Published online Dec 12, 2012. doi: 10.5501/wjv.v1.i6.154
Revised: October 16, 2012
Accepted: November 7, 2012
Published online: December 12, 2012
Viruses have been shown to be responsible for 10%-15% of cancer cases. Epstein-Barr virus (EBV) is the first virus to be associated with human malignancies. EBV can cause many cancers, including Burkett’s lymphoma, Hodgkin’s lymphoma, post-transplant lymphoproliferative disorders, nasopharyngeal carcinoma and gastric cancer. Evidence shows that phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) plays a key role in EBV-induced malignancies. The main EBV oncoproteins latent membrane proteins (LMP) 1 and LMP2A can activate the PI3K/Akt pathway, which, in turn, affects cell survival, apoptosis, proliferation and genomic instability via its downstream target proteins to cause cancer. It has also been demonstrated that the activation of the PI3K/Akt pathway can result in drug resistance to chemotherapy. Thus, the inhibition of this pathway can increase the therapeutic efficacy of EBV-associated cancers. For example, PI3K inhibitor Ly294002 has been shown to increase the effect of 5-fluorouracil in an EBV-associated gastric cancer cell line. At present, dual inhibitors of PI3K and its downstream target mammalian target of rapamycin have been used in clinical trials and may be included in treatment regimens for EBV-associated cancers.
- Citation: Chen J. Roles of the PI3K/Akt pathway in Epstein-Barr virus-induced cancers and therapeutic implications. World J Virol 2012; 1(6): 154-161
- URL: https://www.wjgnet.com/2220-3249/full/v1/i6/154.htm
- DOI: https://dx.doi.org/10.5501/wjv.v1.i6.154
It is now evident that virus-induced cancers account for 10%-15% of all cancer cases[1,2]. Studies of viruses as causes of cancer have played an important role in the elucidation of the mechanisms of carcinogenesis, as indicated by several Nobel Prizes being awarded to scientists in the field of oncoviruses. The initial work to demonstrate that viruses can induce cancer was done by Peyton Rous[3,4]. He identified Rous sarcoma virus as the cause of chicken sarcoma in 1911, and the discovery earned him the 1966 Nobel Prize. The human analogue of the viral oncogene v-Src was found and named c-Src, which was the first human oncogene[5,6]. This work led to the awarding of a Nobel Prize to John Michael Bishop and Harold E. Varmus in 1989. More recently, Harald zur Hausen identified human papillomavirus (HPV) as the cause of cervical cancer (Nobel Prize, 2008)[7]. This discovery led to the invention of the vaccines Gardasil and Cervarix which can effectively prevent HPV-associated cervical cancer[8,9]. The Epstein-Barr virus (EBV); [also called human herpesvirus 4 (HHV-4)] is the first virus identified (in 1964) to be associated with human cancers[1]. It belongs to the B-lymphotropic γ-herpesvirus family with a genome consisting of 172 kb of linear double-stranded DNA[1,10,11]. EBV infects both epithelial and B-cells and, thus, can induce both epithelial cancers and lymphoma[12,13]. After EBV infection, there are two viral phases: lytic and latent[14]. In its lytic phase, the virus replicates in epithelial cells, and, in its latent phase, it transforms B-cells.
Cancer is characterized by the loss of the balance between cell proliferation and apoptosis[15-17]. It has been demonstrated that EBV can increase cell proliferation and decrease apoptosis[18]. EBV has been shown to cause several B-cell lymphomas, including Burkitt’s lymphoma, Hodgkin’s lymphoma and post-transplant lymphoproliferative disorders (PTLDs). This notion is demonstrated by the detection of EBV virus in these cancers, the replication of the virus and its ability to transform B-cells[18,19]. EBV is also closely associated with epithelial cancers. For example, EBV can cause nasopharyngeal carcinoma (NPC), a highly metastatic cancer[20]. The EBV latent membrane proteins (LMP) 1 and LMP2A are frequently detected in NPC[21]. LMP1 may also lead to metastasis of the cancer, as it has been demonstrated that LMP1 can cause epithelial-mesenchymal transition (EMT) via transcription factor Snail[22]. Both LMP1 and Snail are correlated with NPC metastasis[22]. Overall, EBV has been shown to be responsible for about 10% of gastric cancers worldwide[23-25]. However, the mechanisms for EBV-induced gastric cancer are not clear.
Many EBV proteins are expressed in the latent phases and are potentially related to carcinogenesis. These proteins include EBV nuclear antigen (EBNA)-1, -2, -3A, -3B, -3C and leader protein, and LMP-1, -2A and -2B[14]. However, the major identified oncoproteins in EBV are LMP1 and LMP2A[20,26]. These proteins can activate multiple signal pathways, such as the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), the mitogen-activated protein kinase (MAPK) and the signal transducer and activator of transcription 3, all of which are important for carcinogenesis[15,27,28]. LMP1 is considered as an analog of the tumor necrosis factor receptor 1, and it can transform human B-lymphocytes and rodent fibroblasts via activation of multiple intracellular signal pathways through its two signaling domains, the carboxyl-terminal activating regions 1 and 2 (CTAR1 and CTAR2)[29]. Activated pathways include the nuclear factor κB (NF-κB), PI3K/Akt, Notch, MAPK and Jun N-terminal protein kinase (JNK) signaling pathways[27,30-32]. It has been demonstrated that point mutations in the C-terminal region of the LMP1 cytoplasmic domain can influence the transforming potential of the EBV by reducing the ability of LMP1 to activate PI3K/Akt, NF-κB and AP1[29]. LMP1 is essential for EBV-mediated B-cell transformation and is sufficient to transform several cell lines, such as rodent fibroblasts[33]. A recent study showed that LMP1 expression is regulated by C/EBP in addition to EBNA2[34]. This article will discuss how EBV-expressed proteins activate the PI3K/Akt pathway to cause carcinogenesis in EBV-associated cancers. Although EBV oncogenes can affect many signal pathways, such as NF-κB, MAPK, and JNK, it seems that the PI3K/Akt pathway is the most important. In an LMP1-mediated transformation of rodent fibroblasts, inhibition of PI3K activity by Ly294002 induced apoptosis and inhibited cell growth, however, the NF-κB inhibitor BAY 11-7085 had no such effect[35]. Another study also showed that the PI3K/Akt pathway, but not the MAPK or NF-κB pathways, can account for the LMP-1-induced transformation[36].
