2007;26:615-617. [PubMed] [DOI] [Cited in This Article: ] |
| 61. | Cho YK, Kwon JW, Konda S, Ambati BK. Epithelial Keratitis After Cataract Surgery. Cornea. 2018;37:755-759. [PubMed] [DOI] [Cited in This Article: ] |
| 62. | Zou M, Zhang Y, Huang X, Gao S, Liu C. Epithelial keratitis mimicking herpes simplex keratitis in a patient after cataract surgery: A case report. Medicine (Baltimore). 2019;98:e16591. [PubMed] [DOI] [Cited in This Article: ] |
| 63. | Al-Lozi A, Cai S, Chen X, Perez VL, Venkateswaran N. Granulicatella Adiacens as an Unusual Cause of Microbial Keratitis and Endophthalmitis: A Case Series and Literature Review. Ocul Immunol Inflamm. 2022;30:1181-1185. [PubMed] [DOI] [Cited in This Article: ] |
| 64. | Park BC, Lim HR, Park SJ, Koh JW. Clinical Features and Management of Stenotrophomonas Maltophilia Keratitis. Ophthalmol Ther. 2021;10:525-533. [PubMed] [DOI] [Cited in This Article: ] |
| 65. | McCormick I, James C, Welton NJ, Mayaud P, Turner KME, Gottlieb SL, Foster A, Looker KJ. Incidence of herpes simplex virus keratitis and other ocular disease: global review and estimates. Ophthalmic Epidemiol. 2022;29:353-362. [PubMed] [DOI] [Cited in This Article: ] |
| 66. | Chodosh J, Ung L. Adoption of Innovation in Herpes Simplex Virus Keratitis. Cornea. 2020;39:S7-S18. [PubMed] [DOI] [Cited in This Article: ] |
| 67. | Harris KD. Herpes Simplex Virus Keratitis. Home Healthc Now. 2019;37:281-284. [PubMed] [DOI] [Cited in This Article: ] |
| 68. | Foster A. Case study: Clinical research. Community Eye Health. 2022;35:4. [PubMed] [Cited in This Article: ] |
| 69. | Chaloulis SK, Mousteris G, Tsaousis KT. Incidence and Risk Factors of Bilateral Herpetic Keratitis: 2022 Update. Trop Med Infect Dis. 2022;7. [PubMed] [DOI] [Cited in This Article: ] |
| 70. | Gauthier AS, Noureddine S, Delbosc B. Interstitial keratitis diagnosis and treatment. J Fr Ophtalmol. 2019;42:e229-e237. [PubMed] [DOI] [Cited in This Article: ] |
| 71. | Farooq AV, Paley GL, Lubniewski AJ, Gonzales JA, Margolis TP. Unilateral Posterior Interstitial Keratitis as a Clinical Presentation of Herpes Simplex Virus Disease. Cornea. 2018;37:375-378. [PubMed] [DOI] [Cited in This Article: ] |
| 72. | Acharya M, Dave A, Farooqui JH. Commentary: Herpes keratitis: A diagnostic challenge. Indian J Ophthalmol. 2019;67:1046-1047. [PubMed] [DOI] [Cited in This Article: ] |
| 73. | Shrestha P, Paudel S. Stromal Keratitis among Herpes Simplex Keratitis Patients in a Tertiary Eye Hospital: A Descriptive Cross-sectional Study. JNMA J Nepal Med Assoc. 2022;60:1008-1010. [PubMed] [DOI] [Cited in This Article: ] |
| 74. | Antony F, Pundkar C, Sandey M, Jaiswal AK, Mishra A, Kumar A, Channappanavar R, Suryawanshi A. IFN-λ Regulates Neutrophil Biology to Suppress Inflammation in Herpes Simplex Virus-1-Induced Corneal Immunopathology. J Immunol. 2021;206:1866-1877. [PubMed] [DOI] [Cited in This Article: ] |
| 75. | Yun H, Yin XT, Stuart PM, St Leger AJ. Sensory Nerve Retraction and Sympathetic Nerve Innervation Contribute to Immunopathology of Murine Recurrent Herpes Stromal Keratitis. Invest Ophthalmol Vis Sci. 2022;63:4. [PubMed] [DOI] [Cited in This Article: ] |
| 76. | Dana R, Farid M, Gupta PK, Hamrah P, Karpecki P, McCabe CM, Nijm L, Pepose JS, Pflugfelder S, Rapuano CJ, Saini A, Gibbs SN, Broder MS. Expert consensus on the identification, diagnosis, and treatment of neurotrophic keratopathy. BMC Ophthalmol. 2021;21:327. [PubMed] [DOI] [Cited in This Article: ] |
| 77. | Pérez-Bartolomé F, Mingo Botín D, de Dompablo E, de Arriba P, Arnalich Montiel F, Muñoz Negrete FJ. Post-herpes neurotrophic keratopathy: Aetiopathogenesis, clinical signs and current therapies. Arch Soc Esp Oftalmol (Engl Ed). 2019;94:171-183. [PubMed] [DOI] [Cited in This Article: ] |
| 78. | Singh RB, Naderi A, Cho W, Ortiz G, Musayeva A, Dohlman TH, Chen Y, Ferrari G, Dana R. Modulating the tachykinin: Role of substance P and neurokinin receptor expression in ocular surface disorders. Ocul Surf. 2022;25:142-153. [PubMed] [DOI] [Cited in This Article: ] |
| 79. | Fortin P, Wickas T, Perry HD, Wawrzusin P, Morcos M. Bell's palsy with Herpes simplex disciform keratitis: A case report. Am J Ophthalmol Case Rep. 2022;27:101575. [PubMed] [DOI] [Cited in This Article: ] |
| 80. | Charron AJ, Ward SL, North BJ, Ceron S, Leib DA. The US11 Gene of Herpes Simplex Virus 1 Promotes Neuroinvasion and Periocular Replication following Corneal Infection. J Virol. 2019;93. [PubMed] [DOI] [Cited in This Article: ] |
| 81. | Farooq AV, Shukla D. Corneal latency and transmission of herpes simplex virus-1. Future Virol. 2011;6:101-108. [PubMed] [DOI] [Cited in This Article: ] |
| 82. | Dvivedi A, Murthy SI, Garudadri C, Sheba E, Sharma S. Bilateral Severe Herpes Simplex Endotheliitis with a Possible Association with Latanoprost. Ocul Immunol Inflamm. 2023;31:1073-1075. [PubMed] [DOI] [Cited in This Article: ] |
| 83. | Wand M, Gilbert CM, Liesegang TJ. Latanoprost and herpes simplex keratitis. Am J Ophthalmol. 1999;127:602-604. [PubMed] [DOI] [Cited in This Article: ] |
| 84. | Hashizume K, Nabeshima T, Fujiwara T, Machida S, Kurosaka D. A case of herpetic epithelial keratitis after triamcinolone acetonide subtenon injection. Cornea. 2009;28:463-464. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Narang P, Singh S, Mittal V. Bilateral herpes simplex keratitis reactivation after lacrimal gland botulinum toxin injection. Indian J Ophthalmol. 2018;66:697-699. [PubMed] [DOI] [Cited in This Article: ] |
| 86. | Ishimaru H, Hosokawa K, Sugimoto A, Tanaka R, Watanabe T, Fujimuro M. MG132 exerts anti-viral activity against HSV-1 by overcoming virus-mediated suppression of the ERK signaling pathway. Sci Rep. 2020;10:6671. [PubMed] [DOI] [Cited in This Article: ] |
| 87. | Abente S, Fariña N, Samudio M, Duré C, Bordón M, Franco L. [Keratitis due to Mycobaterium abscessus. First case report in Paraguay]. Rev Chilena Infectol. 2022;39:86-90. [PubMed] [DOI] [Cited in This Article: ] |
| 88. | Behaegel J, Ní Dhubhghaill S, Koppen C. Diagnostic Challenges in Nocardia Keratitis. Eye Contact Lens. 2018;44:S370-S372. [PubMed] [DOI] [Cited in This Article: ] |
| 89. | Alabduljabbar M, Sirajuddin F, Maktabi A, AlShabeeb R. A Rare Microsporidial Infection in Lamellar Corneal Tissue, following Transepithelial Photorefractive Keratectomy. Case Rep Ophthalmol. 2023;14:127-133. [PubMed] [DOI] [Cited in This Article: ] |
| 90. | Ting DSJ, Mckenna M, Sadiq SN, Martin J, Mudhar HS, Meeney A, Patel T. Arthrographis kalrae Keratitis Complicated by Endophthalmitis: A Case Report With Literature Review. Eye Contact Lens. 2020;46:e59-e65. [PubMed] [DOI] [Cited in This Article: ] |
| 91. | Dong PN, Hang DTT, Duong NTN, Lien MT, Chen AC, Aldave AJ. Infectious keratitis in Vietnam: etiology, organisms, and management at Vietnam National Eye Hospital. Int J Ophthalmol. 2022;15:128-134. [PubMed] [DOI] [Cited in This Article: ] |
| 92. | Ming W, Dewan N, Yeung SN, Iovieno A. Concomitant herpetic keratitis and acute retinal necrosis: clinical features and outcomes. Eye (Lond). 2020;34:2322-2327. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Ansari WH, Pichi F, Pecen PE, Lowder CY, Srivistava SK. Herpes zoster keratitis development after acute retinal necrosis. Int Ophthalmol. 2018;38:829-832. [PubMed] [DOI] [Cited in This Article: ] |
| 94. | Ren J, Antony F, Rouse BT, Suryawanshi A. Role of Innate Interferon Responses at the Ocular Surface in Herpes Simplex Virus-1-Induced Herpetic Stromal Keratitis. Pathogens. 2023;12. [PubMed] [DOI] [Cited in This Article: ] |
| 95. | Mohankrishnan A, Parmar R, Bhurani V, Dalai SK. Lack of TNF-α signaling through p55 makes the mice more susceptible to acute infection but does not alter state of latency and reactivation of HSV-1. Virus Res. 2018;244:1-5. [PubMed] [DOI] [Cited in This Article: ] |
| 96. | Tsai YE, Weng TH. Dendritic epithelial keratitis in primary herpes simplex infection. QJM. 2022;114:820-821. [PubMed] [DOI] [Cited in This Article: ] |
| 97. | Rana M, Setia M, Suvas PK, Chakraborty A, Suvas S. Diphenyleneiodonium Treatment Inhibits the Development of Severe Herpes Stromal Keratitis Lesions. J Virol. 2022;96:e0101422. [PubMed] [DOI] [Cited in This Article: ] |
| 98. | Filiberti A, Gmyrek GB, Berube AN, Carr DJJ. Osteopontin contributes to virus resistance associated with type I IFN expression, activation of downstream ifn-inducible effector genes, and CCR2(+)CD115(+)CD206(+) macrophage infiltration following ocular HSV-1 infection of mice. Front Immunol. 2022;13:1028341. [PubMed] [DOI] [Cited in This Article: ] |
| 99. | Ikeda M, Ito A, Sekine Y, Fujimuro M. UBE1a Suppresses Herpes Simplex Virus-1 Replication. Viruses. 2020;12. [PubMed] [DOI] [Cited in This Article: ] |
| 100. | Ikeda M, Watanabe T, Ito A, Fujimuro M. Herpes simplex virus 1 infection induces ubiquitination of UBE1a. Biochem J. 2021;478:261-279. [PubMed] [DOI] [Cited in This Article: ] |
| 101. | Poon SHL, Wong WHL, Lo ACY, Yuan H, Chen CF, Jhanji V, Chan YK, Shih KC. A systematic review on advances in diagnostics for herpes simplex keratitis. Surv Ophthalmol. 2021;66:514-530. [PubMed] [DOI] [Cited in This Article: ] |
| 102. | Hooker EA, Faulkner WJ, Kelly LD, Whitford RC. Prospective study of the sensitivity of the Wood's lamp for common eye abnormalities. Emerg Med J. 2019;36:159-162. [PubMed] [DOI] [Cited in This Article: ] |
| 103. | Peng RM, Guo YX, Xiao GG, Li CD, Hong J. Characteristics of Corneal Endotheliitis among Different Viruses by in Vivo Confocal Microscopy. Ocul Immunol Inflamm. 2021;29:324-332. [PubMed] [DOI] [Cited in This Article: ] |
| 104. | Erdem E, Harbiyeli İİ, Öztürk G, Oruz O, Açıkalın A, Yağmur M, Ersöz R, Yarkın F. Atypical herpes simplex keratitis: frequency, clinical presentations and treatment results. Int Ophthalmol. 2020;40:659-665. [PubMed] [DOI] [Cited in This Article: ] |
| 105. | Bayraktutar BN, Ozmen MC, Muzaaya N, Dieckmann G, Koseoglu ND, Müller RT, Cruzat A, Cavalcanti BM, Hamrah P. Comparison of clinical characteristics of post-refractive surgery-related and post-herpetic neuropathic corneal pain. Ocul Surf. 2020;18:641-650. [PubMed] [DOI] [Cited in This Article: ] |
| 106. | Natarajan R, Matai HD, Raman S, Kumar S, Ravichandran S, Swaminathan S, Rani Alex JS. Advances in the diagnosis of herpes simplex stromal necrotising keratitis: A feasibility study on deep learning approach. Indian J Ophthalmol. 2022;70:3279-3283. [PubMed] [DOI] [Cited in This Article: ] |
| 107. | Koyama A, Miyazaki D, Nakagawa Y, Ayatsuka Y, Miyake H, Ehara F, Sasaki SI, Shimizu Y, Inoue Y. Determination of probability of causative pathogen in infectious keratitis using deep learning algorithm of slit-lamp images. Sci Rep. 2021;11:22642. [PubMed] [DOI] [Cited in This Article: ] |
| 108. | Wang L, Chen K, Wen H, Zheng Q, Chen Y, Pu J, Chen W. Feasibility assessment of infectious keratitis depicted on slit-lamp and smartphone photographs using deep learning. Int J Med Inform. 2021;155:104583. [PubMed] [DOI] [Cited in This Article: ] |
| 109. | Zhang Z, Wang H, Wang S, Wei Z, Zhang Y, Wang Z, Chen K, Ou Z, Liang Q. Deep learning-based classification of infectious keratitis on slit-lamp images. Ther Adv Chronic Dis. 2022;13:20406223221136071. [PubMed] [DOI] [Cited in This Article: ] |
| 110. | Heiligenhaus A, Rothaus K, Pleyer U. [Development of classification criteria for uveitis by the standardization of uveitis nomenclature (SUN) working group]. Ophthalmologe. 2021;118:913-918. [PubMed] [DOI] [Cited in This Article: ] |
| 111. | Almeida I, Dias L, Jesus J, Fonseca I, Matias MJ, Pedro JC. Optical coherence tomography angiography in herpetic leucoma. BMC Med Imaging. 2022;22:17. [PubMed] [DOI] [Cited in This Article: ] |
| 112. | Yu S, You D, Agrawal R, Feng Y. Noninvasive Diagnosis of Viral Keratouveitis with Retro-corneal Endothelial Plaques: A Case Series. Ocul Immunol Inflamm. 2022;30:1482-1488. [PubMed] [DOI] [Cited in This Article: ] |
| 113. | Soliman W, Nassr MA, Abdelazeem K, Al-Hussaini AK. Appearance of herpes simplex keratitis on anterior segment optical coherence tomography. Int Ophthalmol. 2019;39:2923-2928. [PubMed] [DOI] [Cited in This Article: ] |
