Copyright
©The Author(s) 2015.
World J Virology. May 12, 2015; 4(2): 124-133
Published online May 12, 2015. doi: 10.5501/wjv.v4.i2.124
Published online May 12, 2015. doi: 10.5501/wjv.v4.i2.124
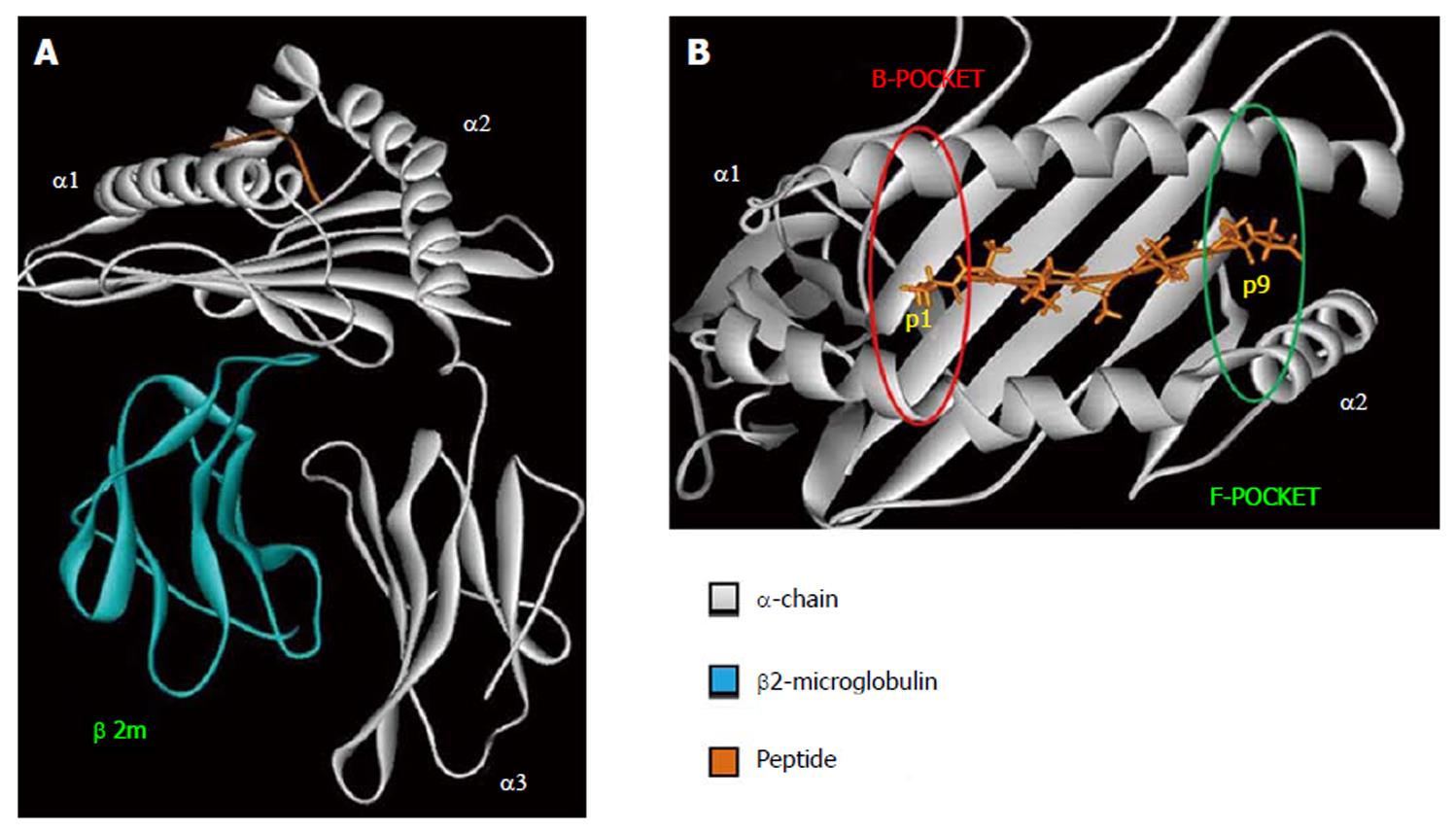
Figure 1 Human leukocyte antigen class I tridimensional structure.
Crystal structure of the HLA-B*57:03 (PDB ID: 2YPK); HLA α-chain in gray, β2-microglobulin in blue, the peptide in orange. A: HLA class I overall structure; B: HLA class I peptide binding pocket. In red is shown the HLA pocket B, in green HLA pocket F the most polymorphic regions in HLA peptide binding pocket. The figure has been made using WebLab Viewer Pro 3.7. HLA: Human leukocyte antigen.