In 1985, Lewis Cantley initially discovered that PI3K plays an important role in cancer[37-41]. PI3K has now been extensively studied with investigation determining its role in carcinogenesis and the potential use of its inhibitors in the treatment of cancers[42-44]. This kinase phosphorylates the 3’ OH position of phosphatidylinositol 4,5-biphosphate (PIP2) and converts it to phosphatidylinositol 3,4,5-triphosphate (PIP3), leading to activation of Akt[45,46], which causes a cascade of cellular signal alterations via its downstream target proteins[39].
Many factors, such as insulin, insulin-like growth factor-1, vascular endothelial growth factor, and cytokines interleukin (IL)-6, IL-17 can increase the activity of the PI3K/Akt pathway[6,47-52]. Mutations of genes encoding key components in the pathway have been found to cause the pathway activation in many cancers[38,53]. Many cancer-related viruses can also activate the PI3K/Akt pathway and rely on it for their transformations[38,39]. Such viral oncoproteins include polyoma virus middle-T antigen, Rous sarcoma virus oncoprotein v-Src, HPV oncoproteins E6, E7 and the human T-cell leukemia virus type 1 oncoprotein Tax[54-57]. It has also been demonstrated that the PI3K/Akt pathway plays a critical role in the carcinogenesis of EBV viral oncoproteins[27].
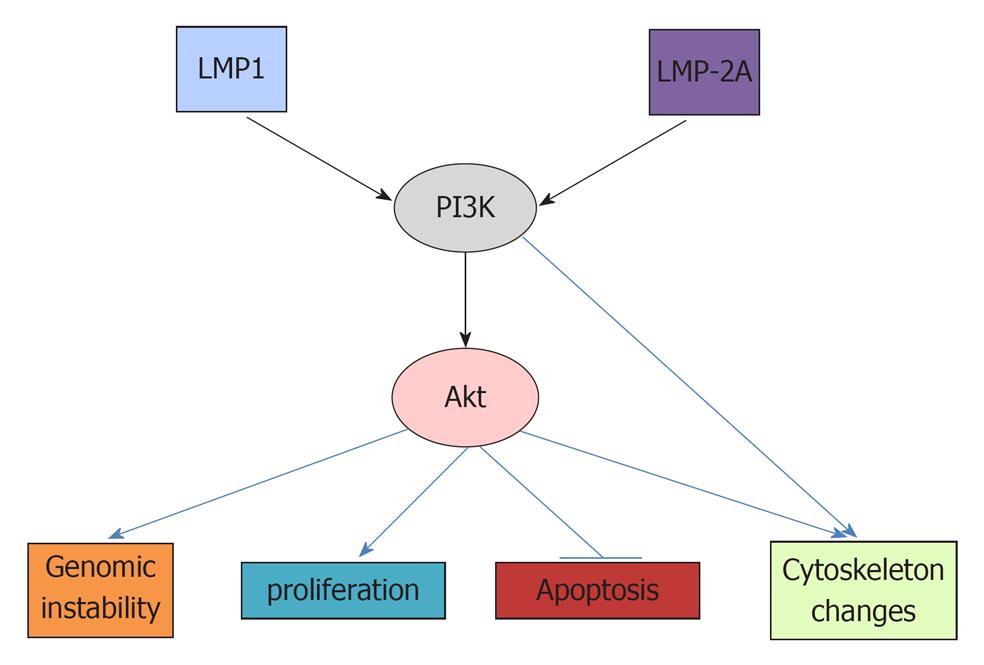
Activated Akt, which is phosphorylated by PDK1, can affect many downstream targets[38,42]. The resulting biological effects include increased genomic instability, increased proliferation, decreased apoptosis and changed cytoskeleton. (Figure 1)[58]. Genomic instability is important for the accumulation of genetic mutations necessary for carcinogenesis[59,60]. Recently, it was reported that constitutively active (CA) Met tyrosine kinase (hepatocyte growth factor receptor) can induce chromosomal instability (CIN), as indicated by increased centrosome counts, multinucleated cells and micronuclei formation[61-63]. While CA-Met increased both phosphorylated Akt and phosphorylated Erk, only phosphorylated Akt is critical in CA-Met-induced CIN. The PI3K inhibitor Ly294002, PTEN (an inhibitor of PI3K), and siRNA against Akt all abolished CA-met mediated CIN[62]. It has also been demonstrated that phosphorylation of Akt can block checkpoint kinase 1 (Chk1), which controls cell cycle progression and maintains genomic stability[61,63,64]. The activation of Chk1 will phosphorylate cdc25A and induce the transient arrest of cells in G1 and S phase before the onset of mitosis[65]. The inhibition of Chk1 has been shown to increase double-strand DNA breaks[66].