| 114. | Rodriguez-Garcia A, Alfaro-Rangel R, Bustamante-Arias A, Hernandez-Camarena JC. In Vivo Corneal Microstructural Changes in Herpetic Stromal Keratitis: A Spectral Domain Optical Coherence Tomography Analysis. J Ophthalmic Vis Res. 2020;15:279-288. [PubMed] [DOI] [Cited in This Article: ] |
| 115. | Singh RB, Batta P. Herpes simplex virus keratitis mimicking Acanthamoeba keratitis: a clinicopathological correlation. BMJ Case Rep. 2018;2018. [PubMed] [DOI] [Cited in This Article: ] |
| 116. | Park YM, Lee JS, Yoo JM, Park JM, Seo SW, Chung IY, Kim SJ. Comparison of anterior segment optical coherence tomography findings in acanthamoeba keratitis and herpetic epithelial keratitis. Int J Ophthalmol. 2018;11:1416-1420. [PubMed] [DOI] [Cited in This Article: ] |
| 117. | Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. JAMA. 2022;327:161-172. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Moshirfar M, Hopping GC, Vaidyanathan U, Liu H, Somani AN, Ronquillo YC, Hoopes PC. Biological Staining and Culturing in Infectious Keratitis: Controversy in Clinical Utility. Med Hypothesis Discov Innov Ophthalmol. 2019;8:145-151. [PubMed] [Cited in This Article: ] |
| 119. | Satpathy G, Behera HS, Sharma A, Mishra AK, Mishra D, Sharma N, Tandon R, Agarwal T, Titiyal JS. A 20-year experience of ocular herpes virus detection using immunofluorescence and polymerase chain reaction. Clin Exp Optom. 2018;101:648-651. [PubMed] [DOI] [Cited in This Article: ] |
| 120. | Hirota A, Inada N, Shiraki Y, Shoji J, Yamagami S. Herpes Simplex DNA in Tears of Atypical Dendritic Keratitis and Multiple Punctate Subepithelial Stromal Opacity: A Case Report. Cornea. 2020;39:1177-1180. [PubMed] [DOI] [Cited in This Article: ] |
| 121. | Wang J, Cherfan DG, Goshe JM. Utility of HSV Serology for Chronic Corneal Pathology. Eye Contact Lens. 2020;46:190-193. [PubMed] [DOI] [Cited in This Article: ] |
| 122. | Rosenberg CR, Abazari A, Chou TY, Weissbart SB. Comparison of Comorbid Associations and Ocular Complications in Herpes Simplex and Zoster Keratitis. Ocul Immunol Inflamm. 2022;30:57-61. [PubMed] [DOI] [Cited in This Article: ] |
| 123. | Aljasser AA, Almulhim A, Alsarhani WK. Neonatal herpetic stromal keratitis confirmed by polymerase chain reaction. J Fr Ophtalmol. 2022;45:e71-e73. [PubMed] [DOI] [Cited in This Article: ] |
| 124. | Guda SJM, Sontam B, Bagga B, Ranjith K, Sharma S, Joseph J. Evaluation of multiplex real-time polymerase chain reaction for the detection of herpes simplex virus-1 and 2 and varicella-zoster virus in corneal cells from normal subjects and patients with keratitis in India. Indian J Ophthalmol. 2019;67:1040-1046. [PubMed] [DOI] [Cited in This Article: ] |
| 125. | Yoshida M, Hariya T, Yokokura S, Maruyama K, Sato K, Sugita S, Tomaru Y, Shimizu N, Nakazawa T. Diagnosing superinfection keratitis with multiplex polymerase chain reaction. J Infect Chemother. 2018;24:1004-1008. [PubMed] [DOI] [Cited in This Article: ] |
| 126. | Chang HL, Kuo BI, Wu JH, Huang WL, Su CC, Chen WL. Anti-glaucoma agents-induced pseudodendritic keratitis presumed to be herpetic simplex keratitis: a clinical case series. Sci Rep. 2021;11:21443. [PubMed] [DOI] [Cited in This Article: ] |
| 127. | Haidar H, Biberoğlu Çelik E, Akkaya Turhan S. Intraocular foreign body in the anterior chamber angle misdiagnosed as herpetic stromal keratitis. Ulus Travma Acil Cerrahi Derg. 2023;29:830-833. [PubMed] [DOI] [Cited in This Article: ] |
| 128. | Austin A, Lietman T, Rose-Nussbaumer J. Update on the Management of Infectious Keratitis. Ophthalmology. 2017;124:1678-1689. [PubMed] [DOI] [Cited in This Article: ] |
| 129. | Bajracharya L, Sapkota J. An Unusual Presentation of Corneal Intraepithelial Neoplasia: A Case Report. Nepal J Ophthalmol. 2022;14:178-182. [PubMed] [DOI] [Cited in This Article: ] |
| 130. | Lee MJ, Srikumaran D, Zafar S, Salehi M, Liu TS, Woreta FA. Case series: Delayed diagnoses of Acanthamoeba keratitis. Am J Ophthalmol Case Rep. 2020;19:100778. [PubMed] [DOI] [Cited in This Article: ] |
| 131. | Hsu CW, Liu CH, Hsiao CH. Corneal Endotheliitis as a Presentation of Acanthamoeba Keratitis. Eye Contact Lens. 2020;46:e30-e32. [PubMed] [DOI] [Cited in This Article: ] |
| 132. | Dos Santos DL, Kwitko S, Marinho DR, de Araújo BS, Locatelli CI, Rott MB. Acanthamoeba keratitis in Porto Alegre (southern Brazil): 28 cases and risk factors. Parasitol Res. 2018;117:747-750. [PubMed] [DOI] [Cited in This Article: ] |
| 133. | Oremosu J, Ung L, Chodosh J, Cañete-Gibas C, Wiederhold NP, Davies EC, Bispo PJM. Fungal keratitis caused by Coniochaeta mutabilis-A case report. J Mycol Med. 2023;33:101384. [PubMed] [DOI] [Cited in This Article: ] |
| 134. | Yeh TC, Kuo YS, Wang LC, Tai TY, Lin PY. Chlorhexidine in the treatment of microsporidial stromal keratitis and the effect of host immunity: A case series and literature review. J Chin Med Assoc. 2022;85:532-536. [PubMed] [DOI] [Cited in This Article: ] |
| 135. | Porcar Plana CA, Matarredona Muñoz J, Moya Roca J, Campos Mollo E. Moraxella nonliquefaciens superinfecting herpes simplex keratitis. Eur J Ophthalmol. 2022;32:NP24-NP27. [PubMed] [DOI] [Cited in This Article: ] |
| 136. | Qiu JN, Huang FF, Liu CH, Cao WJ, Zhang CR. Atypical stromal herpes simplex keratitis: clinical features and diagnosis. Jpn J Ophthalmol. 2023;67:43-49. [PubMed] [DOI] [Cited in This Article: ] |
| 137. | Fa F, Laup L, Mandelbrot L, Sibiude J, Picone O. Fetal and neonatal abnormalities due to congenital herpes simplex virus infection: a literature review. Prenat Diagn. 2020;40:408-414. [PubMed] [DOI] [Cited in This Article: ] |
| 138. | Mohammadpour M, Alizadeh L, Jabbarvand Behrouz M, Khorrami-Nejad M. Quantitative real-time polymerase chain reaction analysis in herpes simplex virus keratitis with and without epithelial involvement. Int Ophthalmol. 2021;41:1807-1813. [PubMed] [DOI] [Cited in This Article: ] |
| 139. | Tóth G, Berkó-Göttel B, Seitz B, Langenbucher A, Stachon T, Pluzsik MT, Nagy ZZ, Smola S, Szentmáry N. Herpes simplex virus PCR in 2230 explanted corneal buttons. Acta Ophthalmol. 2022;100:e77-e82. [PubMed] [DOI] [Cited in This Article: ] |
| 140. | Parekh M, Romano V, Franch A, Leon P, Birattari F, Borroni D, Kaye SB, Ponzin D, Ahmad S, Ferrari S. Shotgun sequencing to determine corneal infection. Am J Ophthalmol Case Rep. 2020;19:100737. [PubMed] [DOI] [Cited in This Article: ] |
| 141. | Cabrera-Aguas M, Kerdraon Y, Watson SL. Diagnosis using polymerase chain reaction and outcomes in herpes simplex keratitis. Acta Ophthalmol. 2021;99:e770-e771. [PubMed] [DOI] [Cited in This Article: ] |
| 142. | Todokoro D, Hosogai M, Nakano S, Akiyama H. Effective diagnosis by real-time PCR of herpes simplex diffuse endotheliitis that is similar in appearance to fungal keratitis: case series. J Ophthalmic Inflamm Infect. 2021;11:20. [PubMed] [DOI] [Cited in This Article: ] |
| 143. | Shoji J, Sakimoto T, Inada N, Kamei Y, Matsubara M, Takamura E, Sawa M. A diagnostic method for herpes simplex keratitis by simultaneous measurement of viral DNA and virus-specific secretory IgA in tears: an evaluation. Jpn J Ophthalmol. 2016;60:294-301. [PubMed] [DOI] [Cited in This Article: ] |
| 144. | Yang H, Yang X, Wang Y, Zheng X, Zhang Y, Shao Y. Comparative analysis of the tear protein profile in herpes simplex virus type 1 epithelial keratitis. BMC Ophthalmol. 2020;20:355. [PubMed] [DOI] [Cited in This Article: ] |
| 145. | Armstrong S, Arroyo M, Decker-Pulice K, Lane M, Mckinney M, Molesworth-Kenyon SJ. IL-1α Modulates IFN-γ-Induced Production of CXCL9/MIG during Herpes Simplex Virus Type-1 Corneal Infection. Curr Eye Res. 2021;46:309-317. [PubMed] [DOI] [Cited in This Article: ] |
| 146. | Azher TN, Yin XT, Tajfirouz D, Huang AJ, Stuart PM. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol. 2017;11:185-191. [PubMed] [DOI] [Cited in This Article: ] |
| 147. | Chatterjee S. Commentary: Diagnostic markers for suspected herpes simplex virus keratitis - A bridge too far. Indian J Ophthalmol. 2021;69:858-859. [PubMed] [DOI] [Cited in This Article: ] |
| 148. | Anjum S, Sen S, Agarwal R, Sharma N, Kashyap S, Sharma A. Quantitative analysis of herpes simplex virus-1 transcript in suspected viral keratitis corneal buttons and its clinical significance. Indian J Ophthalmol. 2021;69:852-858. [PubMed] [DOI] [Cited in This Article: ] |
| 149. | Lu L, Palioura S. Management of Stromal Herpes Simplex Virus Keratitis With Epithelial Ulceration Using Optical Coherence Tomography-Generated Corneal Thickness Maps. Cornea. 2020;39:1566-1570. [PubMed] [DOI] [Cited in This Article: ] |
| 150. | Ledbetter EC, Joslin AR, Spertus CB, Badanes Z, Mohammed HO. In vivo confocal microscopic features of naturally acquired canine herpesvirus-1 and feline herpesvirus-1 dendritic and punctate ulcerative keratitis. Am J Vet Res. 2021;82:903-911. [PubMed] [DOI] [Cited in This Article: ] |
| 151. | Valerio GS, Lin CC. Ocular manifestations of herpes simplex virus. Curr Opin Ophthalmol. 2019;30:525-531. [PubMed] [DOI] [Cited in This Article: ] |
| 152. | Ferreira CS, Figueira L, Moreira-Gonçalves N, Moreira R, Torrão L, Falcão-Reis F. Clinical and Microbiological Profile of Bacterial Microbial Keratitis in a Portuguese Tertiary Referral Center-Where Are We in 2015? Eye Contact Lens. 2018;44:15-20. [PubMed] [DOI] [Cited in This Article: ] |
| 153. | Wensing B, Mochizuki M, De Boer JH. Clinical Characteristics of Herpes Simplex Virus Associated Anterior Uveitis. Ocul Immunol Inflamm. 2018;26:333-337. [PubMed] [DOI] [Cited in This Article: ] |
| 154. | Danileviciene V, Zemaitiene R, Gintauskiene VM, Nedzelskiene I, Zaliuniene D. The Role of C21orf91 in Herpes Simplex Virus Keratitis. Medicina (Kaunas). 2019;55. [PubMed] [DOI] [Cited in This Article: ] |
| 155. | Borivoje S, Svetlana S, Milan HM, Nela Đ, Olivera MĐ, Filip M, Milenko S, Srbislav P. IL28B Genetic Variations in Patients with Recurrent Herpes Simplex Keratitis. Medicina (Kaunas). 2019;55. [PubMed] [DOI] [Cited in This Article: ] |
| 156. | Marcos-Fernández MÁ, Tabernero SS, Herreras JM, Galarreta DJ. Impact of herpetic stromal immune keratitis in corneal biomechanics and innervation. Graefes Arch Clin Exp Ophthalmol. 2018;256:155-161. [PubMed] [DOI] [Cited in This Article: ] |
| 157. | Zemaitiene R, Rakauskiene M, Danileviciene V, Use V, Kriauciuniene L, Zaliuniene D. Corneal esthesiometry and sub-basal nerves morphological changes in herpes simplex virus keratitis/uveitis patients. Int J Ophthalmol. 2019;12:407-411. [PubMed] [DOI] [Cited in This Article: ] |
| 158. | Hsu CJ, Hung JH, Lin IH, Tseng SH, Lin SH, Huang YH. Overweight and Obesity as Risk Factors for Recurrent Herpetic Stromal Keratitis during Long-Term Antiviral Prophylaxis. Viruses. 2022;14. [PubMed] [DOI] [Cited in This Article: ] |
| 159. | Brunner M, Somerville T, Corless CE, Myneni J, Rajhbeharrysingh T, Tiew S, Neal T, Kaye SB. Use of a corneal impression membrane and PCR for the detection of herpes simplex virus type-1. J Med Microbiol. 2019;68:1324-1329. [PubMed] [DOI] [Cited in This Article: ] |
| 160. | Somerville TF, Corless CE, Neal T, Kaye SB. Effect of storage time and temperature on the detection of Pseudomonas aeruginosa, Acanthamoeba and Herpes Simplex Virus from corneal impression membranes. J Med Microbiol. 2018;67:1321-1325. [PubMed] [DOI] [Cited in This Article: ] |
| 161. | Li Z, Breitwieser FP, Lu J, Jun AS, Asnaghi L, Salzberg SL, Eberhart CG. Identifying Corneal Infections in Formalin-Fixed Specimens Using Next Generation Sequencing. Invest Ophthalmol Vis Sci. 2018;59:280-288. [PubMed] [DOI] [Cited in This Article: ] |
| 162. | Dridi S, Richerioux N, Gonzalez Suarez CE, Vanharen M, Sanabria-Solano C, Pearson A. A Mutation in the UL24 Gene Abolishes Expression of the Newly Identified UL24.5 Protein of Herpes Simplex Virus 1 and Leads to an Increase in Pathogenicity in Mice. J Virol. 2018;92. [PubMed] [DOI] [Cited in This Article: ] |
| 163. | Miyazaki D, Kandori-Inoue M, Shimizu Y, Ohtani F, Chono I, Inoue Y, Yamagami S. Role Played by Receptors for Advanced Glycosylation End Products in Corneal Endothelial Cells after HSV-1 Infection. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] |
| 164. | Cabrera-Aguas M, Kerdraon Y, Symes RJ, McCluskey P, Samarawickrama C, Rawlinson W, Watson SL. Development, Implementation, and Evaluation of Treatment Guidelines for Herpes Simplex Keratitis in Sydney, Australia. Cornea. 2020;39:834-840. [PubMed] [DOI] [Cited in This Article: ] |
