Copyright
©2012 Baishideng.
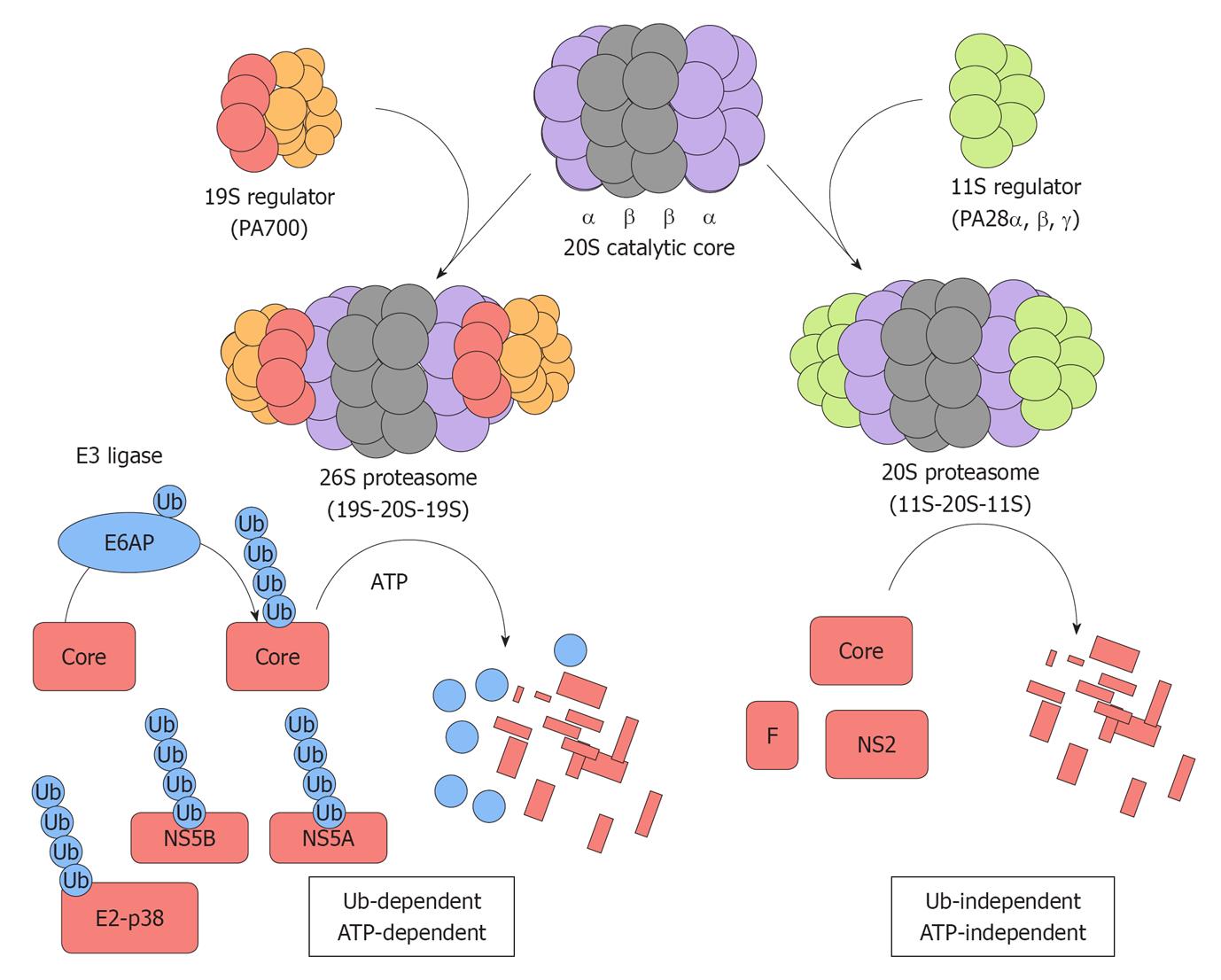
Figure 1 Two distinct proteasome pathways target hepatitis C virus proteins for degradation.
The 20S catalytic core is composed of α and β subunits that form a barrel-like structure. The 19S regulator (PA700) can associate with either or both ends of the 20S catalytic core. The combination of one 20S catalytic core and one or two 19S regulator generates the 26S proteasome that is responsible for ubiquitin-dependent ATP-dependent degradation of specific target substrates. E6AP mediates the polyubiquitylation of the hepatitis C virus (HCV) core protein and thereby targets it for ubiquitin-dependent degradation. E2-p38, NS5A, and NS5B are degraded through this ubiquitin-dependent and ATP-dependent proteasome pathway. The proteasome activator, PA28γ, forms a homoheptamer and is implicated in the ubiquitin-independent turnover of the HCV core protein. The F and NS2 proteins are also degraded through the ubiquitin-independent pathway.
- Citation: Shoji I. Roles of the two distinct proteasome pathways in hepatitis C virus infection. World J Virol 2012; 1(2): 44-50
- URL: https://www.wjgnet.com/2220-3249/full/v1/i2/44.htm
- DOI: https://dx.doi.org/10.5501/wjv.v1.i2.44