The activation of Akt can increase cell proliferation and cell size by accelerating the cell cycle and cell metabolism. Akt can phosphorylate glycogen synthase kinase 3β (GSK3β) and, thus, deactivate it, leading to increased cyclin D1 and Myc[67]. Myc is an oncoprotein that upregulates cyclin-dependent kinase 4 (CDK4)[68]. Additionally, the Akt-mediated inhibition of the forkhead protein results in the downregulation of the cell cycle proteins p27 and p21[69], thus promoting cell cycle progression[70]. Both p27 and p21 are G1-checkpoint CDK inhibitors which can promote G1/S transition and thus, accelerate cell cycle[17,71,72]. Another target activated by the activation of Akt is mTORC1, which plays an important role in the carcinogenesis of many cancers, including Burkitt’s lymphoma and NPC[73-75]. Phosphorylated Akt blocks TSC1 and 2 (tuberous sclerosis complex 1 and 2) and, thus, activates Rheb (Ras homolog enriched in brain), thereby activating the mTOC1 complex[38]. The mTORC1 is composed of mammalian target of rapamycin (mTOR), regulatory associated protein of mTOR (Raptor), mammalian LST8/G-protein β-subunit like protein (mLST8/GβL), PRAS40 and Deptor[73]. The activation of mTORC1 can increase protein synthesis, cell growth and cell metabolism via its downstream targets[76-78]. The mTORC1 increases protein translation by activating the 70 kDa ribosomal S6 kinase (S6K), and inhibiting the elongation-initiation factor 4E binding protein[79,80]. A recent study using phosphopromeotic technique and new inhibitor Torin1 revealed many more proteins regulated by mTORC1 including protein Grb 10 which feedback inhibits PI3K[76]. Further study may elucidate the roles of these proteins in mTORC1 mediated carcinogenesis.
The activation of Akt can decrease apoptosis by decreasing Fas ligand transcription via blocking the forkhead protein and thus affecting FasL-mediated apoptosis[58]. Akt decreases the pro-apoptotic proteins BAD and BAX and increases anti-apoptotic Bcl-xl, Bcl-2 and Mcl1 to promote cell survival[81]. Akt also inhibits the p53 tumor-suppressor, which can cause apoptosis under stimulation of DNA damage or environmental factors[82,83].
Akt can also regulate cytoskeleton, which is important for cell mobility and the metastasis of cancers[84-86]. The p70 S6K, a downstream target of mTORC1, has been demonstrated to promote actin cytoskeleton change to increase cancer cell migration[87]. In addition, PI3K can cause the change of cytoskeleton independent of Akt. It can activate Rac1, which also causes reorganization of actin cytoskeleton[88-90].
Examination of activated PI3K in EBV-associated cancers provides evidence for the critical role of the PI3K/Akt pathway in the carcinogenesis of EBV. Adams et al[91] (2009) examined eight cases of post-transplant Hodgkin lymphoma and found that all of them expressed PI3K. Analyses of NPC biopsy samples using microarray and affymetrix assays showed PI3K mediated LMP2A-induced expression of the carcinogenic UDP-glucose dehydrogenase (UGDH) gene[92,93]. The overexpression of LMP2A in HEK293 cells increased the expression of UGDH which was abolished by the inhibition of the PI3K/Akt pathway[92]. Proteomic analyses of the EBV-infected gastric carcinoma cell line NU-GC-3 [EBV (+)] showed that EBV infection upregulated the phosphorylated Akt[94]. The fact that the increased phosphorylated HSP27 was reduced by treatment with the PI3K inhibitors Ly294002 and wortmannin suggests that EBV infection can upregulate the phosphorylation of HSP27 via the PI3K/Akt pathway. In PTLDs, protein microarrays of samples from patients showed that PI3K, mTOR and NF-κB were also dysregulated[95].
The activated PI3K/Akt pathway in EBV-associated cancers have been demonstrated to be mediated by LMP1 and LMP2A. A study showed that LMP1 expression in EBV-infected B-cells induced the production of cellular IL-10, an autocrine growth factor for B cell lymphomas, in a PI3K-dependent manner[96]. In these cell lines, PI3K/Akt pathway is activated and the LMP1-mediated IL-10 production is suppressed by mTORC1 inhibitor rapamycin. It has also been demonstrated that expression of dominant negative forms of LMP1 in EBV-immortalized monocytic and lymphocytic cell lines resulted in decreased Akt and NF-κB activities with increased apoptosis[97]. At present, six identified sequence variants of LMP1 including Alaskan, China 1, China 2, Med+, Med-, and NC have been shown to induce the PI3K/Akt signaling pathway to similar extents after being transformed into Rat-1 fibroblasts, HFK cells and BJAB cells[98]. EBV LMP2A has also been shown to activate PI3K in epithelial cells and to affect differentiation[26]. In epithelial cells, the overexpression of LMP2A of Rhesus lymphocryptovirus (LCV), which is highly homologous to EBV LMP2A activated the PI3K/Akt pathway, indicated by Akt activation and GSK3β inactivation[26]. LMP2A was shown to act as a B-cell receptor (BCR) signal, which results in B cells exiting the bone marrow and decreases B cell apoptosis in the periphery via the activation of PI3K[99].
There are many studies demonstrating that EBV can affect the PI3K/Akt pathway to cause cancers. EBV activation of the PI3K/Akt pathway can increase carcinogenesis via multiple downstream targets, including increased genomic instability, cell proliferation, decreased apoptosis and increased cytoskeleton dynamics.
Genomic stability is important to avoid carcinogenesis and is maintained by the DNA repair system[16,59,60,100-102]. It has been demonstrated that genomic instability plays an important role in EBV-induced cancers[103-107]. In human epithelial cells, LMP1 represses DNA repair via the CTAR1-mediated activation of PI3K/Akt pathway[33]. The activated PI3K/Akt pathway resulted in inactivation of FOXO3a, which plays an important role in DNA repair via DNA damage-binding protein 1[33]. The critical role of FOXO3a was further demonstrated by the fact that constitutive expression of an active FOXO3a abolished LMP1-mediated repression of DNA repair[33]. Furthermore, a recent study has shown that phosphorylated Akt can block Chk1 to affect genomic instability[62]. This effect may be involved in LMP-1-induced genomic instability and warrants further study.