| 165. | Cabrera-Aguas M, Robaei D, McCluskey P, Watson S. Clinical translation of recommendations from randomized trials for management of herpes simplex virus keratitis. Clin Exp Ophthalmol. 2018;46:1008-1016. [PubMed] [DOI] [Cited in This Article: ] |
| 166. | Hicks PM, Singh K, Prajna NV, Lu MC, Niziol LM, Greenwald MF, Verkade A, Amescua G, Farsiu S, Woodward MA; Corneal Ulcer Study Group. Quantifying Clinicians' Diagnostic Uncertainty When Making Initial Treatment Decisions for Microbial Keratitis. Cornea. 2023;42:1408-1413. [PubMed] [DOI] [Cited in This Article: ] |
| 167. | Lázaro-Rodríguez V, Berrada H, Capella MJ. A case report of isolated primary herpes-simplex virus neuroretinitis in an immunocompetent adult. BMC Ophthalmol. 2022;22:47. [PubMed] [DOI] [Cited in This Article: ] |
| 168. | Robinet-Perrin A, Tumiotto C, Cornut T, Santoni A, Touboul D, Goupil-Gouyette T, Garrigue I, Boutolleau D, Burrel S. Input of recombinant phenotyping for the characterization of a novel acyclovir-resistance mutation identified in a patient with recurrent herpetic keratitis. Antiviral Res. 2019;168:183-186. [PubMed] [DOI] [Cited in This Article: ] |
| 169. | Koganti R, Yadavalli T, Shukla D. Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections. Microorganisms. 2019;7. [PubMed] [DOI] [Cited in This Article: ] |
| 170. | Zannella C, Chianese A, De Bernardo M, Folliero V, Petrillo F, De Filippis A, Boccia G, Franci G, Rosa N, Galdiero M. Ophthalmic Solutions with a Broad Antiviral Action: Evaluation of Their Potential against Ocular Herpetic Infections. Microorganisms. 2022;10. [PubMed] [DOI] [Cited in This Article: ] |
| 171. | Kalke K, Lund LM, Nyman MC, Levanova AA, Urtti A, Poranen MM, Hukkanen V, Paavilainen H. Swarms of chemically modified antiviral siRNA targeting herpes simplex virus infection in human corneal epithelial cells. PLoS Pathog. 2022;18:e1010688. [PubMed] [DOI] [Cited in This Article: ] |
| 172. | Moshirfar M, Kelkar N, Peterson T, Bradshaw J, Parker L, Ronquillo YC, Hoopes PC. The Impact of Antiviral Resistance on Herpetic Keratitis. Eye Contact Lens. 2023;49:127-134. [PubMed] [DOI] [Cited in This Article: ] |
| 173. | Rousseau A, Pharm SB, Gueudry J, Deback C, Haigh O, Schweitzer C, Boutolleau D, Labetoulle M. Acyclovir-Resistant Herpes Simplex Virus 1 Keratitis: A Concerning and Emerging Clinical Challenge. Am J Ophthalmol. 2022;238:110-119. [PubMed] [DOI] [Cited in This Article: ] |
| 174. | Wang S, Hou F, Yao YF, Pan D. Efficient establishment of reactivatable latency by an acyclovir-resistant herpes simplex virus 1 thymidine kinase substitution mutant with reduced neuronal replication. Virology. 2021;556:140-148. [PubMed] [DOI] [Cited in This Article: ] |
| 175. | Peyman A, Nayebzadeh M, Peyman M, Afshari NA, Pourazizi M. Topical cyclosporine-A vs prednisolone for herpetic stromal keratitis: a randomized controlled trial. Acta Ophthalmol. 2019;97:e194-e198. [PubMed] [DOI] [Cited in This Article: ] |
| 176. | Aramă V. Topical antibiotic therapy in eye infections - myths and certainties in the era of bacterial resistance to antibiotics. Rom J Ophthalmol. 2020;64:245-260. [PubMed] [Cited in This Article: ] |
| 177. | Kuan HC, Ivan Cheng EY, Yong MH, Wan Abdul Halim WH, Othman O. Corneal Nodules and Possible Pathologies: A Case Series. Cureus. 2021;13:e20822. [PubMed] [DOI] [Cited in This Article: ] |
| 178. | Li X, Nayeni M, Malvankar-Mehta MS. Antiviral and Anti-Inflammatory Therapeutic Interventions for Treating Herpes Stromal Keratitis: A Systematic Review. Ophthalmic Epidemiol. 2023;1-19. [PubMed] [DOI] [Cited in This Article: ] |
| 179. | Koseoglu ND, Strauss BR, Hamrah P. Successful Management of Herpes Simplex Keratitis With Oral Valganciclovir in Patients Unresponsive or Allergic to Conventional Antiviral Therapy. Cornea. 2019;38:663-667. [PubMed] [DOI] [Cited in This Article: ] |
| 180. | Watson ZL, Washington SD, Phelan DM, Lewin AS, Tuli SS, Schultz GS, Neumann DM, Bloom DC. In Vivo Knockdown of the Herpes Simplex Virus 1 Latency-Associated Transcript Reduces Reactivation from Latency. J Virol. 2018;92. [PubMed] [DOI] [Cited in This Article: ] |
| 181. | Zinser E, Krawczyk A, Mühl-Zürbes P, Aufderhorst U, Draßner C, Stich L, Zaja M, Strobl S, Steinkasserer A, Heilingloh CS. A new promising candidate to overcome drug resistant herpes simplex virus infections. Antiviral Res. 2018;149:202-210. [PubMed] [DOI] [Cited in This Article: ] |
| 182. | Daley JR, Lee MK, Wang X, Ly M, Samarawickrama C. Epidemiology and Economic Cost Analysis of Microbial Keratitis from a Tertiary Referral Hospital in Australia. Pathogens. 2023;12. [PubMed] [DOI] [Cited in This Article: ] |
| 183. | Pandey M, Choudhury H, Abdul-Aziz A, Bhattamisra SK, Gorain B, Su JST, Tan CL, Chin WY, Yip KY. Advancement on Sustained Antiviral Ocular Drug Delivery for Herpes Simplex Virus Keratitis: Recent Update on Potential Investigation. Pharmaceutics. 2020;13. [PubMed] [DOI] [Cited in This Article: ] |
| 184. | Cabrera-Aguas M, Kerdraon Y, Watson SL. Clinical outcomes of herpes simplex keratitis: Two-year experience from a quaternary eye care centre in Sydney, Australia. Ophthalmic Physiol Opt. 2021;41:961-970. [PubMed] [DOI] [Cited in This Article: ] |
| 185. | Deback C, Rousseau A, Breckler M, Molet L, Boutolleau D, Burrel S, Roque-Afonso AM, Labetoulle M. Antiviral effects of Cacicol(®), a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection. Antivir Ther. 2018;23:665-675. [PubMed] [DOI] [Cited in This Article: ] |
| 186. | Guan H, Nuth M, Lee V, Lin C, Mitchell CH, Lu W, Scott RW, Parker MH, Kulp JL 3rd, Reitz AB, Ricciardi RP. Herpes Simplex Virus-1 infection in human primary corneal epithelial cells is blocked by a stapled peptide that targets processive DNA synthesis. Ocul Surf. 2021;19:313-321. [PubMed] [DOI] [Cited in This Article: ] |
| 187. | De Clerck I, Walgraeve V, Snoeck R, Andrei G, Blanckaert J, Mulliez E, Delbeke H. Putting drug resistant epithelial herpes keratitis in the spotlight: A case series. Am J Ophthalmol Case Rep. 2022;25:101268. [PubMed] [DOI] [Cited in This Article: ] |
| 188. | Sumbria D, Berber E, Miller L, Rouse BT. Modulating glutamine metabolism to control viral immuno-inflammatory lesions. Cell Immunol. 2021;370:104450. [PubMed] [DOI] [Cited in This Article: ] |
| 189. | Goerlitz-Jessen MF, Cummings TJ, Daluvoy MB. Infectious Keratitis in a 23-Year-Old Patient With Netherton Syndrome. JAMA Ophthalmol. 2020;138:210-211. [PubMed] [DOI] [Cited in This Article: ] |
| 190. | Souza PM, Holland EJ, Huang AJ. Bilateral herpetic keratoconjunctivitis. Ophthalmology. 2003;110:493-496. [PubMed] [DOI] [Cited in This Article: ] |
| 191. | Ida T, Furuta S, Takayama A, Tamura J, Hayashi Y, Abe K, Kurihara S, Ishikawa J, Iwamoto T, Ikeda K, Suzuki K, Nakajima H. Efficacy and safety of dose escalation of tofacitinib in refractory anti-MDA5 antibody-positive dermatomyositis. RMD Open. 2023;9. [PubMed] [DOI] [Cited in This Article: ] |
| 192. | Lappin M, Wotman K, Chow L, Williams M, Hawley J, Dow S. Nanoparticle ocular immunotherapy for herpesvirus surface eye infections evaluated in cat infection model. PLoS One. 2023;18:e0279462. [PubMed] [DOI] [Cited in This Article: ] |
| 193. | Davido DJ, Tu EM, Wang H, Korom M, Gazquez Casals A, Reddy PJ, Mostafa HH, Combs B, Haenchen SD, Morrison LA. Attenuated Herpes Simplex Virus 1 (HSV-1) Expressing a Mutant Form of ICP6 Stimulates a Strong Immune Response That Protects Mice against HSV-1-Induced Corneal Disease. J Virol. 2018;92. [PubMed] [DOI] [Cited in This Article: ] |
| 194. | Wang H, Davido DJ, Mostafa HH, Morrison LA. Efficacy of an HSV-1 Neuro-Attenuated Vaccine in Mice Is Reduced by Preventing Viral DNA Replication. Viruses. 2022;14. [PubMed] [DOI] [Cited in This Article: ] |
| 195. | Matundan HH, Jaggi U, Wang S, Ghiasi H. Loss of ICP22 in HSV-1 Elicits Immune Infiltration and Maintains Stromal Keratitis Despite Reduced Primary and Latent Virus Infectivity. Invest Ophthalmol Vis Sci. 2019;60:3398-3406. [PubMed] [DOI] [Cited in This Article: ] |
| 196. | Amador C, Shah R, Ghiam S, Kramerov AA, Ljubimov AV. Gene Therapy in the Anterior Eye Segment. Curr Gene Ther. 2022;22:104-131. [PubMed] [DOI] [Cited in This Article: ] |
| 197. | Naidu SK, Nabi R, Cheemarla NR, Stanfield BA, Rider PJ, Jambunathan N, Chouljenko VN, Carter R, Del Piero F, Langohr I, Kousoulas KG. Intramuscular vaccination of mice with the human herpes simplex virus type-1(HSV-1) VC2 vaccine, but not its parental strain HSV-1(F) confers full protection against lethal ocular HSV-1 (McKrae) pathogenesis. PLoS One. 2020;15:e0228252. [PubMed] [DOI] [Cited in This Article: ] |
| 198. | Nabi R, Lewin AC, Collantes TM, Chouljenko VN, Kousoulas KG. Intramuscular Vaccination With the HSV-1(VC2) Live-Attenuated Vaccine Strain Confers Protection Against Viral Ocular Immunopathogenesis Associated With γδT Cell Intracorneal Infiltration. Front Immunol. 2021;12:789454. [PubMed] [DOI] [Cited in This Article: ] |
| 199. | Grinage E, Shukla D. Optineurin in ocular herpes infection. Exp Eye Res. 2022;219:109059. [PubMed] [DOI] [Cited in This Article: ] |
| 200. | Hirose S, Jaggi U, Wang S, Tormanen K, Nagaoka Y, Katsumata M, Ghiasi H. Role of TH17 Responses in Increasing Herpetic Keratitis in the Eyes of Mice Infected with HSV-1. Invest Ophthalmol Vis Sci. 2020;61:20. [PubMed] [DOI] [Cited in This Article: ] |
| 201. | Li F, Zhang Q. Hypopyon after Periocular Corticosteroid Injection: A Case Series. Ocul Immunol Inflamm. 2023;31:955-960. [PubMed] [DOI] [Cited in This Article: ] |
| 202. | Bansode YD, Chattopadhyay D, Saha B. Transcriptomic Analysis of Interferon Response in Toll-Like Receptor 2 Ligand-Treated and Herpes Simplex Virus 1-Infected Neurons and Astrocytes. Viral Immunol. 2021;34:256-266. [PubMed] [DOI] [Cited in This Article: ] |
| 203. | Kim YJ, Yeon Y, Lee WJ, Shin YU, Cho H, Lim HW, Kang MH. Analysis of MicroRNA Expression in Tears of Patients with Herpes Epithelial Keratitis: A Preliminary Study. Invest Ophthalmol Vis Sci. 2022;63:21. [PubMed] [DOI] [Cited in This Article: ] |
| 204. | Módis LV, Varkoly G, Bencze J, Hortobágyi TG, Módis L Jr, Hortobágyi T. Extracellular matrix changes in corneal opacification vary depending on etiology. Mol Vis. 2021;27:26-36. [PubMed] [Cited in This Article: ] |
| 205. | Dhanushkodi NR, Prakash S, Srivastava R, Coulon PA, Arellano D, Kapadia RV, Fahim R, Suzer B, Jamal L, Vahed H, BenMohamed L. Antiviral CD19(+)CD27(+) Memory B Cells Are Associated with Protection from Recurrent Asymptomatic Ocular Herpesvirus Infection. J Virol. 2022;96:e0205721. [PubMed] [DOI] [Cited in This Article: ] |
| 206. | Jaggi U, Matundan HH, Yu J, Hirose S, Mueller M, Wormley FL Jr, Ghiasi H. Essential role of M1 macrophages in blocking cytokine storm and pathology associated with murine HSV-1 infection. PLoS Pathog. 2021;17:e1009999. [PubMed] [DOI] [Cited in This Article: ] |
| 207. | Prakash S, Roy S, Srivastava R, Coulon PG, Dhanushkodi NR, Vahed H, Jankeel A, Geertsema R, Amezquita C, Nguyen L, Messaoudi I, Burkhardt AM, BenMohamed L. Unique molecular signatures of antiviral memory CD8(+) T cells associated with asymptomatic recurrent ocular herpes. Sci Rep. 2020;10:13843. [PubMed] [DOI] [Cited in This Article: ] |
| 208. | Srivastava R, Coulon PA, Prakash S, Dhanushkodi NR, Roy S, Nguyen AM, Alomari NI, Mai UT, Amezquita C, Ye C, Maillère B, BenMohamed L. Human Epitopes Identified from Herpes Simplex Virus Tegument Protein VP11/12 (UL46) Recall Multifunctional Effector Memory CD4(+) T(EM) Cells in Asymptomatic Individuals and Protect from Ocular Herpes Infection and Disease in "Humanized" HLA-DR Transgenic Mice. J Virol. 2020;94. [PubMed] [DOI] [Cited in This Article: ] |
| 209. | Rajasagi NK, Rouse BT. The Role of T Cells in Herpes Stromal Keratitis. Front Immunol. 2019;10:512. [PubMed] [DOI] [Cited in This Article: ] |
| 210. | O'Neil TR, Hu K, Truong NR, Arshad S, Shacklett BL, Cunningham AL, Nasr N. The Role of Tissue Resident Memory CD4 T Cells in Herpes Simplex Viral and HIV Infection. Viruses. 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 211. | Suvas PK, Setia M, Rana M, Chakraborty A, Suvas S. Novel characterization of CXCR4 expressing cells in uninfected and herpes simplex virus-1 infected corneas. Ocul Surf. 2023;28:99-107. [PubMed] [DOI] [Cited in This Article: ] |