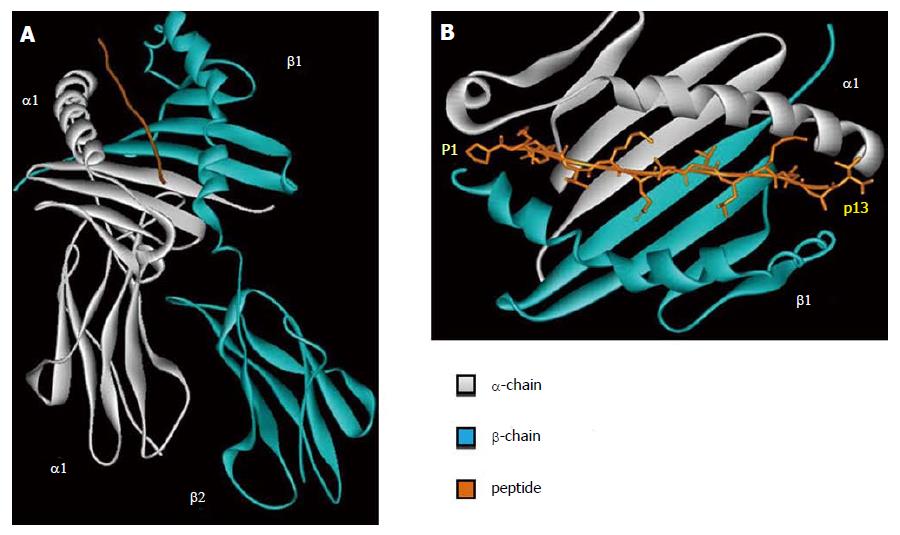
Figure 2 Human leukocyte antigen class II tridimensional structure.
Crystal structure of the HLA-DR1 (PDB ID: 1DLH) in gray HLA α-chain, in blue HLA β-chain, in orange the peptide. A: HLA class II overall structure; B HLA class II peptide binding pocket. The figure has been made using WebLab Viewer Pro 3.7. HLA: Human leukocyte antigen.
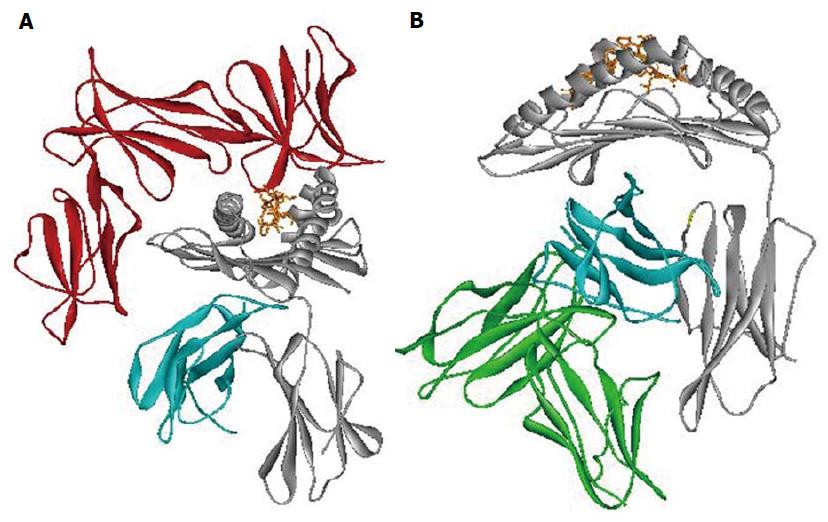
Figure 3 Human leukocyte antigen class I interaction with innate receptor.
Crystal structure models of the HLA-B*57:01 interacting respectively with (A): KIR3DL1 receptor (red) (PDB ID: 3HV8); (B): LILRB1 receptor (green). HLA α-chain in gray, β2-microglobulin in blue, the peptide in orange. The figure has been made using WebLab Viewer Pro 3.7. HLA: Human leukocyte antigen.
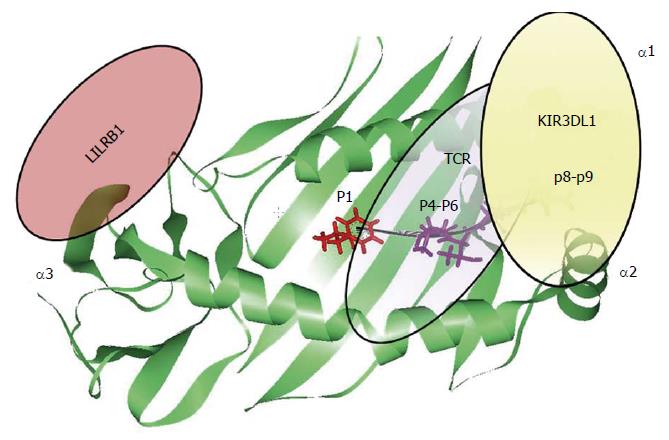
Figure 4 Peptide recognition.
Schematic representation of the contribution of each HLA bound peptide position in the modulation of the interaction between HLA-B molecules (green) with TCR (violet) and KIR3DL1 (yellow), respectively. The figure has been made using WebLab Viewer Pro 3.7. HLA: Human leukocyte antigen; TCR: T cell receptor.
- Citation: Grifoni A, Montesano C, Colizzi V, Amicosante M. Key role of human leukocyte antigen in modulating human immunodeficiency virus progression: An overview of the possible applications. World J Virology 2015; 4(2): 124-133
- URL: https://www.wjgnet.com/2220-3249/full/v4/i2/124.htm
- DOI: https://dx.doi.org/10.5501/wjv.v4.i2.124