In EBV-immortalized B-cells, also known as lymphoblastoid cell lines, the activation of the PI3K/Akt pathway can promote E2F transcriptional activity to affect the cell cycle and increase proliferation[108]. Inhibition of the PI3K by Ly294002 in these cells reduced both cyclin D2 and cyclin D3, which are two key regulators of cell cycle and increased p27, a cyclin-dependent kinase inhibitor[108]. CTAR1 of LMP1 has been identified to mediate the activation of PI3K signaling and associated induction of cell cycle markers in G1/S transition[30]. This PI3K activating effect was mapped to the TRAF-binding domain within CTAR1. In Rat-1 fibroblast cells, PI3K/Akt has been demonstrated to be a key factor in LMP1 mediated rodent fibroblast transformation[35]. Inhibition of the pathway abolished LMP1-induced cell growth. CTAR1 but not CTAR2 is critical for the activation of the PI3K/Akt pathway and associated cell growth. In human fibroblasts, LMP1 also caused phosphoryaltion of Akt and decreased levels of p27 and thus increased cell proliferation[35]. A study showed that, in an EBV-positive NPC cell line, LMP1 enhanced cell growth and migration through the activation of PI3K/Akt and NF-κB signaling which was reduced by the inhibition of PI3K, Akt, and NF-κB[109]. However, it has been shown that constitutive activation of Akt alone is not sufficient to promote cell growth; NF-κB activation is also required by LMP1 for its effect. Activation of PI3K/Akt and NF-κB has also been demonstrated to increase glucose import which is necessary for increased cell proliferation[110].
Several studies have shown that LMP2A can decrease apoptosis via the activation of the PI3K/Akt pathway. In LMP2A transgenic mice, peripheral BCR-negative B-cells have CA Ras, an upstream protein of PI3K with correlated increased expression of Bcl-xL, a dowm-stream target protein of PI3K[111]. The specific inhibitors of PI3K and Akt can cause apoptosis of these cells, suggesting the important role of the PI3K/Akt in LMP2A mediated B-cell survival. In an EBV-associated gastric cancer cell line, LMP2A activated PI3K/Akt pathway has been associated with the resistance to apoptosis induced by chemotherapy[112]. In PTLD-derived EBV+ B cell lines, LMP2A increased caspase inhibitor XIAP to block apoptosis via the activation of PI3K/Akt pathway[113]. In NPC cell lines, expression of LMP1 activated the PI3K/Akt pathway and its downstream Bcl-2, which in turn suppressed the pro-apoptotic activity of prostate apoptosis response-4[114]. These studies provide sufficient evidence that PI3K/Akt is a key pathway in LMP1 and LMP2A-mediated decreased apoptosis.
The cytoskeleton plays an important role in carcinogenesis through the control of cell mobility[84-86], and several cancer therapies have been developed targeting the proteins regulating the cytoskeleton[115,116]. The PI3K/Akt pathway has been shown to play a key role in LMP1-induced actin stress-fiber formation[36]. This pathway may be also important in microtubule activity. A study has shown that EBV LMP1 can activate cdc2, which, in turn, phosphorylates Op18/stathmin, a regulator of microtubules[117]. It is possible that this process is mediated by the PI3K/Akt pathway, as Akt has been shown to increase cdc2 activity[118].
The PI3K/Akt pathway is not only important in carcinogenesis and maintenance of cancer but is important in metastasis and drug resistance to chemotherapy[119-121]. For example, insulin can increase drug resistance via this pathway[47,122-124]. Many studies have been performed to test PI3K/Akt inhibitors and their utilization in combination with chemotherapeutic agents[125-131]. In EBV-associated cancer, the PI3K/Akt pathway is increased, as described above. Thus, the inhibition of the pathway may be effective for the treatment of these cancers. Indeed, some preliminary studies have shown that inhibiting the pathway increased the effect of chemotherapy on EBV-associated cancers.
In an EBV-positive gastric cancer cell line, SNU-719, Ly294002 was tested in combination with 5-fluorouracil (5-FU), a common chemotherapeutic agent[112,132]. In these cells, the use of 5-FU alone increased phosphorylation levels of Akt and NF-κB. The increased activity of the PI3K/Akt is known to cause drug resistance to chemotherapy[120,121]. By contract, the sequential treatment of 5-FU and Ly294002 decreased their levels, as well as bcl-2 expression, and increased the sensitivity of these cancer cells to 5-FU. The therapeutic efficacy of the mTOR inhibitor rapamycin has been demonstrated; it decreased tumor growth and metastasis in a mouse model of EBV-associated Burkitt’s lymphoma established by over-expression of both LMP2A and myc[74]. Ly294002 and Akt inhibitor II also induced the apoptosis of EBV-associated NK/T-cell lymphoma cell lines Hank-1 and NK-YS, which have high levels of activated PI3K[133]. NPC is usually treated by radiotherapy, and studies have shown that inhibition of the PI3K/Akt/mTOR pathway can increase the sensitivity of cancer cells to radiotherapy[131]. Thus, it may be useful to apply PI3K inhibitors in the treatment of EBV-associated NPC.
At present, dual inhibitors of PI3K and mTOR including BEZ235, PI-103, SF1126 and XL756 have been developed and some of them are in clinical trials to treat cancers with activated PI3K[38,134,135]. These inhibitors may be ideal compounds to be added into treatment regimens for EBV-associated cancers. Compounds from traditional medicine have been studied to inhibit signaling pathways; specifically, curcumin and flavonoids can inhibit either the PI3K/Akt pathway or its downstream targets cyclooxygenase-2 and NF-κB[136-142]. These compounds could also be tested for their effects on EBV-associated cancers.
The PI3K/Akt pathway can be activated by the EBV virus proteins LMP1 and LMP-2A and plays an important role in the carcinogenesis of EBV-associated cancers. This pathway is also known to be involved in drug resistance to chemotherapy. Thus, the inhibition of the pathway may have therapeutic implications for EBV-associated cancers. Indeed, some inhibitors of the PI3K/Akt pathway have been tested in EBV-associated cancer cell lines. At present, dual inhibitors of PI3K and mTOR have been developed and may be useful in the treatment of EBV-associated cancers.