| 212. | Treat BR, Bidula SM, St Leger AJ, Hendricks RL, Kinchington PR. Herpes Simplex Virus 1-Specific CD8(+) T Cell Priming and Latent Ganglionic Retention Are Shaped by Viral Epitope Promoter Kinetics. J Virol. 2020;94. [PubMed] [DOI] [Cited in This Article: ] |
| 213. | Khan AA, Srivastava R, Vahed H, Roy S, Walia SS, Kim GJ, Fouladi MA, Yamada T, Ly VT, Lam C, Lou A, Nguyen V, Boldbaatar U, Geertsema R, Fraser NW, BenMohamed L. Human Asymptomatic Epitope Peptide/CXCL10-Based Prime/Pull Vaccine Induces Herpes Simplex Virus-Specific Gamma Interferon-Positive CD107(+) CD8(+) T Cells That Infiltrate the Corneas and Trigeminal Ganglia of Humanized HLA Transgenic Rabbits and Protect against Ocular Herpes Challenge. J Virol. 2018;92. [PubMed] [DOI] [Cited in This Article: ] |
| 214. | Jamali A, Hu K, Sendra VG, Blanco T, Lopez MJ, Ortiz G, Qazi Y, Zheng L, Turhan A, Harris DL, Hamrah P. Characterization of Resident Corneal Plasmacytoid Dendritic Cells and Their Pivotal Role in Herpes Simplex Keratitis. Cell Rep. 2020;32:108099. [PubMed] [DOI] [Cited in This Article: ] |
| 215. | Ní Gabhann-Dromgoole J, de Chaumont C, Shahnazaryan D, Smith S, Malone C, Hassan J, De Gascun CF, Jefferies CA, Murphy CC. Systemic IL-1β production as a consequence of corneal HSV-1 infection-contribution to the development of herpes simplex keratitis. Int J Ophthalmol. 2019;12:1493-1497. [PubMed] [DOI] [Cited in This Article: ] |
| 216. | Antony F, Pundkar C, Sandey M, Mishra A, Suryawanshi A. Role of IL-27 in HSV-1-Induced Herpetic Stromal Keratitis. J Immunol. 2023;211:474-485. [PubMed] [DOI] [Cited in This Article: ] |
| 217. | Zhu JY, Zhang X, Zheng X, Luo LL, Mao CY, Lin S, Ye J. Dry eye symptoms in interferon regulatory factor 3-deficient mice due to herpes simplex virus infection in harderian gland and lacrimal gland. Exp Eye Res. 2022;219:109053. [PubMed] [DOI] [Cited in This Article: ] |
| 218. | Berube A, Gmyrek GB, Royer DJ, Carr DJJ. Tripartite-Motif 21 (TRIM21) Deficiency Results in a Modest Loss of Herpes Simplex Virus (HSV)-1 Surveillance in the Trigeminal Ganglia Following Cornea Infection. Viruses. 2022;14. [PubMed] [DOI] [Cited in This Article: ] |
| 219. | Ramsey NLM, Visciano M, Hunte R, Loh LN, Burn Aschner C, Jacobs WR Jr, Herold BC. A Single-Cycle Glycoprotein D Deletion Viral Vaccine Candidate, ΔgD-2, Elicits Polyfunctional Antibodies That Protect against Ocular Herpes Simplex Virus. J Virol. 2020;94. [PubMed] [DOI] [Cited in This Article: ] |
| 220. | Wang S, Hirose S, Ghiasi H. The Absence of Lymphotoxin-α, a Herpesvirus Entry Mediator (HVEM) Ligand, Affects Herpes Simplex Virus 1 Infection In Vivo Differently than the Absence of Other HVEM Cellular Ligands. J Virol. 2019;93. [PubMed] [DOI] [Cited in This Article: ] |
| 221. | Dhanushkodi NR, Srivastava R, Prakash S, Roy S, Coulon PA, Vahed H, Nguyen AM, Salazar S, Nguyen L, Amezquita C, Ye C, Nguyen V, BenMohamed L. High Frequency of Gamma Interferon-Producing PLZF(lo)RORγt(lo) Invariant Natural Killer 1 Cells Infiltrating Herpes Simplex Virus 1-Infected Corneas Is Associated with Asymptomatic Ocular Herpesvirus Infection. J Virol. 2020;94. [PubMed] [DOI] [Cited in This Article: ] |
| 222. | Ohtani F, Miyazaki D, Shimizu Y, Haruki T, Yamagami S, Inoue Y. Role of interferon regulatory factor 7 in corneal endothelial cells after HSV-1 infection. Sci Rep. 2021;11:16487. [PubMed] [DOI] [Cited in This Article: ] |
| 223. | Yin XT, Baugnon NK, Krishnan R, Potter CA, Yarlagadda S, Keadle TL, Stuart PM. CD137 costimulation is associated with reduced herpetic stromal keratitis and with developing normal CD8(+) T cells in trigeminal ganglia. J Gen Virol. 2022;103. [PubMed] [DOI] [Cited in This Article: ] |
| 224. | Azeem A, Baartman B, Conrady CD, Meier JL, El-Herte R. Herpes simplex virus dissemination with necrotizing hepatitis following Descemet membrane endothelial keratoplasty. BMC Infect Dis. 2023;23:465. [PubMed] [DOI] [Cited in This Article: ] |
| 225. | Li S, Li M, Gu L, Peng L, Deng Y, Zhong J, Wang B, Wang Q, Xiao Y, Yuan J. Risk factors influencing survival of acellular porcine corneal stroma in infectious keratitis: a prospective clinical study. J Transl Med. 2019;17:434. [PubMed] [DOI] [Cited in This Article: ] |
| 226. | Feizi S, Azari AA. Approaches toward enhancing survival probability following deep anterior lamellar keratoplasty. Ther Adv Ophthalmol. 2020;12:2515841420913014. [PubMed] [DOI] [Cited in This Article: ] |
| 227. | Shin J, Ra H, Rho CR. Herpes simplex virus linear endotheliitis in a post-keratoplasty patient: A case report. Medicine (Baltimore). 2019;98:e14191. [PubMed] [DOI] [Cited in This Article: ] |
| 228. | Suzuki T, Yamaguchi T, Tomida D, Fukui M, Shimazaki J. Outcome of Lamellar Graft Patching for the Treatment of Noninfectious Corneal Perforations. Cornea. 2022;41:1122-1128. [PubMed] [DOI] [Cited in This Article: ] |
| 229. | Tourkmani AK, Ansari AS, Hossain PN, Konstantopoulos A, Anderson DF. Tectonic Descemet Stripping Endothelial Keratoplasty for the Management of Corneal Perforation: A Case Series. Cornea. 2020;39:1571-1575. [PubMed] [DOI] [Cited in This Article: ] |
| 230. | LoBue SA, Tailor P, Carlson SM, Mano F, Giovane RA, Schaefer E, LoBue TD. Recurrent herpes zoster ophthalmicus in a young, healthy individual taking high doses of l-Arginine. Am J Ophthalmol Case Rep. 2019;16:100547. [PubMed] [DOI] [Cited in This Article: ] |
| 231. | Amini-Salehi E, Eslami N, Tamimi A, Sedighi N, Moghdam SS, Yaghubi-Kalurazi T, Hassanipour S, Joukar F, Mansour-Ghanaei F, Eftekhari H. Unusual herpetic reactivation in a young female following botox injection: a case report study. BMC Infect Dis. 2023;23:647. [PubMed] [DOI] [Cited in This Article: ] |
| 232. | Das N, Das J, Basak S. A Case of Reactivation of Herpes Simplex Virus Corneal Endotheliitis Following Periocular Botulinum Toxin A Injection. Ophthalmic Plast Reconstr Surg. 2020;36:e73-e75. [PubMed] [DOI] [Cited in This Article: ] |
| 233. | Ramappa M, Jiya PY, Chaurasia S, Naik M, Sharma S. Reactivation of herpes simplex viral keratitis following the botulinum toxin injection. Indian J Ophthalmol. 2018;66:306-308. [PubMed] [DOI] [Cited in This Article: ] |
| 234. | Wang L, Deng Y, Ma K, Yin H, Sun C, Tang J. Herpetic Keratitis Following Corneal Crosslinking for Keratoconus: A Case Series. Infect Drug Resist. 2022;15:6555-6562. [PubMed] [DOI] [Cited in This Article: ] |
| 235. | Papaioannou L, Miligkos M, Papathanassiou M. Corneal Collagen Cross-Linking for Infectious Keratitis: A Systematic Review and Meta-Analysis. Cornea. 2016;35:62-71. [PubMed] [DOI] [Cited in This Article: ] |
| 236. | Bevara A, Potti S. Herpetic stromal keratitis after collagen cross-linking for keratoconus: A unique presentation. Indian J Ophthalmol. 2020;68:1156-1158. [PubMed] [DOI] [Cited in This Article: ] |
| 237. | Potti S, Bagad PA, Khatib N, Bevara A. Precipitation of herpetic stromal keratitis after collagen cross-linking for keratoconus. Indian J Ophthalmol. 2020;68:207. [PubMed] [DOI] [Cited in This Article: ] |
| 238. | Manns RPC, Achiron A, Knyazer B, Elhaddad O, Darcy K, Yahalomi T, Tole D, Avadhanam VS. Use of corneal cross-linking beyond keratoconus: a systemic literature review. Graefes Arch Clin Exp Ophthalmol. 2023;261:2435-2453. [PubMed] [DOI] [Cited in This Article: ] |
| 239. | Mohanty A, Behera HS, Barik MR, Kaur A, Sharma S, Das S, Fernandes M, Panda S, Sahu SK. Microsporidia-induced stromal keratitis: a new cause of presumed immune stromal (interstitial) keratitis. Br J Ophthalmol. 2023;107:607-613. [PubMed] [DOI] [Cited in This Article: ] |
| 240. | Wilhelmus KR, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, Barron BA, Kaufman HE, Sugar J, Hyndiuk RA, Laibson PR, Stulting RD, Asbell PA; Herpetic Eye Disease Study Group. Herpetic Eye Disease Study: A Controlled Trial of Topical Corticosteroids for Herpes Simplex Stromal Keratitis. Ophthalmology. 2020;127:S5-S18. [PubMed] [DOI] [Cited in This Article: ] |
| 241. | Denier M, Gabison E, Sahyoun M, Labetoulle M, Dureau P, Cochereau I, Doan S. Stromal Keratitis After Varicella in Children. Cornea. 2020;39:680-684. [PubMed] [DOI] [Cited in This Article: ] |
| 242. | Hirano K, Tanaka H, Kato K, Araki-Sasaki K. Topical Corticosteroids for Infectious Keratitis Before Culture-Proven Diagnosis. Clin Ophthalmol. 2021;15:609-616. [PubMed] [DOI] [Cited in This Article: ] |
| 243. | Berman T, O'Connor A, Yeo DCM, Nayak H. Herpes simplex keratoconjunctivitis in the immediate postoperative period after strabismus surgery. Strabismus. 2021;29:86-89. [PubMed] [DOI] [Cited in This Article: ] |
| 244. | Atia R, Jouve L, Knoeri J, Georgeon C, Laroche L, Borderie V, Bouheraoua N. [Corneal collagen cross-linking to treat infectious keratitis]. J Fr Ophtalmol. 2018;41:560-568. [PubMed] [DOI] [Cited in This Article: ] |
| 245. | Natarajan R, Mohan M. Decision-making in Herpes Simplex viral keratitis grafts. Indian J Ophthalmol. 2023;71:2629-2630. [PubMed] [DOI] [Cited in This Article: ] |
| 246. | Szkodny D, Wróblewska-Czajka E, Wylęgała A, Nandzik M, Wylęgała E. Incidence of Complications Related to Corneal Graft in a Group of 758 Patients. J Clin Med. 2022;12. [PubMed] [DOI] [Cited in This Article: ] |
| 247. | Vasquez-Perez A, Nanavaty MA. Modified Allogenic Simple Limbal Epithelial Transplantation Followed by Keratoplasty as Treatment for Total Limbal Stem Cell Deficiency. Ocul Immunol Inflamm. 2018;26:1189-1191. [PubMed] [DOI] [Cited in This Article: ] |
| 248. | Hu F, Guan W, Zhang Y, Peng X. Herpetic uveitis caused by herpes simplex virus after cataract surgery in a patient without prior viral keratitis or uveitis: a case report. BMC Ophthalmol. 2022;22:104. [PubMed] [DOI] [Cited in This Article: ] |
| 249. | Nche EN, Katzir A, Solomon A, Wolf D, Panet A, Lavy I. Occurrence of Herpes Viruses in Morphologically Normal Corneas. Cornea. 2023;42:412-415. [PubMed] [DOI] [Cited in This Article: ] |
| 250. | Gessa-Sorroche M, Kanclerz P, Alio J. Evidence in the prevention of the recurrence of herpes simplex and herpes zoster keratitis after eye surgery. Arch Soc Esp Oftalmol (Engl Ed). 2022;97:149-160. [PubMed] [DOI] [Cited in This Article: ] |
| 251. | Wróblewska-Czajka E, Nowińska A, Dobrowolski D, Szkodny D, Wylęgała E. A Retrospective Study of Herpetic Keratitis in Patients with Keratoconus after Crosslinking Surgery. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] |
| 252. | Ramirez-Miranda A, Mangwani-Mordani S, Quiroz-Casian N, Oliva-Bienzobas V, Cabral-Macias J, Navas A, Graue-Hernandez EO. Combined Bacterial and Herpes Simplex Virus Keratitis following Small-Incision Lenticule Extraction for the Correction of Myopia. Case Rep Ophthalmol. 2021;12:227-231. [PubMed] [DOI] [Cited in This Article: ] |
| 253. | Moshirfar M, Ziari M, Peterson C, Kelkar N, Ronquillo Y, Hoopes P. Herpes endotheliitis following laser-assisted in situ keratomileusis and photorefractive keratectomy. Taiwan J Ophthalmol. 2023;13:93-96. [PubMed] [DOI] [Cited in This Article: ] |
| 254. | Basak SK, Basak S. Recurrence of herpes simplex virus endotheliitis in a Descemet membrane endothelial keratoplasty graft: mimicking fungal interface infection. BMJ Case Rep. 2019;12. [PubMed] [DOI] [Cited in This Article: ] |
| 255. | Musa M, Zeppieri M, Atuanya GN, Enaholo ES, Topah EK, Ojo OM, Salati C. Nutritional Factors: Benefits in Glaucoma and Ophthalmologic Pathologies. Life (Basel). 2023;13. [PubMed] [DOI] [Cited in This Article: ] |
| 256. | Hirota A, Shoji J, Inada N, Adachi R, Tonozuka Y, Yamagami S. Rapid detection and diagnosis of herpetic keratitis using quantitative microfluidic polymerase chain reaction system for herpes simplex and varicella-zoster virus DNA: a case series. BMC Ophthalmol. 2023;23:177. [PubMed] [DOI] [Cited in This Article: ] |
| 257. | Mirenayat MS, Heshmatnia J, Saghebi SR, Sheikhy K, Marjani M, Fakharian A, Jamaati H. Uncommon Complications of Lung Transplantation in a Referral Center. Tanaffos. 2022;21:179-185. [PubMed] [Cited in This Article: ] |
| 258. | Li TH, Lai CC, Wang WH, Chen WS, Tsao YP, Tsai CY, Chang YS. Risk of severe herpes simplex virus infection in systemic lupus erythematosus: analysis of epidemiology and risk factors analysis in Taiwan. Ann Rheum Dis. 2019;78:941-946. [PubMed] [DOI] [Cited in This Article: ] |
| 259. | Bernauer W, Schuler S, Borradori L. Rituximab and bilateral HSV epithelial keratitis in a patient with mucous membrane pemphigoid. J Ophthalmic Inflamm Infect. 2018;8:12. [PubMed] [DOI] [Cited in This Article: ] |
| 260. | Ott M, Nagamany T, Zandi S, Pichi F, Agarwal A, Carreño E, Gupta V, Grewal DS, Cunningham ET, Munk MR. Herpetic anterior uveitis following COVID-19 vaccines: a case series. Front Med (Lausanne). 2023;10:1242225. [PubMed] [DOI] [Cited in This Article: ] |
| 261. | Fard AM, Desilets J, Patel S. Recurrence of Herpetic Keratitis after COVID-19 Vaccination: A Report of Two Cases. Case Rep Ophthalmol Med. 2022;2022:7094893. [PubMed] [DOI] [Cited in This Article: ] |
| 262. | Mohammadpour M, Farrokhpour H, Sadeghi R. Herpetic endotheliitis and stromal keratitis following inactivated COVID-19 vaccination. Clin Case Rep. 2022;10:e6397. [PubMed] [DOI] [Cited in This Article: ] |
| 263. | Rallis KI, Fausto R, Ting DSJ, Al-Aqaba MA, Said DG, Dua HS. Manifestation of Herpetic Eye Disease after COVID-19 Vaccine: A UK Case Series. Ocul Immunol Inflamm. 2022;30:1136-1141. [PubMed] [DOI] [Cited in This Article: ] |
| 264. | Alkwikbi H, Alenazi M, Alanazi W, Alruwaili S. Herpetic Keratitis and Corneal Endothelitis Following COVID-19 Vaccination: A Case Series. Cureus. 2022;14:e20967. [PubMed] [DOI] [Cited in This Article: ] |
| 265. | Alkhalifah MI, Alsobki HE, Alwael HM, Al Fawaz AM, Al-Mezaine HS. Herpes Simplex Virus Keratitis Reactivation after SARS-CoV-2 BNT162b2 mRNA Vaccination: A Report of Two Cases. Ocul Immunol Inflamm. 2021;29:1238-1240. [PubMed] [DOI] [Cited in This Article: ] |
| 266. | Richardson-May J, Rothwell A, Rashid M. Reactivation of herpes simplex keratitis following vaccination for COVID-19. BMJ Case Rep. 2021;14. [PubMed] [DOI] [Cited in This Article: ] |
| 267. | Hung KH, Lan YH, Lin JY, Kang EY, Tan HY, Chen HC, Hsiao CH, Yeh LK. Potential Role and Significance of Ocular Demodicosis in Patients with Concomitant Refractory Herpetic Keratitis. Clin Ophthalmol. 2020;14:4469-4482. [PubMed] [DOI] [Cited in This Article: ] |
| 268. | He B, Tavakoli H, Etminan M, Shokoohi S, Iovieno A, Yeung SN. Impact of the use of anti-glaucoma medications on the risk of herpetic keratitis recurrence. Int Ophthalmol. 2023;43:1559-1564. [PubMed] [DOI] [Cited in This Article: ] |
| 269. | Ozturk T, Arikan G, Oner H. Herpetic Keratouveitis following Intravitreal Ranibizumab Injection in a Case with Diabetic Macular Edema. Ocul Immunol Inflamm. 2021;29:1645-1647. [PubMed] [DOI] [Cited in This Article: ] |
| 270. | Behera G, Gokhale T, Deb AK, Babu KR. Meta-herpetic ulcer following intravitreal bevacizumab. Eur J Ophthalmol. 2022;32:NP24-NP26. [PubMed] [DOI] [Cited in This Article: ] |
| 271. | Al-Kaabi A, Choremis J. HSV epithelial keratitis reactivation after subconjunctival bevacizumab injection: A case report. Can J Ophthalmol. 2019;54:e180-e182. [PubMed] [DOI] [Cited in This Article: ] |
| 272. | Lu LM, McGhee CNJ, Sims JL, Niederer RL. High rate of recurrence of herpes zoster-related ocular disease after phacoemulsification cataract surgery. J Cataract Refract Surg. 2019;45:810-815. [PubMed] [DOI] [Cited in This Article: ] |
| 273. | Kim GN, Yoo WS, Park MH, Chung JK, Han YS, Chung IY, Seo SW, Yoo JM, Kim SJ. Clinical Features of Herpes Simplex Keratitis in a Korean Tertiary Referral Center: Efficacy of Oral Antiviral and Ascorbic Acid on Recurrence. Korean J Ophthalmol. 2018;32:353-360. [PubMed] [DOI] [Cited in This Article: ] |
| 274. | Ravn NH, Ahmadzay ZF, Christensen TA, Larsen HHP, Loft N, Rævdal P, Heegaard S, Kolko M, Egeberg A, Silverberg JI, Halling AS, Thyssen JP. Bidirectional association between atopic dermatitis, conjunctivitis, and other ocular surface diseases: A systematic review and meta-analysis. J Am Acad Dermatol. 2021;85:453-461. [PubMed] [DOI] [Cited in This Article: ] |
| 275. | Parameshwarappa DC, Nanda S, Kavya N, Matada R, Murthy GJ, Murthy PR. Endoilluminator-aided cataract surgery in eyes with corneal opacity - A modified surgical approach. Indian J Ophthalmol. 2022;70:1868. [PubMed] [DOI] [Cited in This Article: ] |
| 276. | Rao P, McKown RL, Laurie GW, Suvas S. Development of lacrimal gland inflammation in the mouse model of herpes stromal keratitis. Exp Eye Res. 2019;184:101-106. [PubMed] [DOI] [Cited in This Article: ] |
| 277. | Wang L, Wang R, Xu C, Zhou H. Pathogenesis of Herpes Stromal Keratitis: Immune Inflammatory Response Mediated by Inflammatory Regulators. Front Immunol. 2020;11:766. [PubMed] [DOI] [Cited in This Article: ] |
| 278. | Neuman M, Spittler J. Photo Rounds: Painful facial blisters, fever, and conjunctivitis. J Fam Pract. 2018;67:573-575. [PubMed] [Cited in This Article: ] |
| 279. | Greenan E, Gallagher S, Khalil R, Murphy CC, Ní Gabhann-Dromgoole J. Advancing Our Understanding of Corneal Herpes Simplex Virus-1 Immune Evasion Mechanisms and Future Therapeutics. Viruses. 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 280. | Lobo AM, Agelidis AM, Shukla D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019;17:40-49. [PubMed] [DOI] [Cited in This Article: ] |
| 281. | He J, Neumann D, Kakazu A, Pham TL, Musarrat F, Cortina MS, Bazan HEP. PEDF plus DHA modulate inflammation and stimulate nerve regeneration after HSV-1 infection. Exp Eye Res. 2017;161:153-162. [PubMed] [DOI] [Cited in This Article: ] |
| 282. | Danileviciene V, Zemaitiene R, Gintauskiene VM, Nedzelskiene I, Zaliuniene D. Corneal Sub-Basal Nerve Changes in Patients with Herpetic Keratitis During Acute Phase and after 6 Months. Medicina (Kaunas). 2019;55. [PubMed] [DOI] [Cited in This Article: ] |
| 283. | Yu T, Schuette F, Christofi M, Forrester JV, Graham GJ, Kuffova L. The atypical chemokine receptor-2 fine-tunes the immune response in herpes stromal keratitis. Front Immunol. 2022;13:1054260. [PubMed] [DOI] [Cited in This Article: ] |
| 284. | Yoshida M, Hosogai M, Yokokura S, Sato K, Hariya T, Kobayashi W, Okabe T, Todokoro D, Nakazawa T. Bilateral Necrotizing Herpes Simplex Keratitis in an Immunocompetent Patient With Genetic Analysis of Herpes Simplex Virus 1. Cornea. 2019;38:1185-1188. [PubMed] [DOI] [Cited in This Article: ] |
| 285. | Pisitpayat P, Jongkhajornpong P, Lekhanont K, Nonpassopon M. Role of Intravenous Acyclovir in Treatment of Herpes Simplex Virus Stromal Keratitis with Ulceration: A Review of 2 Cases. Am J Case Rep. 2021;22:e930467. [PubMed] [DOI] [Cited in This Article: ] |
| 286. | Glickman A, Hunter A, Greenberg PB, Galler E, Mega J, Sellechio J. A Patient With Recurrent Immune Stromal Keratitis and Adherence Challenges. Fed Pract. 2022;39:403-405. [PubMed] [DOI] [Cited in This Article: ] |
| 287. | Roth M, Dierse S, Alder J, Holtmann C, Geerling G. Incidence, prevalence, and outcome of moderate to severe neurotrophic keratopathy in a German tertiary referral center from 2013 to 2017. Graefes Arch Clin Exp Ophthalmol. 2022;260:1961-1973. [PubMed] [DOI] [Cited in This Article: ] |
| 288. | Gadjeva M. Looking into nerve damage in the cornea. Elife. 2019;8. [PubMed] [DOI] [Cited in This Article: ] |
| 289. | Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. 2018;10:37-45. [PubMed] [DOI] [Cited in This Article: ] |
| 290. | Borik K, Mohney BG, Hodge D, Reynolds MM. Birth prevalence and characteristics of congenital corneal opacities. Eur J Ophthalmol. 2023;11206721231202900. [PubMed] [DOI] [Cited in This Article: ] |
| 291. | Roszkowska AM, Inferrera L, Aragona E, Gargano R, Postorino EI, Aragona P. Clinical and instrumental assessment of the corneal healing in moderate and severe neurotrophic keratopathy treated with rh-NGF (Cenegermin). Eur J Ophthalmol. 2022;32:3402-3410. [PubMed] [DOI] [Cited in This Article: ] |
| 292. | Trinh T, Santaella G, Mimouni M, Mednick Z, Cohen E, Sorkin N, Rootman DS, Slomovic AR, Chan CC. Assessment of response to multimodal management of neurotrophic corneal disease. Ocul Surf. 2021;19:330-335. [PubMed] [DOI] [Cited in This Article: ] |
| 293. | Jaggi U, Wang S, Tormanen K, Matundan H, Ljubimov AV, Ghiasi H. Role of Herpes Simplex Virus Type 1 (HSV-1) Glycoprotein K (gK) Pathogenic CD8(+) T Cells in Exacerbation of Eye Disease. Front Immunol. 2018;9:2895. [PubMed] [DOI] [Cited in This Article: ] |
| 294. | Musa M, Zeppieri M, Enaholo ES, Chukwuyem E, Salati C. An Overview of Corneal Transplantation in the Past Decade. Clin Pract. 2023;13:264-279. [PubMed] [DOI] [Cited in This Article: ] |
| 295. | da Costa Paula CA, Gore DM, Shah K, Kuit G, Angunawela RI, Barnett JP, Tuft SJ. Cytomegalovirus infection is not a major cause of corneal graft failure in the United Kingdom. Eye (Lond). 2019;33:833-837. [PubMed] [DOI] [Cited in This Article: ] |
| 296. | Ichikawa K, Ono T, Chen L, Kitamoto K, Taketatni Y, Toyono T, Yoshida J, Aihara M, Miyai T. Quantitative evaluation of corneal irregularity and scarring after infectious keratitis using anterior segment optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2023;. [PubMed] [DOI] [Cited in This Article: ] |
| 297. | Chan NS, Chee SP. Demystifying viral anterior uveitis: A review. Clin Exp Ophthalmol. 2019;47:320-333. [PubMed] [DOI] [Cited in This Article: ] |
| 298. | Chen N, Chen D, Cheng J. Alternate Herpes Simplex Virus-Associated Inflammation in the Cornea and Retina Within a Span of over Two Decades: A Case Report. Ocul Immunol Inflamm. 2023;1-3. [PubMed] [DOI] [Cited in This Article: ] |
| 299. | Tananuvat N, Apivatthakakul A, Tangmonkongvoragul C. Corneal perforation after noncontact tonometry in patients with active recurrent herpes simplex keratitis: case report. Int Ophthalmol. 2019;39:697-701. [PubMed] [DOI] [Cited in This Article: ] |
| 300. | Sendra VG, Tau J, Zapata G, Lasagni Vitar RM, Illian E, Chiaradía P, Berra A. Polluted Air Exposure Compromises Corneal Immunity and Exacerbates Inflammation in Acute Herpes Simplex Keratitis. Front Immunol. 2021;12:618597. [PubMed] [DOI] [Cited in This Article: ] |
| 301. | Moein HR, Kheirkhah A, Muller RT, Cruzat AC, Pavan-Langston D, Hamrah P. Corneal nerve regeneration after herpes simplex keratitis: A longitudinal in vivo confocal microscopy study. Ocul Surf. 2018;16:218-225. [PubMed] [DOI] [Cited in This Article: ] |
| 302. | Zhang Q, Martin-Caraballo M, Hsia SV. Pathophysiological roles and therapeutic potential of voltage-gated ion channels (VGICs) in pain associated with herpesvirus infection. Cell Biosci. 2020;10:70. [PubMed] [DOI] [Cited in This Article: ] |
| 303. | Carreno-Galeano JT, Dohlman TH, Yin J, Dana R. Limbal Stem Cell Deficiency Associated With Herpes Keratitis. Cornea. 2021;40:967-971. [PubMed] [DOI] [Cited in This Article: ] |
| 304. | Cabrera-Aguas M, Khoo P, George CRR, Lahra MM, Watson SL. Predisposing factors, microbiological features and outcomes of patients with clinical presumed concomitant microbial and herpes simplex keratitis. Eye (Lond). 2022;36:86-94. [PubMed] [DOI] [Cited in This Article: ] |
| 305. | Liu X, Xu S, Wang Y, Jin X, Shi Y, Zhang H. Bilateral Limbal Stem Cell Alterations in Patients With Unilateral Herpes Simplex Keratitis and Herpes Zoster Ophthalmicus as Shown by In Vivo Confocal Microscopy. Invest Ophthalmol Vis Sci. 2021;62:12. [PubMed] [DOI] [Cited in This Article: ] |
| 306. | Moein HR, Sendra VG, Jamali A, Kheirkhah A, Harris DL, Hamrah P. Herpes simplex virus-1 KOS-63 strain is virulent and causes titer-dependent corneal nerve damage and keratitis. Sci Rep. 2021;11:4267. [PubMed] [DOI] [Cited in This Article: ] |
| 307. | Chirapapaisan C, Muller RT, Sahin A, Cruzat A, Cavalcanti BM, Jamali A, Pavan-Langston D, Hamrah P. Effect of herpes simplex keratitis scar location on bilateral corneal nerve alterations: an in vivo confocal microscopy study. Br J Ophthalmol. 2022;106:319-325. [PubMed] [DOI] [Cited in This Article: ] |
| 308. | Posarelli M, Chirapapaisan C, Muller R, Abbouda A, Pondelis N, Cruzat A, Cavalcanti BM, Cox SM, Jamali A, Pavan-Langston D, Hamrah P. Corneal nerve regeneration is affected by scar location in herpes simplex keratitis: A longitudinal in vivo confocal microscopy study. Ocul Surf. 2023;28:42-52. [PubMed] [DOI] [Cited in This Article: ] |