Peer reviewer: Jaime Gómez-Laguna, DVM, PhD, Researcher, Department of R and D, Centre of Research and Agrifood Quality - CICAP, Ctra de la Canaleja s/n Apdo Correos 105, Pozoblanco, Córdoba, 14400, Spain
S- Editor Wang JL L- Editor A E- Editor Zheng XM
| 1. | Moore PS, Chang Y. Why do viruses cause cancer Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 443] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 2. | Martin D, Gutkind JS. Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. Oncogene. 2008;27 Suppl 2:S31-S42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. 1911;13:397-411. [PubMed] [Cited in This Article: ] |
| 4. | Becsei-Kilborn E. Scientific discovery and scientific reputation: the reception of Peyton Rous' discovery of the chicken sarcoma virus. J Hist Biol. 2010;43:111-157. [PubMed] [Cited in This Article: ] |
| 5. | Parker RC, Varmus HE, Bishop JM. Cellular homologue (c-src) of the transforming gene of Rous sarcoma virus: isolation, mapping, and transcriptional analysis of c-src and flanking regions. Proc Natl Acad Sci USA. 1981;78:5842-5846. [PubMed] [Cited in This Article: ] |
| 6. | Chen J. Is Src the key to understanding metastasis and developing new treatments for colon cancer. Nat Clin Pract Gastroenterol Hepatol. 2008;5:306-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2728] [Cited by in F6Publishing: 2648] [Article Influence: 120.4] [Reference Citation Analysis (0)] |
| 8. | Schiller JT, Castellsagué X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26 Suppl 10:K53-K61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Zhao KN, Chen J. Codon usage roles in human papillomavirus. Rev Med Virol. 2011;21:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Morissette G, Flamand L. Herpesviruses and chromosomal integration. J Virol. 2010;84:12100-12109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Odumade OA, Hogquist KA, Balfour HH. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev. 2011;24:193-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Vereide D, Sugden B. Insights into the evolution of lymphomas induced by Epstein-Barr virus. Adv Cancer Res. 2010;108:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 14. | Petrova M, Kamburov V. Epstein-Barr virus: silent companion or causative agent of chronic liver disease. World J Gastroenterol. 2010;16:4130-4134. [PubMed] [Cited in This Article: ] |
| 15. | Chen J, McMillan NA. Molecular basis of pathogenesis, prognosis and therapy in chronic lymphocytic leukaemia. Cancer Biol Ther. 2008;7:174-179. [PubMed] [Cited in This Article: ] |
| 16. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39812] [Cited by in F6Publishing: 43011] [Article Influence: 3308.5] [Reference Citation Analysis (4)] |
| 17. | Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 18. | Vereide D, Sugden B. Proof for EBV's sustaining role in Burkitt's lymphomas. Semin Cancer Biol. 2009;19:389-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Klein G, Klein E, Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun. 2010;396:67-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Zheng ZM. Viral oncogenes, noncoding RNAs, and RNA splicing in human tumor viruses. Int J Biol Sci. 2010;6:730-755. [PubMed] [Cited in This Article: ] |
| 21. | Brooks L, Yao QY, Rickinson AB, Young LS. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689-2697. [PubMed] [Cited in This Article: ] |
| 22. | Horikawa T, Yoshizaki T, Kondo S, Furukawa M, Kaizaki Y, Pagano JS. Epstein-Barr Virus latent membrane protein 1 induces Snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer. 2011;104:1160-1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Ryan JL, Jones RJ, Kenney SC, Rivenbark AG, Tang W, Knight ER, Coleman WB, Gulley ML. Epstein-Barr virus-specific methylation of human genes in gastric cancer cells. Infect Agent Cancer. 2010;5:27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Fukayama M. Epstein-Barr virus and gastric carcinoma. Pathol Int. 2010;60:337-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996;27:20-27. [PubMed] [Cited in This Article: ] |
| 26. | Siler CA, Raab-Traub N. Rhesus lymphocryptovirus latent membrane protein 2A activates beta-catenin signaling and inhibits differentiation in epithelial cells. Virology. 2008;377:273-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Thornburg NJ, Kulwichit W, Edwards RH, Shair KH, Bendt KM, Raab-Traub N. LMP1 signaling and activation of NF-kappaB in LMP1 transgenic mice. Oncogene. 2006;25:288-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther. 2009;8:1313-1317. [PubMed] [Cited in This Article: ] |
| 29. | Diduk SV, Smirnova KV, Pavlish OA, Gurtsevitch VE. Functionally significant mutations in the Epstein-Barr virus LMP1 gene and their role in activation of cell signaling pathways. Biochemistry (Mosc). 2008;73:1134-1139. [PubMed] [Cited in This Article: ] |
| 30. | Mainou BA, Everly DN, Raab-Traub N. Unique signaling properties of CTAR1 in LMP1-mediated transformation. J Virol. 2007;81:9680-9692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Everly DN, Kusano S, Raab-Traub N. Accumulation of cytoplasmic beta-catenin and nuclear glycogen synthase kinase 3beta in Epstein-Barr virus-infected cells. J Virol. 2004;78:11648-11655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Li SS, Yang S, Wang S, Yang XM, Tang QL, Wang SH. Latent membrane protein 1 mediates the resistance of nasopharyngeal carcinoma cells to TRAIL-induced apoptosis by activation of the PI3K/Akt signaling pathway. Oncol Rep. 2011;26:1573-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Chen YR, Liu MT, Chang YT, Wu CC, Hu CY, Chen JY. Epstein-Barr virus latent membrane protein 1 represses DNA repair through the PI3K/Akt/FOXO3a pathway in human epithelial cells. J Virol. 2008;82:8124-8137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Noda C, Murata T, Kanda T, Yoshiyama H, Sugimoto A, Kawashima D, Saito S, Isomura H, Tsurumi T. Identification and characterization of CCAAT enhancer-binding protein (C/EBP) as a transcriptional activator for Epstein-Barr virus oncogene latent membrane protein 1. J Biol Chem. 2011;286:42524-42533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Mainou BA, Everly DN, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 CTAR1 mediates rodent and human fibroblast transformation through activation of PI3K. Oncogene. 2005;24:6917-6924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Dawson CW, Tramountanis G, Eliopoulos AG, Young LS. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem. 2003;278:3694-3704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486-5496. [PubMed] [Cited in This Article: ] |