| 309. | Wang W, Ye W, Chen S, Tang Y, Chen D, Lu Y, Wu Z, Huang Z, Ge Y. METTL3-mediated m(6)A RNA modification promotes corneal neovascularization by upregulating the canonical Wnt pathway during HSV-1 infection. Cell Signal. 2023;109:110784. [PubMed] [DOI] [Cited in This Article: ] |
| 310. | Sitaula S, Singh SK, Gurung A. Bilateral viral keratitis following corneal collagen crosslinking for progressive keratoconus. J Ophthalmic Inflamm Infect. 2019;9:16. [PubMed] [DOI] [Cited in This Article: ] |
| 311. | Ho MC, Kang EY, Yeh LK, Ma DHK, Lin HC, Tan HY, Chen HC, Hsiao CH. Clinico-microbiological profile of Burkholderia cepacia keratitis: a case series. Ann Clin Microbiol Antimicrob. 2021;20:6. [PubMed] [DOI] [Cited in This Article: ] |
| 312. | Montgomery ML, Callegan MC, Fuller KK, Carr DJJ. Ocular Glands Become Infected Secondarily to Infectious Keratitis and Play a Role in Corneal Resistance to Infection. J Virol. 2019;93. [PubMed] [DOI] [Cited in This Article: ] |
| 313. | Rousseau A, Haigh O, Legrand R, Palgen JL, Lemaitre J, Deback C, Oziol N, Lomonte P, Labetoulle M. Initial TK-deficient HSV-1 infection in the lip alters contralateral lip challenge immune dynamics. Sci Rep. 2022;12:8489. [PubMed] [DOI] [Cited in This Article: ] |
| 314. | Pasternak J. Vaccine against herpes zoster. Einstein (Sao Paulo). 2013;11:133-134. [PubMed] [DOI] [Cited in This Article: ] |
| 315. | Carr DJJ, Gmyrek GB, Filiberti A, Berube AN, Browne WP, Gudgel BM, Sjoelund VH. Distinguishing Features of High- and Low-Dose Vaccine against Ocular HSV-1 Infection Correlates with Recognition of Specific HSV-1-Encoded Proteins. Immunohorizons. 2020;4:608-626. [PubMed] [DOI] [Cited in This Article: ] |
| 316. | Gupta AA, Mammo DA, Page MA. Intrastromal bevacizumab in the management of corneal neovascularization: a retrospective review. Graefes Arch Clin Exp Ophthalmol. 2020;258:167-173. [PubMed] [DOI] [Cited in This Article: ] |
| 317. | Sridhar U, Tripathy K. Commentary: Herpes simplex virus stromal keratitis preferred practice patterns among ophthalmologists vis-à-vis the Herpetic Eye Diseases Study. Indian J Ophthalmol. 2021;69:1340-1341. [PubMed] [DOI] [Cited in This Article: ] |
| 318. | Labetoulle M, Boutolleau D, Burrel S, Haigh O, Rousseau A. Herpes simplex virus, varicella-zoster virus and cytomegalovirus keratitis: Facts for the clinician. Ocul Surf. 2023;28:336-350. [PubMed] [DOI] [Cited in This Article: ] |
| 319. | Kalezic T, Mazen M, Kuklinski E, Asbell P. Herpetic eye disease study: lessons learned. Curr Opin Ophthalmol. 2018;29:340-346. [PubMed] [DOI] [Cited in This Article: ] |
| 320. | . A controlled trial of oral acyclovir for iridocyclitis caused by herpes simplex virus. The Herpetic Eye Disease Study Group. Arch Ophthalmol. 1996;114:1065-1072. [PubMed] [DOI] [Cited in This Article: ] |
| 321. | You IC, Ahn M, Cho NC. A Case Report of Herpes Zoster Ophthalmicus and Meningitis After COVID-19 Vaccination. J Korean Med Sci. 2022;37:e165. [PubMed] [DOI] [Cited in This Article: ] |
| 322. | Jaggi U, Varanasi SK, Bhela S, Rouse BT. On the role of retinoic acid in virus induced inflammatory response in cornea. Microbes Infect. 2018;20:337-345. [PubMed] [DOI] [Cited in This Article: ] |
| 323. | Wang S, Jaggi U, Tormanen K, Hirose S, Ghiasi H. Absence of signal peptide peptidase in peripheral sensory neurons affects latency-reactivation in HSV-1 ocularly infected mice. PLoS Pathog. 2022;18:e1010281. [PubMed] [DOI] [Cited in This Article: ] |
| 324. | Chen H, Zhang J, Dai Y, Xu J. Nerve growth factor inhibits TLR3-induced inflammatory cascades in human corneal epithelial cells. J Inflamm (Lond). 2019;16:27. [PubMed] [DOI] [Cited in This Article: ] |
| 325. | Li J, Cheng C, Lin T, Xue R, Liu X, Wu K. Efficacy of sodium polyanethol sulfonate on herpes simplex virus-1 infection in vitro. Mol Vis. 2022;28:516-525. [PubMed] [Cited in This Article: ] |
| 326. | Roy S, Coulon PG, Srivastava R, Vahed H, Kim GJ, Walia SS, Yamada T, Fouladi MA, Ly VT, BenMohamed L. Blockade of LAG-3 Immune Checkpoint Combined With Therapeutic Vaccination Restore the Function of Tissue-Resident Anti-viral CD8(+) T Cells and Protect Against Recurrent Ocular Herpes Simplex Infection and Disease. Front Immunol. 2018;9:2922. [PubMed] [DOI] [Cited in This Article: ] |
| 327. | Hasan M, Islam S, Chakraborty S, Mustafa AH, Azim KF, Joy ZF, Hossain MN, Foysal SH, Hasan MN. Contriving a chimeric polyvalent vaccine to prevent infections caused by herpes simplex virus (type-1 and type-2): an exploratory immunoinformatic approach. J Biomol Struct Dyn. 2020;38:2898-2915. [PubMed] [DOI] [Cited in This Article: ] |
| 328. | Wei A, Yin D, Zhai Z, Ling S, Le H, Tian L, Xu J, Paludan SR, Cai Y, Hong J. In vivo CRISPR gene editing in patients with herpetic stromal keratitis. Mol Ther. 2023;31:3163-3175. [PubMed] [DOI] [Cited in This Article: ] |
| 329. | Li Y, Wei Y, Li G, Huang S, Xu J, Ding Q, Hong J. Targeting NECTIN-1 Based on CRISPR/Cas9 System Attenuated the Herpes Simplex Virus Infection in Human Corneal Epithelial Cells In Vitro. Transl Vis Sci Technol. 2022;11:8. [PubMed] [DOI] [Cited in This Article: ] |
| 330. | Zhang I, Hsiao Z, Liu F. Development of Genome Editing Approaches against Herpes Simplex Virus Infections. Viruses. 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 331. | Neuhausser WM, Oh HS, Eggan P, Angelova M, Kirchner R, Eggan KC, Knipe DM. Screening Method for CRISPR/Cas9 Inhibition of a Human DNA Virus: Herpes Simplex Virus. Bio Protoc. 2020;10:e3748. [PubMed] [DOI] [Cited in This Article: ] |
| 332. | Chen Y, Zhi S, Liang P, Zheng Q, Liu M, Zhao Q, Ren J, Cui J, Huang J, Liu Y, Songyang Z. Single AAV-Mediated CRISPR-SaCas9 Inhibits HSV-1 Replication by Editing ICP4 in Trigeminal Ganglion Neurons. Mol Ther Methods Clin Dev. 2020;18:33-43. [PubMed] [DOI] [Cited in This Article: ] |
| 333. | Whitley R, Baines J. Clinical management of herpes simplex virus infections: past, present, and future. F1000Res. 2018;7. [PubMed] [DOI] [Cited in This Article: ] |
| 334. | Wu Y, Song X, Qin S, Chen P, Huang L, Wang Q, Shan T, Liang F, Liao X, Liu Q, Huang Y, Wang Y. Subacute toxicological evaluation of AT-533 and AT-533 gel in Sprague-Dawley rats. Exp Ther Med. 2021;21:632. [PubMed] [DOI] [Cited in This Article: ] |
| 335. | Chan LP, Tseng YP, Liu C, Liang CH. Fermented pomegranate extracts protect against oxidative stress and aging of skin. J Cosmet Dermatol. 2022;21:2236-2245. [PubMed] [DOI] [Cited in This Article: ] |
| 336. | Zannella C, Chianese A, Annunziata G, Ambrosino A, De Filippis A, Tenore GC, Novellino E, Stornaiuolo M, Galdiero M. Antiherpetic Activity of Taurisolo(®), a Grape Pomace Polyphenolic Extract. Microorganisms. 2023;11. [PubMed] [DOI] [Cited in This Article: ] |
| 337. | Ribelato EV, Wouk J, Celestino GG, Rodrigues BCD, Darido MLG, Barboza MGL, Botura TJ, de Oliveira MC, de Andrade FG, Lonni AASG, de Mello JCP, da Rocha SPD, Faccin-Galhardi LC. Topical formulations containing Trichilia catigua extract as therapeutic options for a genital and an acyclovir-resistant strain of herpes recurrent infection. Braz J Microbiol. 2023;54:1501-1511. [PubMed] [DOI] [Cited in This Article: ] |
| 338. | Diaz-Valle D, Burgos-Blasco B, Gegundez-Fernandez JA, Garcia-Caride S, Puebla-Garcia V, Peña-Urbina P, Benitez-Del-Castillo JM. Topical insulin for refractory persistent corneal epithelial defects. Eur J Ophthalmol. 2021;31:2280-2286. [PubMed] [DOI] [Cited in This Article: ] |
| 339. | Kannan L, Kumar A, Jacobs B, Langland J. Anti-herpes virus activity of the carnivorous botanical, Sarracenia purpurea. Sci Rep. 2020;10:18953. [PubMed] [DOI] [Cited in This Article: ] |
| 340. | Maqsood S, Elsawah K, Dhillon N, Soliman S, Laginaf M, Lodhia V, Lake D, Hamada S, Elalfy M. Management of Persistent Corneal Epithelial Defects with Human Amniotic Membrane-derived Dry Matrix. Clin Ophthalmol. 2021;15:2231-2238. [PubMed] [DOI] [Cited in This Article: ] |
| 341. | Varela-Garcia A, Gomez-Amoza JL, Concheiro A, Alvarez-Lorenzo C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] |
| 342. | Wang LQ, Wu TY, Chen XN, Xu ZQ, Yang M, Xiang R, Ma X, Zhang S, Huang YF. Long-term outcomes of Boston keratoprosthesis type I: the Chinese People's Liberation Army General Hospital experience. Br J Ophthalmol. 2022;106:781-785. [PubMed] [DOI] [Cited in This Article: ] |
| 343. | Cressey A, Jacobs DS, Remington C, Carrasquillo KG. Improvement of chronic corneal opacity in ocular surface disease with prosthetic replacement of the ocular surface ecosystem (PROSE) treatment. Am J Ophthalmol Case Rep. 2018;10:108-113. [PubMed] [DOI] [Cited in This Article: ] |
| 344. | Shadambikar G, Marathe S, Patil A, Joshi R, Bandari S, Majumdar S, Repka M. Novel Application of Hot Melt Extrusion Technology for Preparation and Evaluation of Valacyclovir Hydrochloride Ocular Inserts. AAPS PharmSciTech. 2021;22:48. [PubMed] [DOI] [Cited in This Article: ] |
| 345. | Gilger BC. Developing advanced therapeutics through the study of naturally occurring immune-mediated ocular disease in domestic animals. Am J Vet Res. 2022;83. [PubMed] [DOI] [Cited in This Article: ] |
| 346. | Musa M, Zeppieri M, Enaholo ES, Salati C, Parodi PC. Adipose Stem Cells in Modern-Day Ophthalmology. Clin Pract. 2023;13:230-245. [PubMed] [DOI] [Cited in This Article: ] |
| 347. | Roozbahani M, Hammersmith KM. Management of herpes simplex virus epithelial keratitis. Curr Opin Ophthalmol. 2018;29:360-364. [PubMed] [DOI] [Cited in This Article: ] |
| 348. | Rao P, Suvas PK, Jerome AD, Steinle JJ, Suvas S. Role of Insulin-Like Growth Factor Binding Protein-3 in the Pathogenesis of Herpes Stromal Keratitis. Invest Ophthalmol Vis Sci. 2020;61:46. [PubMed] [DOI] [Cited in This Article: ] |
| 349. | P R S, K S, S Y. Cold atmospheric plasma-induced oxidative stress and ensuing immunological response - a Neo-Vista in immunotherapy. Free Radic Res. 2022;56:498-510. [PubMed] [DOI] [Cited in This Article: ] |
| 350. | Cochener B, Zagnoli C, Hugny-Larroque C, Derrien S. Healing of resistant corneal neurotrophic ulcers using a matrix regenerating agent. J Fr Ophtalmol. 2019;42:159-165. [PubMed] [DOI] [Cited in This Article: ] |
| 351. | Bhela S, Rouse BT. Are miRNAs critical determinants in herpes simplex virus pathogenesis? Microbes Infect. 2018;20:461-465. [PubMed] [DOI] [Cited in This Article: ] |
| 352. | Wang Y, Li D, Su W, Dai Y. Clinical Features, Risk Factors, and Therapy of Epithelial Keratitis after Cataract Surgery. J Ophthalmol. 2021;2021:6636228. [PubMed] [DOI] [Cited in This Article: ] |
| 353. | Balal S, Nitiahpapand R, Hassan A, Than J, Patel A, Kumar B, Sharma A. Finger-Prick Autologous Blood in the Treatment of Persistent Corneal Epithelial Defects. Cornea. 2020;39:594-597. [PubMed] [DOI] [Cited in This Article: ] |
| 354. | Faria-E-Sousa SJ, Antunes-Foschini R. Herpes simplex keratitis revisited. Arq Bras Oftalmol. 2021;84:506-512. [PubMed] [DOI] [Cited in This Article: ] |
| 355. | Xu S, Hazlett LD. MicroRNAs in Ocular Infection. Microorganisms. 2019;7. [PubMed] [DOI] [Cited in This Article: ] |
| 356. | Yin D, Ling S, Wang D, Dai Y, Jiang H, Zhou X, Paludan SR, Hong J, Cai Y. Targeting herpes simplex virus with CRISPR-Cas9 cures herpetic stromal keratitis in mice. Nat Biotechnol. 2021;39:567-577. [PubMed] [DOI] [Cited in This Article: ] |
| 357. | Yadavalli T, Suryawanshi R, Ali M, Iqbal A, Koganti R, Ames J, Aakalu VK, Shukla D. Prior inhibition of AKT phosphorylation by BX795 can define a safer strategy to prevent herpes simplex virus-1 infection of the eye. Ocul Surf. 2020;18:221-230. [PubMed] [DOI] [Cited in This Article: ] |
| 358. | Zhang F, Liu Y, You Q, Yang E, Liu B, Wang H, Xu S, Nawaz W, Chen D, Wu Z. NSC23766 and Ehop016 Suppress Herpes Simplex Virus-1 Replication by Inhibiting Rac1 Activity. Biol Pharm Bull. 2021;44:1263-1271. [PubMed] [DOI] [Cited in This Article: ] |
| 359. | Qin Q, Wang Y, Huang X, Jin X. SHIP-1 affects herpetic simplex keratitis prognosis by mediating CD4(+) T lymphocytes migration through PI3K signaling and transcription factor KLF2 in the cornea. Antiviral Res. 2022;207:105424. [PubMed] [DOI] [Cited in This Article: ] |
| 360. | Stenton GR, Mackenzie LF, Tam P, Cross JL, Harwig C, Raymond J, Toews J, Chernoff D, MacRury T, Szabo C. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 2. Efficacy studies in allergic and pulmonary inflammation models in vivo. Br J Pharmacol. 2013;168:1519-1529. [PubMed] [DOI] [Cited in This Article: ] |
| 361. | Hu S, Sheng WS, Schachtele SJ, Lokensgard JR. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J Neuroinflammation. 2011;8:123. [PubMed] [DOI] [Cited in This Article: ] |
| 362. | Liu Y, You Q, Zhang F, Chen D, Huang Z, Wu Z. Harringtonine Inhibits Herpes Simplex Virus Type 1 Infection by Reducing Herpes Virus Entry Mediator Expression. Front Microbiol. 2021;12:722748. [PubMed] [DOI] [Cited in This Article: ] |
| 363. | Fujimoto Y, Hikita SI, Takeda K, Ozaki K, Inoue H, Takakuwa H, Sonoda KH, Ono E. Evaluation of the antiviral potential of the soluble forms of glycoprotein D receptors on ocular herpes caused by HSV-1 and HSV-2 infections in a transgenic mouse model. J Med Virol. 2019;91:820-828. [PubMed] [DOI] [Cited in This Article: ] |
| 364. | He Y, Wang C, Liang Q, Guo R, Jiang J, Shen W, Hu K. PKHB1 peptide induces antiviral effects through induction of immunogenic cell death in herpes simplex keratitis. Front Pharmacol. 2022;13:1048978. [PubMed] [DOI] [Cited in This Article: ] |
| 365. | Alimbarova L, Egorova A, Riabova O, Monakhova N, Makarov V. A proof-of-concept study for the efficacy of dispirotripiperazine PDSTP in a rabbit model of herpes simplex epithelial keratitis. Antiviral Res. 2022;202:105327. [PubMed] [DOI] [Cited in This Article: ] |
| 366. | Chen L, Pan ZQ, Zhai CB. Adenovirus-mediated RNA interference against herpes simplex virus infection in vitro. Folia Histochem Cytobiol. 2021;59:302-310. [PubMed] [DOI] [Cited in This Article: ] |
| 367. | Wan S, Zhou Y, Huang Q, Yang Y. Dot1 L Aggravates Keratitis Induced by Herpes Simplex Virus Type 1 in Mice via p38 MAPK-Mediated Oxidative Stress. Oxid Med Cell Longev. 2021;2021:6612689. [PubMed] [DOI] [Cited in This Article: ] |
| 368. | Tang R, Zhai Y, Dong L, Malla T, Hu K. Immunization with dendritic cell-based DNA vaccine pRSC-NLDC145.gD-IL21 protects mice against herpes simplex virus keratitis. Immunotherapy. 2018;10:189-200. [PubMed] [DOI] [Cited in This Article: ] |
| 369. | Muniruzzaman S, McIntosh M, Hossain A, Izumori K, Bhattacharjee PS. A novel rare sugar inhibitor of murine herpes simplex keratitis. J Pharmacol Sci. 2016;131:126-130. [PubMed] [DOI] [Cited in This Article: ] |
| 370. | Tian X, Wang T, Zhang S, Wang Q, Hu X, Ge C, Xie L, Zhou Q. PEDF Reduces the Severity of Herpetic Simplex Keratitis in Mice. Invest Ophthalmol Vis Sci. 2018;59:2923-2931. [PubMed] [DOI] [Cited in This Article: ] |
| 371. | Lin TL, Cheng C, Zeng WT, Duan F, Pei YH, Liu XP, Shang F, Wu KL. Anti-viral activity of Staphylococcus aureus lysates against herpes simplex virus type-I infection: an in vitro and in vivo study. Int J Ophthalmol. 2021;14:1463-1472. [PubMed] [DOI] [Cited in This Article: ] |
| 372. | Ke L, Yang Y, Li JW, Wang B, Wang Y, Yang W, Yan J. Modulation of Corneal FAK/PI3K/Akt Signaling Expression and of Metalloproteinase-2 and Metalloproteinase-9 during the Development of Herpes Simplex Keratitis. Biomed Res Int. 2019;2019:4143981. [PubMed] [DOI] [Cited in This Article: ] |
| 373. | Rajasagi NK, Rouse BT. Application of our understanding of pathogenesis of herpetic stromal keratitis for novel therapy. Microbes Infect. 2018;20:526-530. [PubMed] [DOI] [Cited in This Article: ] |
| 374. | Akbari M, Soltani Moghadam R, Elmi R, Nosrati A, Taghiabadi E, Aghdami N. Topical Tacrolimus as an adjunct to Conventional Therapy for Stromal Herpetic Keratitis: a Randomized Clinical Trial. J Ophthalmic Vis Res. 2019;14:400-411. [PubMed] [DOI] [Cited in This Article: ] |
| 375. | Levy N, Carbonnel E, Bertrand E, Mairot K, Gonzalvez M, Denis D. [Clinical variant of herpetic keratitis: Archipelago keratitis]. J Fr Ophtalmol. 2021;44:609-610. [PubMed] [DOI] [Cited in This Article: ] |
| 376. | Chapellier B, Guindolet D, Pereira D, Galetto R, Sahel JA, Labetoulle M, Gabison EE. Meganuclease targeting HSV-1 protects against herpetic keratitis: Application to corneal transplants. Mol Ther Nucleic Acids. 2022;30:511-521. [PubMed] [DOI] [Cited in This Article: ] |
| 377. | Berber E, Rouse BT. Controlling Herpes Simplex Virus-Induced Immunoinflammatory Lesions Using Metabolic Therapy: a Comparison of 2-Deoxy-d-Glucose with Metformin. J Virol. 2022;96:e0068822. [PubMed] [DOI] [Cited in This Article: ] |
| 378. | Zhang C, Hu Z, Wang K, Yang L, Li Y, Schlüter H, Yang P, Hong J, Yu H. Lipidomic profiling of virus infection identifies mediators that resolve herpes simplex virus-induced corneal inflammatory lesions. Analyst. 2020;145:3967-3976. [PubMed] [DOI] [Cited in This Article: ] |
| 379. | Klarlund JK, Callaghan JD, Stella NA, Kowalski RP, McNamara NA, Shanks RMQ. Use of Collagen Binding Domains to Deliver Molecules to the Cornea. J Ocul Pharmacol Ther. 2019;35:491-496. [PubMed] [DOI] [Cited in This Article: ] |
| 380. | Dhanushkodi NR, Srivastava R, Coulon PA, Prakash S, Roy S, Bagnol D, David ED, BenMohamed L. Healing of Ocular Herpetic Disease Following Treatment With an Engineered FGF-1 Is Associated With Increased Corneal Anti-Inflammatory M2 Macrophages. Front Immunol. 2021;12:673763. [PubMed] [DOI] [Cited in This Article: ] |
| 381. | Gurung HR, Carr MM, Bryant K, Chucair-Elliott AJ, Carr DJ. Fibroblast growth factor-2 drives and maintains progressive corneal neovascularization following HSV-1 infection. Mucosal Immunol. 2018;11:172-185. [PubMed] [DOI] [Cited in This Article: ] |
| 382. | Shan T, Ye J, Jia J, Wang Z, Jiang Y, Wang Y, Zheng K, Ren Z. Viral UL8 Is Involved in the Antiviral Activity of Oleanolic Acid Against HSV-1 Infection. Front Microbiol. 2021;12:689607. [PubMed] [DOI] [Cited in This Article: ] |
| 383. | Fitzpatrick S, Lausch R, Barrington RA. CCR6-Positive γδ T Cells Provide Protection Against Intracorneal HSV-1 Infection. Invest Ophthalmol Vis Sci. 2019;60:3952-3962. [PubMed] [DOI] [Cited in This Article: ] |
| 384. | Cui YH, Song XL, Hu ZX, Yang G, Li ZJ, Pan HW. Transcriptional Profile and Integrative Analyses of Long Noncoding RNAs in Primary Human Corneal Epithelial Cells in Response to HSV-1 Infection. Curr Eye Res. 2018;43:1422-1431. [PubMed] [DOI] [Cited in This Article: ] |
| 385. | Xia L, Tan T, Li Y, Zhong Q, Shi M. Blockade of IL-27 signaling ameliorates herpes stromal keratitis with upregulated CD4(+) Foxp3(+) regulatory T cells influx in mice. Indian J Ophthalmol. 2019;67:1821-1828. [PubMed] [DOI] [Cited in This Article: ] |
| 386. | Sarkar R, Mathew A, Sehrawat S. Myeloid-Derived Suppressor Cells Confer Infectious Tolerance to Dampen Virus-Induced Tissue Immunoinflammation. J Immunol. 2019;203:1325-1337. [PubMed] [DOI] [Cited in This Article: ] |
| 387. | Minegaki N, Koshizuka T, Hatasa K, Kondo H, Kato H, Tannaka M, Takahashi K, Tsuji M, Inoue N. The C-Terminal Penta-Peptide Repeats of Major Royal Jelly Protein 3 Ameliorate the Progression of Inflammation in Vivo and in Vitro. Biol Pharm Bull. 2022;45:583-589. [PubMed] [DOI] [Cited in This Article: ] |
| 388. | Rao P, Suvas S. Development of Inflammatory Hypoxia and Prevalence of Glycolytic Metabolism in Progressing Herpes Stromal Keratitis Lesions. J Immunol. 2019;202:514-526. [PubMed] [DOI] [Cited in This Article: ] |
| 389. | Wang S, Ghiasi H. Absence of Signal Peptide Peptidase, an Essential Herpes Simplex Virus 1 Glycoprotein K Binding Partner, Reduces Virus Infectivity In Vivo. J Virol. 2019;93. [PubMed] [DOI] [Cited in This Article: ] |
| 390. | Jiang L, Yu Y, Li Z, Gao Y, Zhang H, Zhang M, Cao W, Peng Q, Chen X. BMS-265246, a Cyclin-Dependent Kinase Inhibitor, Inhibits the Infection of Herpes Simplex Virus Type 1. Viruses. 2023;15. [PubMed] [DOI] [Cited in This Article: ] |
| 391. | Sumbria D, Berber E, Rouse BT. Supplementing the Diet with Sodium Propionate Suppresses the Severity of Viral Immuno-inflammatory Lesions. J Virol. 2021;95. [PubMed] [DOI] [Cited in This Article: ] |
| 392. | Majmudar H, Hao M, Sankaranarayanan NV, Zanotti B, Volin MV, Desai UR, Tiwari V. A synthetic glycosaminoglycan mimetic blocks HSV-1 infection in human iris stromal cells. Antiviral Res. 2019;161:154-162. [PubMed] [DOI] [Cited in This Article: ] |
| 393. | Murchison CE, Petroll WM, Robertson DM. Infectious keratitis after corneal crosslinking: systematic review. J Cataract Refract Surg. 2021;47:1075-1080. [PubMed] [DOI] [Cited in This Article: ] |
| 394. | Subramaniam R, Sonny Teo KS, Muhammed J. Atypical Presentation of Herpes Stromal Keratitis in a Contact Lens Wearer. Cureus. 2023;15:e38438. [PubMed] [DOI] [Cited in This Article: ] |
| 395. | Feizi S, Karjou Z, Esfandiari H. Lately Diagnosed Acanthamoeba Keratitis Manifesting as an Intrastromal Corneal Abscess: A Case Report. Eye Contact Lens. 2023;49:569-571. [PubMed] [DOI] [Cited in This Article: ] |
| 396. | Sanchez S, Faraj LA, Wajnsztajn D, Dart JKG, Milligan AL. Acanthamoeba more commonly causes epithelial keratitis than herpes simplex in South-East England contact lens users. Infection. 2022;50:1619-1622. [PubMed] [DOI] [Cited in This Article: ] |
| 397. | Carnt N, Robaei D, Minassian DC, Dart JKG. Acanthamoeba keratitis in 194 patients: risk factors for bad outcomes and severe inflammatory complications. Br J Ophthalmol. 2018;102:1431-1435. [PubMed] [DOI] [Cited in This Article: ] |
| 398. | Li G, Shekhawat N. Acanthamoeba epitheliopathy: Importance of early diagnosis. Am J Ophthalmol Case Rep. 2022;26:101499. [PubMed] [DOI] [Cited in This Article: ] |
| 399. | Connelly L, Anijeet D, Alexander CL. A descriptive case of persistent Acanthamoeba keratitis: raising awareness of this complex ocular disease. Access Microbiol. 2020;2:acmi000084. [PubMed] [DOI] [Cited in This Article: ] |
| 400. | Toshida H, Sadamatsu Y. A Case of Herpetic Keratitis in an Orthokeratology Contact Lens Wearer. Cureus. 2022;14:e27388. [PubMed] [DOI] [Cited in This Article: ] |
| 401. | Stanfield BA, Kousoulas KG, Fernandez A, Gershburg E. Rational Design of Live-Attenuated Vaccines against Herpes Simplex Viruses. Viruses. 2021;13. [PubMed] [DOI] [Cited in This Article: ] |
| 402. | Ulman EA, Selver OB, Biler ED, Palamar M. Clinical Features of Pediatric Age Herpes Simplex Virus Keratitis. Cornea. 2023;42:1099-1103. [PubMed] [DOI] [Cited in This Article: ] |
| 403. | Thibault LP, Mitchell GA, Parisien B, Hamel P, Blanchard AC. An Infant with Bilateral Keratitis: From Infectious to Genetic Diagnosis. Am J Case Rep. 2022;23:e937967. [PubMed] [DOI] [Cited in This Article: ] |
| 404. | Vadoothker S, Andrews L, Jeng BH, Levin MR. Management of Herpes Simplex Virus Keratitis in the Pediatric Population. Pediatr Infect Dis J. 2018;37:949-951. [PubMed] [DOI] [Cited in This Article: ] |
| 405. | Bodack MI. Case Series: Pediatric Herpes Simplex Keratitis. Optom Vis Sci. 2019;96:221-226. [PubMed] [DOI] [Cited in This Article: ] |
| 406. | Luccarelli SV, Lucentini S, Martellucci CA, Marelli L, Sacchi M, Nucci P. Impact of Adherence (Compliance) to Oral Acyclovir Prophylaxis in the Recurrence of Herpetic Keratitis: Long-Term Results From a Pediatric Cohort. Cornea. 2021;40:1126-1131. [PubMed] [DOI] [Cited in This Article: ] |
| 407. | Moshirfar M, Milner DC, Baker PA, McCabe SE, Ronquillo YC, Hoopes PC. Corneal Refractive Surgery in Patients with a History of Herpes Simplex Keratitis: A Narrative Review. Clin Ophthalmol. 2020;14:3891-3901. [PubMed] [DOI] [Cited in This Article: ] |
| 408. | Velásquez-Monzón K, Navarro-Peña MC, Klunder-Klunder M, Tsatsos M, Ramírez-Ortiz MA. Pediatric penetrating keratoplasty and graft rejection: experience at the Hospital Infantil de México Federico Gómez. Bol Med Hosp Infant Mex. 2020;77:23-27. [PubMed] [DOI] [Cited in This Article: ] |
| 409. | Lang SJ, Böhringer D, Reinhard T. [Keratoplasty in children : Indications and results]. Ophthalmologe. 2020;117:215-217. [PubMed] [DOI] [Cited in This Article: ] |
| 410. | Pinninti SG, Kimberlin DW. Neonatal herpes simplex virus infections. Semin Perinatol. 2018;42:168-175. [PubMed] [DOI] [Cited in This Article: ] |
| 411. | Chen D, Liu Y, Zhang F, You Q, Ma W, Wu J, Wu Z. 6-Thioguanine Inhibits Herpes Simplex Virus 1 Infection of Eyes. Microbiol Spectr. 2021;9:e0064621. [PubMed] [DOI] [Cited in This Article: ] |
| 412. | Marchenko NR, Kasparova EA, Budnikova EA, Makarova MA. [Anterior eye segment damage in coronavirus infection (COVID-19)]. Vestn Oftalmol. 2021;137:142-148. [PubMed] [DOI] [Cited in This Article: ] |