| 38. | Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075-1083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 968] [Cited by in F6Publishing: 950] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 39. | Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1785] [Cited by in F6Publishing: 1832] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 40. | Fulda S. The PI3K/Akt/mTOR pathway as therapeutic target in neuroblastoma. Curr Cancer Drug Targets. 2009;9:729-737. [PubMed] [Cited in This Article: ] |
| 41. | Vogt PK, Hart JR, Gymnopoulos M, Jiang H, Kang S, Bader AG, Zhao L, Denley A. Phosphatidylinositol 3-kinase: the oncoprotein. Curr Top Microbiol Immunol. 2010;347:79-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Chen JZ. Targeted therapy of obesity-associated colon cancer. Transl Gastrointest Cancer. 2012;1:44-57. [DOI] [Cited in This Article: ] |
| 44. | Chen JZ, Wang MB. The roles of miRNA-143 in colon cancer and therapeutic implications. Transl Gastrointest Cancer. 2012;1:169-174. [DOI] [Cited in This Article: ] |
| 45. | Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2247] [Cited by in F6Publishing: 2407] [Article Influence: 133.7] [Reference Citation Analysis (0)] |
| 46. | Lee JY, Engelman JA, Cantley LC. Biochemistry. PI3K charges ahead. Science. 2007;317:206-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Chen J, Huang XF, Qiao L, Katsifis A. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol. 2011;2:27-33. [PubMed] [Cited in This Article: ] |
| 48. | Gislette T, Chen J. The possible role of IL-17 in obesity-associated cancer. ScientificWorldJournal. 2010;10:2265-2271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346-1353. [PubMed] [Cited in This Article: ] |
| 50. | Renehan AG, Egger M, Minder C, O'Dwyer ST, Shalet SM, Zwahlen M. IGF-I, IGF binding protein-3 and breast cancer risk: comparison of 3 meta-analyses. Int J Cancer. 2005;115:1006-107; author reply 1008. [PubMed] [Cited in This Article: ] |
| 51. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [PubMed] [Cited in This Article: ] |
| 52. | Johnson C, Han Y, Hughart N, McCarra J, Alpini G, Meng F. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer. 2012;1:58-70. [PubMed] [Cited in This Article: ] |
| 53. | Samuels Y, Waldman T. Oncogenic mutations of PIK3CA in human cancers. Curr Top Microbiol Immunol. 2010;347:21-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Whitman M, Kaplan DR, Schaffhausen B, Cantley L, Roberts TM. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985;315:239-242. [PubMed] [Cited in This Article: ] |
| 55. | Ling LE, Druker BJ, Cantley LC, Roberts TM. Transformation-defective mutants of polyomavirus middle T antigen associate with phosphatidylinositol 3-kinase (PI 3-kinase) but are unable to maintain wild-type levels of PI 3-kinase products in intact cells. J Virol. 1992;66:1702-1708. [PubMed] [Cited in This Article: ] |
| 56. | Fukuda RI, Tsuchiya K, Suzuki K, Itoh K, Fujita J, Utsunomiya A, Tsuji T. Human T-cell leukemia virus type I tax down-regulates the expression of phosphatidylinositol 3,4,5-trisphosphate inositol phosphatases via the NF-kappaB pathway. J Biol Chem. 2009;284:2680-2689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Chen JZ, McMillan N, Gu WY. Intra-tumor injection of lentiviral-vector delivered shRNA targeting human papillomavirus E6 and E7 oncogenes reduces tumor growth in a xenograft cervical cancer model in mice. J Solid Tumors. 2012;2:4-10. [Cited in This Article: ] |
| 58. | Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10:610-616. [PubMed] [Cited in This Article: ] |
| 59. | Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20:R285-R295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 403] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 60. | Murnane JP. Telomere loss as a mechanism for chromosome instability in human cancer. Cancer Res. 2010;70:4255-4259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Liu W, Zhou Y, Reske SN, Shen C. PTEN mutation: many birds with one stone in tumorigenesis. Anticancer Res. 2008;28:3613-3619. [PubMed] [Cited in This Article: ] |
| 62. | Nam HJ, Chae S, Jang SH, Cho H, Lee JH. The PI3K-Akt mediates oncogenic Met-induced centrosome amplification and chromosome instability. Carcinogenesis. 2010;31:1531-1540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7:193-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 64. | Li L, Ross AH. Why is PTEN an important tumor suppressor. J Cell Biochem. 2007;102:1368-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421-429. [PubMed] [Cited in This Article: ] |
| 66. | Syljuåsen RG, Sørensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553-3562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 421] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 67. | Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501-2514. [PubMed] [Cited in This Article: ] |
| 68. | Miliani de Marval PL, Macias E, Rounbehler R, Sicinski P, Kiyokawa H, Johnson DG, Conti CJ, Rodriguez-Puebla ML. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol Cell Biol. 2004;24:7538-7547. [PubMed] [Cited in This Article: ] |
| 69. | Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339-345. [PubMed] [Cited in This Article: ] |
| 70. | Martínez-Gac L, Alvarez B, García Z, Marqués M, Arrizabalaga M, Carrera AC. Phosphoinositide 3-kinase and Forkhead, a switch for cell division. Biochem Soc Trans. 2004;32:360-361. [PubMed] [Cited in This Article: ] |
| 71. | Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996;85:721-732. [PubMed] [Cited in This Article: ] |
| 72. | Hsieh FF, Barnett LA, Green WF, Freedman K, Matushansky I, Skoultchi AI, Kelley LL. Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase. Blood. 2000;96:2746-2754. [PubMed] [Cited in This Article: ] |
| 73. | Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471-484. [PubMed] [Cited in This Article: ] |