| 413. | Sookaromdee P, Wiwanitkit V. Relapsed Disciform Stromal Herpetic Keratitis and mRNA COVID-19 Vaccination. Korean J Ophthalmol. 2022;36:176-177. [PubMed] [DOI] [Cited in This Article: ] |
| 414. | Das N, Das J, Pal D. Stromal and endothelial herpes simplex virus keratitis reactivation in the convalescent period of COVID-19 - A case report. Indian J Ophthalmol. 2022;70:1410-1412. [PubMed] [DOI] [Cited in This Article: ] |
| 415. | Song MY, Koh KM, Hwang KY, Kwon YA, Kim KY. Relapsed Disciform Stromal Herpetic Keratitis Following mRNA COVID-19 Vaccination: A Case Report. Korean J Ophthalmol. 2022;36:80-82. [PubMed] [DOI] [Cited in This Article: ] |
| 416. | Singh RB, Parmar UPS, Ichhpujani P, Jeng BH, Jhanji V. Herpetic Eye Disease After SARS-CoV-2 Vaccination: A CDC-VAERS Database Analysis. Cornea. 2023;42:731-738. [PubMed] [DOI] [Cited in This Article: ] |
| 417. | Guindolet D, Gemahling A, Rousseau A, Nguyen Kim P, Azar G, Martin GC, Cochereau I, Labetoulle M, Gabison EE. Clinical course and treatment of archipelago keratitis: a Herpesviridae keratitis subtype. Br J Ophthalmol. 2022;. [PubMed] [DOI] [Cited in This Article: ] |
| 418. | Matalia HP, Nandini C, Saishree M, Matalia J. Archipelago keratitis. Indian J Ophthalmol. 2019;67:555. [PubMed] [DOI] [Cited in This Article: ] |
| 419. | Gupta V, Pal H, Das S, Pathuri DS, Vathulya M. Varicella Zoster Reactivation Manifesting as Serpiginous Peripheral Keratitis and Disciform Keratitis Occurring After Necrotizing Fasciitis in an Immunocompromised Male: A Case Report. Cureus. 2023;15:e40787. [PubMed] [DOI] [Cited in This Article: ] |
| 420. | Murgova S, Balchev G. Ophthalmic manifestation after SARS-CoV-2 vaccination: a case series. J Ophthalmic Inflamm Infect. 2022;12:20. [PubMed] [DOI] [Cited in This Article: ] |
| 421. | Yildiz BK, Ozkan D, Tellioglu A, Demirok A. Is COVID-19 Infection a Trigger for Herpetic Stromal Keratitis? Beyoglu Eye J. 2022;7:140-142. [PubMed] [DOI] [Cited in This Article: ] |
| 422. | Huang LY, Chiang CC, Li YL, Lai HY, Hsieh YC, Wu YH, Tsai YY. Corneal Complications after COVID-19 Vaccination: A Systemic Review. J Clin Med. 2022;11. [PubMed] [DOI] [Cited in This Article: ] |
| 423. | Matharu KS, Mammen A, Jhanji V, Kinchington PR, Kowalski RP. Double Trouble: An Unusual Keratitis Case of Herpes Simplex Virus and Varicella-Zoster Virus Co-infection. Cornea. 2023;42:1451-1453. [PubMed] [DOI] [Cited in This Article: ] |
| 424. | Cohen S, Olshaker H, Fischer N, Vishnevskia-Dai V, Hagin D, Rosenblatt A, Zur D, Habot-Wilner Z. Herpetic Eye Disease Following the SARS-CoV-2 Vaccinations. Ocul Immunol Inflamm. 2023;31:1151-1162. [PubMed] [DOI] [Cited in This Article: ] |
| 425. | Yoshida M, Hariya T, Yokokura S, Kobayashi W, Watanabe R, Ishii T, Nakazawa T. Concomitant herpes simplex keratitis and autoimmune-associated ulcerative keratitis in rheumatoid arthritis patients. Am J Ophthalmol Case Rep. 2020;18:100648. [PubMed] [DOI] [Cited in This Article: ] |
| 426. | Fei P, Feng H, Li J, Liu M, Luo J, Ye H, Zhao P. Inflammatory ocular events after inactivated COVID-19 vaccination. Hum Vaccin Immunother. 2022;18:2138051. [PubMed] [DOI] [Cited in This Article: ] |
| 427. | Al-Dwairi RA, Aleshawi A, Adi S, Abu-Zreig L. Reactivation of Herpes Simplex Keratitis on a Corneal Graft Following SARS-CoV-2 mRNA Vaccination. Med Arch. 2022;76:146-148. [PubMed] [DOI] [Cited in This Article: ] |
| 428. | Majtanova N, Kriskova P, Keri P, Fellner Z, Majtan J, Kolar P. Herpes Simplex Keratitis in Patients with SARS-CoV-2 Infection: A Series of Five Cases. Medicina (Kaunas). 2021;57. [PubMed] [DOI] [Cited in This Article: ] |
| 429. | Roberts HW, Akram H, Myerscough J. Negative polymerase chain reaction for SARS-CoV-2 in aqueous sample of patient with confirmed SARS-CoV-2 and recurrence of herpetic stromal keratitis. J Cataract Refract Surg. 2020;46:e61-e63. [PubMed] [DOI] [Cited in This Article: ] |
| 430. | Kuziez L, Eleiwa TK, Chauhan MZ, Sallam AB, Elhusseiny AM, Saeed HN. Corneal Adverse Events Associated with SARS-CoV-2/COVID-19 Vaccination: A Systematic Review. Vaccines (Basel). 2023;11. [PubMed] [DOI] [Cited in This Article: ] |
| 431. | Mohammadzadeh M, Hooshmandi S, Jafari M, Hassanpour K. Presumably Corneal Graft Rejection after COVID-19 Vaccination. Case Rep Ophthalmol. 2022;13:562-569. [PubMed] [DOI] [Cited in This Article: ] |
| 432. | Ichhpujani P, Parmar UPS, Duggal S, Kumar S. COVID-19 Vaccine-Associated Ocular Adverse Effects: An Overview. Vaccines (Basel). 2022;10. [PubMed] [DOI] [Cited in This Article: ] |
| 433. | Ono T, Iwasaki T, Terada Y, Mori Y, Nejima R, Ozaki M, Mochizuki M, Miyata K. Long-term outcome in corneal endotheliitis with molecular detection of herpes simplex virus 1 and human herpes virus 6: a case report. BMC Ophthalmol. 2022;22:48. [PubMed] [DOI] [Cited in This Article: ] |
| 434. | Sinha P, Dash M, Bhatkoti B, Krishnan L. Epithelial herpes simplex keratitis in a patient on treatment with secukinumab for psoriasis: An effect of interleukin-17 blockade? Indian J Dermatol Venereol Leprol. 2022;88:225-227. [PubMed] [DOI] [Cited in This Article: ] |
| 435. | Alfaro Rangel R, Lepper S, Szentmáry N, Langenbucher A, Seitz B. Herpes Simplex Virus Keratitis in a University Tertiary Referral Centre - Clinical Features and Surgical Approaches. Klin Monbl Augenheilkd. 2021;238:989-995. [PubMed] [DOI] [Cited in This Article: ] |
| 436. | Basak SK, Basak S. Descemet Membrane Endothelial Keratoplasty in Irreversible Corneal Edema Due to Herpes Simplex Virus Endotheliitis. Cornea. 2020;39:8-12. [PubMed] [DOI] [Cited in This Article: ] |
| 437. | Abdelmassih Y, Dubrulle P, Sitbon C, El-Khoury S, Guindolet D, Doan S, Labetoulle M, Cochereau I, Gabison EE. Therapeutic Challenges and Prognosis of Descemet's Membrane Endothelial Keratoplasty in Herpes Simplex Eye Disease. Cornea. 2019;38:553-558. [PubMed] [DOI] [Cited in This Article: ] |
| 438. | Asi F, Milioti G, Seitz B. Descemet membrane endothelial keratoplasty for corneal decompensation caused by herpes simplex virus endotheliitis. J Cataract Refract Surg. 2018;44:106-108. [PubMed] [DOI] [Cited in This Article: ] |
| 439. | Ying LY, Qiu WY, Wang BH, Zhou P, Zhang B, Yao YF. Corneal endothelial regeneration in human eyes using endothelium-free grafts. BMC Ophthalmol. 2022;22:32. [PubMed] [DOI] [Cited in This Article: ] |
| 440. | Friehmann A, Myerscough J, Giannaccare G, Mazzoni M, Bovone C, Busin M. Successful Descemet Membrane Endothelial Keratoplasty in Proven Herpetic Endothelial Decompensation Requires Intensive Antiviral Therapy. Cornea. 2020;39:196-199. [PubMed] [DOI] [Cited in This Article: ] |
| 441. | Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog Retin Eye Res. 2013;32:88-101. [PubMed] [DOI] [Cited in This Article: ] |
| 442. | Mekonnen B, Kossler AL, Lin CC. Modified Gundersen Flap Using Inferior Palpebral-Bulbar Conjunctiva. Cornea. 2022;41:260-263. [PubMed] [DOI] [Cited in This Article: ] |
| 443. | Kim JS, Rafailov L, Leyngold IM. Corneal Neurotization for Postherpetic Neurotrophic Keratopathy: Initial Experience and Clinical Outcomes. Ophthalmic Plast Reconstr Surg. 2021;37:42-50. [PubMed] [DOI] [Cited in This Article: ] |
| 444. | Tuli S, Gray M, Shah A. Surgical management of herpetic keratitis. Curr Opin Ophthalmol. 2018;29:347-354. [PubMed] [DOI] [Cited in This Article: ] |
| 445. | Lin CH, Lai LJ. Herpetic Corneal Keratopathy Management Using Ipsilateral Supratrochlear Nerve Transfer for Corneal Neurotization. Ann Plast Surg. 2019;83:553-557. [PubMed] [DOI] [Cited in This Article: ] |
| 446. | Bourcier T, Henrat C, Heitz A, Kremer SF, Labetoulle M, Liverneaux P. Lateral Antebrachial Cutaneous Nerve as Autologous Graft for Mini-Invasive Corneal Neurotization (MICORNE). Cornea. 2019;38:1029-1032. [PubMed] [DOI] [Cited in This Article: ] |
| 447. | Roberts HW, Gunasekera CD, Law EM, Seifelnasr M, Giannaccare G, Busin M, Myerscough J. Sutureless Tectonic Mini-Descemet's Stripping Automated Endothelial Keratoplasty ("mini-DSAEK") for the management of corneal perforations. Eur J Ophthalmol. 2022;32:2133-2140. [PubMed] [DOI] [Cited in This Article: ] |
| 448. | Qi X, Wang M, Li X, Jia Y, Li S, Shi W, Gao H. Characteristics of New Onset Herpes Simplex Keratitis after Keratoplasty. J Ophthalmol. 2018;2018:4351460. [PubMed] [DOI] [Cited in This Article: ] |
| 449. | Dapena I, Musayeva A, Dragnea DC, Groeneveld-van Beek EA, Ní Dhubhghaill S, Parker JS, van Dijk K, Melles GRJ. Bowman Layer Onlay Transplantation to Manage Herpes Corneal Scar. Cornea. 2020;39:1164-1166. [PubMed] [DOI] [Cited in This Article: ] |
| 450. | Domínguez-López A, Magaña-Guerrero FS, Buentello-Volante B, Bautista-Hernández LA, Reyes-Grajeda JP, Bautista-de Lucio VM, Garfias Y. Amniotic membrane conditioned medium (AMCM) reduces inflammatory response on human limbal myofibroblast, and the potential role of lumican. Mol Vis. 2021;27:370-383. [PubMed] [Cited in This Article: ] |
| 451. | Ting DSJ, Henein C, Said DG, Dua HS. Amniotic membrane transplantation for infectious keratitis: a systematic review and meta-analysis. Sci Rep. 2021;11:13007. [PubMed] [DOI] [Cited in This Article: ] |
| 452. | Lamas-Francis D, Navarro D, Moreno C, de-Rojas V, Mansilla R, Dios E, Rigueiro J, Álvarez D, Crego P, Rodríguez-Ares T, Touriño R. Amniotic Membrane Transplantation in the Management of Corneal Ulceration Following Infectious Keratitis. Ocul Immunol Inflamm. 2023;1-7. [PubMed] [DOI] [Cited in This Article: ] |
| 453. | Hayek G, Francois J, Perone JM. Corneal Perforation Repair Using a Novel Lyophilized Amniotic Membrane Graft Technique: Plug and Patch. Am J Case Rep. 2023;24:e939626. [PubMed] [DOI] [Cited in This Article: ] |
| 454. | Kanclerz P, Alio JL. Ocular surgery after herpes simplex and herpes zoster keratitis. Int Ophthalmol. 2020;40:3599-3612. [PubMed] [DOI] [Cited in This Article: ] |
| 455. | Wang Y, Cheng J, Yang N, Li T, Dong Y, Xie L. Combined vs sequential penetrating keratoplasty and cataract surgery for herpes simplex keratitis: a retrospective study. Front Med (Lausanne). 2023;10:1190485. [PubMed] [DOI] [Cited in This Article: ] |
| 456. | Bu JB, Grabitz SD, Pfeiffer N, Wasielica-Poslednik J. Prevalence of Herpesvirus DNA in Corneal Transplant Recipients. J Clin Med. 2022;12. [PubMed] [DOI] [Cited in This Article: ] |
| 457. | Yu AC, Friehmann A, Myerscough J, Socea S, Furiosi L, Giannaccare G, Bovone C, Busin M. Initial High-Dose Prophylaxis and Extended Taper for Mushroom Keratoplasty in Vascularized Herpetic Scars. Am J Ophthalmol. 2020;217:212-223. [PubMed] [DOI] [Cited in This Article: ] |
| 458. | Fry M, Aravena C, Yu F, Kattan J, Aldave AJ. Long-term outcomes of the Boston type I keratoprosthesis in eyes with previous herpes simplex virus keratitis. Br J Ophthalmol. 2018;102:48-53. [PubMed] [DOI] [Cited in This Article: ] |
| 459. | Mimouni M, Liu ES, Din N, Gouvea L, Alshaker S, Cohen E, Kim DB, Chan CC. Tape Splint Tarsorrhaphy for Persistent Corneal Epithelial Defects. Am J Ophthalmol. 2022;237:235-240. [PubMed] [DOI] [Cited in This Article: ] |
| 460. | Hata-Mizuno M, Ingaki E, Mitamura H, Uchino Y, Tsubota K, Shimmura S. Conjunctival Epithelial Ingrowth After Penetrating Keratoplasty. Cornea. 2020;39:1181-1183. [PubMed] [DOI] [Cited in This Article: ] |
| 461. | Liu X, Kolli S, McDonnell P, Patel A, Quinlan M, Skym K, Denniston AK, Shah P, Williams GP. Patient priorities in herpes simplex keratitis. BMJ Open Ophthalmol. 2019;4:e000177. [PubMed] [DOI] [Cited in This Article: ] |
| 462. | Courrier E, Maurin C, Lambert V, Renault D, Bourlet T, Pillet S, Verhoeven PO, Forest F, Perrache C, He Z, Garcin T, Rousseau A, Labetoulle M, Gain P, Thuret G. Ex vivo model of herpes simplex virus type I dendritic and geographic keratitis using a corneal active storage machine. PLoS One. 2020;15:e0236183. [PubMed] [DOI] [Cited in This Article: ] |
| 463. | Poccardi N, Rousseau A, Haigh O, Takissian J, Naas T, Deback C, Trouillaud L, Issa M, Roubille S, Juillard F, Efstathiou S, Lomonte P, Labetoulle M. Herpes Simplex Virus 1 Replication, Ocular Disease, and Reactivations from Latency Are Restricted Unilaterally after Inoculation of Virus into the Lip. J Virol. 2019;93. [PubMed] [DOI] [Cited in This Article: ] |