| 74. | Cen O, Longnecker R. Rapamycin reverses splenomegaly and inhibits tumor development in a transgenic model of Epstein-Barr virus-related Burkitt's lymphoma. Mol Cancer Ther. 2011;10:679-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Chen J, Hu CF, Hou JH, Shao Q, Yan LX, Zhu XF, Zeng YX, Shao JY. Epstein-Barr virus encoded latent membrane protein 1 regulates mTOR signaling pathway genes which predict poor prognosis of nasopharyngeal carcinoma. J Transl Med. 2010;8:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 816] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 77. | Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villén J, Kubica N, Hoffman GR, Cantley LC, Gygi SP. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 700] [Cited by in F6Publishing: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 78. | Yea SS, Fruman DA. Cell signaling. New mTOR targets Grb attention. Science. 2011;332:1270-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423-6435. [PubMed] [Cited in This Article: ] |
| 80. | Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472-1487. [PubMed] [Cited in This Article: ] |
| 81. | Pastorino JG, Tafani M, Farber JL. Tumor necrosis factor induces phosphorylation and translocation of BAD through a phosphatidylinositide-3-OH kinase-dependent pathway. J Biol Chem. 1999;274:19411-19416. [PubMed] [Cited in This Article: ] |
| 82. | Trotman LC, Pandolfi PP. PTEN and p53: who will get the upper hand. Cancer Cell. 2003;3:97-99. [PubMed] [Cited in This Article: ] |
| 83. | Chen J, Huang XF. Activation of p53 for the treatment of cancer. J Cell Biochem. 2009;107:567-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 84. | Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037-3049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 85. | Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 86. | Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1356] [Cited by in F6Publishing: 1376] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 87. | Ip CK, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene. 2011;30:2420-2432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol. 2010;188:863-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 89. | Huang Q, Shen HM, Ong CN. Emodin inhibits tumor cell migration through suppression of the phosphatidylinositol 3-kinase-Cdc42/Rac1 pathway. Cell Mol Life Sci. 2005;62:1167-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445-1455. [PubMed] [Cited in This Article: ] |
| 91. | Adams H, Campidelli C, Dirnhofer S, Pileri SA, Tzankov A. Clinical, phenotypic and genetic similarities and disparities between post-transplant and classical Hodgkin lymphomas with respect to therapeutic targets. Expert Opin Ther Targets. 2009;13:1137-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Pan YR, Vatsyayan J, Chang YS, Chang HY. Epstein-Barr virus latent membrane protein 2A upregulates UDP-glucose dehydrogenase gene expression via ERK and PI3K/Akt pathway. Cell Microbiol. 2008;10:2447-2460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Pegtel DM, Subramanian A, Sheen TS, Tsai CH, Golub TR, Thorley-Lawson DA. Epstein-Barr-virus-encoded LMP2A induces primary epithelial cell migration and invasion: possible role in nasopharyngeal carcinoma metastasis. J Virol. 2005;79:15430-15442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Fukagawa Y, Nishikawa J, Kuramitsu Y, Iwakiri D, Takada K, Imai S, Satake M, Okamoto T, Fujimoto M, Okita K. Epstein-Barr virus upregulates phosphorylated heat shock protein 27 kDa in carcinoma cells using the phosphoinositide 3-kinase/Akt pathway. Electrophoresis. 2008;29:3192-3200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Alsayed Y, Leleu X, Leontovich A, Oton AB, Melhem M, George D, Ghobrial IM. Proteomics analysis in post-transplant lymphoproliferative disorders. Eur J Haematol. 2008;81:298-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Lambert SL, Martinez OM. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol. 2007;179:8225-8234. [PubMed] [Cited in This Article: ] |
| 97. | Ndour PA, Brocqueville G, Ouk TS, Goormachtigh G, Morales O, Mougel A, Bertout J, Melnyk O, Fafeur V, Feuillard J. Inhibition of latent membrane protein 1 impairs the growth and tumorigenesis of latency II Epstein-Barr virus-transformed T cells. J Virol. 2012;86:3934-3943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Mainou BA, Raab-Traub N. LMP1 strain variants: biological and molecular properties. J Virol. 2006;80:6458-6468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Anderson LJ, Longnecker R. EBV LMP2A provides a surrogate pre-B cell receptor signal through constitutive activation of the ERK/MAPK pathway. J Gen Virol. 2008;89:1563-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 100. | Burrell RA, Juul N, Johnston SR, Reis-Filho JS, Szallasi Z, Swanton C. Targeting chromosomal instability and tumour heterogeneity in HER2-positive breast cancer. J Cell Biochem. 2010;111:782-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 101. | Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1120] [Cited by in F6Publishing: 1127] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 102. | Vineis P, Schatzkin A, Potter JD. Models of carcinogenesis: an overview. Carcinogenesis. 2010;31:1703-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 103. | Ferrasi AC, Pinheiro NA, Rabenhorst SH, Caballero OL, Rodrigues MA, de Carvalho F, Leite CV, Ferreira MV, Barros MA, Pardini MI. Helicobacter pylori and EBV in gastric carcinomas: methylation status and microsatellite instability. World J Gastroenterol. 2010;16:312-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Gruhne B, Kamranvar SA, Masucci MG, Sompallae R. EBV and genomic instability--a new look at the role of the virus in the pathogenesis of Burkitt's lymphoma. Semin Cancer Biol. 2009;19:394-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Gruhne B, Sompallae R, Masucci MG. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene. 2009;28:3997-4008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Kamranvar SA, Gruhne B, Szeles A, Masucci MG. Epstein-Barr virus promotes genomic instability in Burkitt's lymphoma. Oncogene. 2007;26:5115-5123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Wu CC, Liu MT, Chang YT, Fang CY, Chou SP, Liao HW, Kuo KL, Hsu SL, Chen YR, Wang PW. Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res. 2010;38:1932-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Brennan P, Mehl AM, Jones M, Rowe M. Phosphatidylinositol 3-kinase is essential for the proliferation of lymphoblastoid cells. Oncogene. 2002;21:1263-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Shair KH, Schnegg CI, Raab-Traub N. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer Res. 2008;68:6997-7005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Sommermann TG, O'Neill K, Plas DR, Cahir-McFarland E. IKKβ and NF-κB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;71:7291-7300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 111. | Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene. 2004;23:8619-8628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 112. | Shin JY, Kim JO, Lee SK, Chae HS, Kang JH. LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein-Barr virus-positive gastric cancer cells. BMC Cancer. 2010;10:425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Hatton O, Phillips LK, Vaysberg M, Hurwich J, Krams SM, Martinez OM. Syk activation of phosphatidylinositol 3-kinase/Akt prevents HtrA2-dependent loss of X-linked inhibitor of apoptosis protein (XIAP) to promote survival of Epstein-Barr virus+ (EBV+) B cell lymphomas. J Biol Chem. 2011;286:37368-37378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Lee JW, Liu PF, Hsu LP, Chen PR, Chang CH, Shih WL. EBV LMP-1 negatively regulates expression and pro-apoptotic activity of Par-4 in nasopharyngeal carcinoma cells. Cancer Lett. 2009;279:193-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1271] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 116. | Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 762] [Cited by in F6Publishing: 816] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 117. | Lin X, Liu S, Luo X, Ma X, Guo L, Li L, Li Z, Tao Y, Cao Y. EBV-encoded LMP1 regulates Op18/stathmin signaling pathway by cdc2 mediation in nasopharyngeal carcinoma cells. Int J Cancer. 2009;124:1020-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | Katayama K, Fujita N, Tsuruo T. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Mol Cell Biol. 2005;25:5725-5737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 119. | Falasca M. PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des. 2010;16:1410-1416. [PubMed] [Cited in This Article: ] |
| 120. | Chen J. The Src/PI3K/Akt signal pathway may play a key role in decreased drug efficacy in obesity-associated cancer. J Cell Biochem. 2010;110:279-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 121. | Chen J, Huang XF, Katsifis A. Activation of signal pathways and the resistance to anti-EGFR treatment in colorectal cancer. J Cell Biochem. 2010;111:1082-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 122. | Chen J, Katsifis A, Hu C, Huang XF. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol. 2011;8:119-125. [PubMed] [Cited in This Article: ] |
| 123. | Renehan AG, Dive C. Obesity, insulin and chemoresistance in colon cancer. J Gastrointest Oncol. 2011;2:8-10. [PubMed] [Cited in This Article: ] |
| 124. | Landriscina M, Esposito F. Insulin-resistant conditions: A favorable milieu for aggressive drug-resistant malignancies. J Gastrointest Oncol. 2011;2:11-12. [PubMed] [Cited in This Article: ] |
| 125. | Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1833] [Cited by in F6Publishing: 2007] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 126. | Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237-249. [PubMed] [Cited in This Article: ] |
| 127. | Paz-Ares L, Blanco-Aparicio C, García-Carbonero R, Carnero A. Inhibiting PI3K as a therapeutic strategy against cancer. Clin Transl Oncol. 2009;11:572-579. [PubMed] [Cited in This Article: ] |
| 128. | Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799-4805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 129. | Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1166] [Cited by in F6Publishing: 1274] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 130. | Vanhaesebroeck B, Vogt PK, Rommel C. PI3K: from the bench to the clinic and back. Curr Top Microbiol Immunol. 2010;347:1-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 131. | Zaidi SH, Huddart RA, Harrington KJ. Novel targeted radiosensitisers in cancer treatment. Curr Drug Discov Technol. 2009;6:103-134. [PubMed] [Cited in This Article: ] |
| 132. | Katkoori VR, Suarez-Cuervo C, Shanmugam C, Jhala NC, Callens T, Messiaen L, Posey J, Bumpers HL, Meleth S, Grizzle WE. Bax expression is a candidate prognostic and predictive marker of colorectal cancer. J Gastrointest Oncol. 2010;1:76-89. [PubMed] [Cited in This Article: ] |
| 133. | Jeon YK, Park CH, Kim KY, Li YC, Kim J, Kim YA, Paik JH, Park BK, Kim CW, Kim YN. The heat-shock protein 90 inhibitor, geldanamycin, induces apoptotic cell death in Epstein-Barr virus-positive NK/T-cell lymphoma by Akt down-regulation. J Pathol. 2007;213:170-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 134. | Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L, Lollini PL, Maira SM, García-Echeverría C, Mercuri M. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res. 2010;16:530-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 135. | Bhende PM, Park SI, Lim MS, Dittmer DP, Damania B. The dual PI3K/mTOR inhibitor, NVP-BEZ235, is efficacious against follicular lymphoma. Leukemia. 2010;24:1781-1784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 136. | Bisht S, Maitra A. Systemic delivery of curcumin: 21st century solutions for an ancient conundrum. Curr Drug Discov Technol. 2009;6:192-199. [PubMed] [Cited in This Article: ] |
| 137. | Tian G, Guo L, Gao W. Use of compound Chinese medicine in the treatment of lung cancer. Curr Drug Discov Technol. 2010;7:32-36. [PubMed] [Cited in This Article: ] |
| 138. | Wang H, Cui Y, Zhao C. Flavonoids of the Genus Iris (Iridaceae). Curr Drug Discov Technol. 2010;6:Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 139. | Wang S, Penchala S, Prabhu S, Wang J, Huang Y. Molecular basis of traditional Chinese medicine in cancer chemoprevention. Curr Drug Discov Technol. 2010;7:67-75. [PubMed] [Cited in This Article: ] |
| 140. | Youns M, Hoheisel JD, Efferth T. Traditional Chinese medicines (TCMs) for molecular targeted therapies of tumours. Curr Drug Discov Technol. 2010;7:37-45. [PubMed] [Cited in This Article: ] |
| 141. | Choi BH, Kim CG, Lim Y, Shin SY, Lee YH. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008;259:111-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 142. | Chen JZ. Prevention of obesity-associated colon cancer by (-)-epigallocatechin-3 gallate and curcumin. Transl Gastrointest Cancer. 2012;1:243-249. [DOI] [Cited in This Article: ] |