INTRODUCTION
Kidney transplantation remains the best renal replacement modality for most patients with end-stage kidney disease[1]. Yet, as with everything else in the medical field, it is not devoid of risk. The patients who manage to get a kidney transplant in a timely fashion face a constant struggle for successful long-lasting survival. The vast majority of graft failure is attributed to alloimmune-mediated injury, recurrent glomerulonephritis, infections, cardiovascular mortality and malignancy[2,3]. Nonetheless, a number of renal allografts are lost due to urological complications, especially in the early post-transplant period. The purpose of this review is to discuss different presentations and provide an evidence-based management plan for patients who present with such complications.
OUTLINE OF SURGICAL AND UROLOGICAL COMPLICATIONS
Complications in the immediate post-transplant period can be broadly subdivided into vascular, urological, fluid collections and wound healing problems. Vascular complications encompass hemorrhage, thrombosis, aneurysm, dissection and stenosis, while urological complications mainly involve leaks and/or obstruction of the collecting system[4,5]. In essence, hematomas form due to poor tissue handling, insecure knot tying and inadequate hemostasis. The lymphoceles result from severed lymph channels, which should be tied or clipped rather than diathermied, leading to extravasation of lymph. Urine leaks can result in the formation of urinomas. These collections can compress vascular structures or urine outflow, causing transplant dysfunction. In addition, urine leaks are associated with increased risk of surgical site infection, which can lead to peri-nephric abscesses[6,7]. Wound healing complications are generally more common when mammalian target of rapamycin (mTOR)-based immunosuppression is used[8].
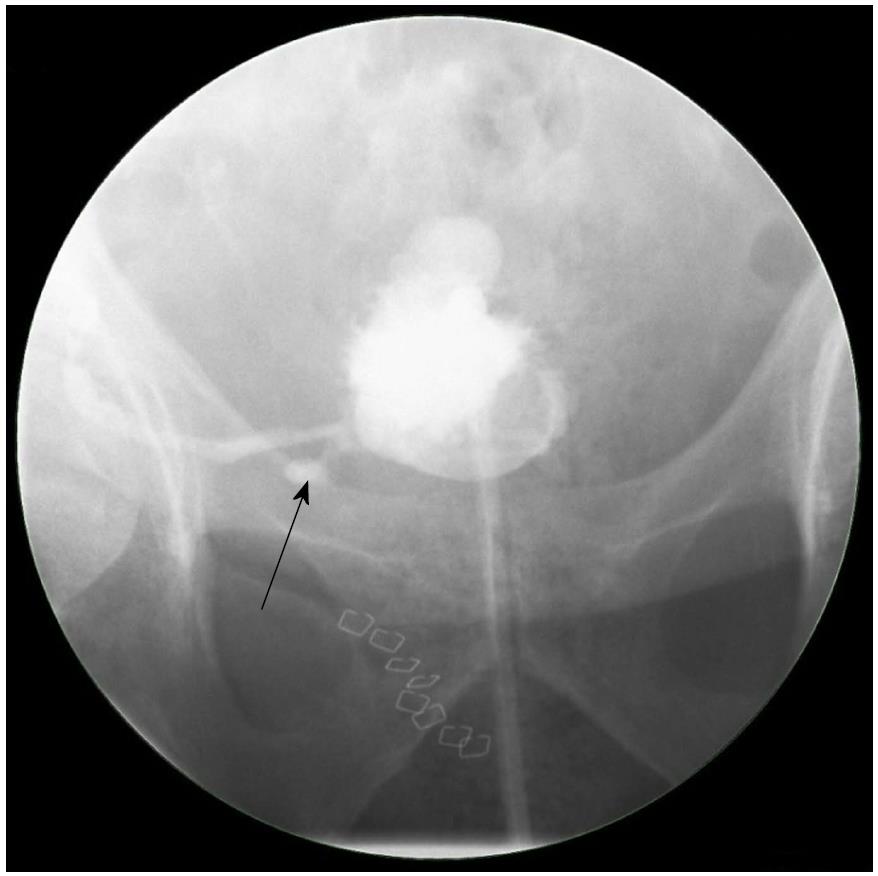
Ultrasonography is the first-line imaging modality for graft evaluation in the immediate post-transplant period, especially when suspecting vascular problems, fluid collections and/or obstruction[9,10]. Apart from being non-invasive, it can provide some additional information on the graft function by measuring the intra-renal resistivity indices[11]. Differentiating between different types of collections on ultrasound can be difficult. A urinoma usually appears as a well-defined, rapidly enlarging non-echoic fluid collection without septations, whereas a hematoma usually has a complex and echogenic appearance with numerous septations[9,12]. Computed tomography may assist in the diagnosis by further elucidating the ultrasound findings such as the extent or exact relationship of the fluid collection to the transplanted kidney[10]. 99mTC-MAG-3 radionuclide isotope scan is useful to confirm the presence of a urine leak outside the anatomical space of the urinary tract, as the radionuclide tracer accumulates in the excreted urine as opposed to other types of fluid collections[13]. A cystogram can provide additional information to establish the exact site of urine leak, especially if it is at the ureterovesical junction (Figure 1). Antegrade pyelography performed during nephrostomy tube insertion remains the investigation of choice to identify the exact site and extent of urine leak. Ultrasound and/or computed tomography-guided needle aspiration followed by biochemical and bacteriological analysis is essential in diagnosing the exact etiology of fluid collections[4]. A fluid creatinine well above the serum level indicates a urine leak as opposed to a lymphocele which has levels similar to that of serum. Gram stain and cultures are important because any fluid collection can potentially become infected[6].
Figure 1 A cystogram showing urinary leak (arrow) at the anastomosis between the newly implanted graft ureter and urinary bladder.
RISK FACTORS AND PRESENTATION OF URINE LEAKS
The incidence of urological complications following kidney transplantation as portrayed in early studies (i.e., including patients between 1970-1990s) ranged between 4.2% to 14.1%[14-18], while in later studies (i.e., including patients between 1990-2000), it ranged between 3.7% to 6.0%[19-21]. The incidence of urine leaks described in studies that included patients between the 1990s and 2000 ranged between 1.5% to 6.0%[19-23]. This variability is probably a reflection of the different transplantation era, diagnostic tools and surgical proficiency. Indeed, the incidence of urological complications has been shown to diminish considerably with increasing center experience[24]. These complications are associated with significant patient morbidity, including graft loss and mortality[17,25].
Urine leaks generally present in the immediate or early post-transplant period (3 mo)[26]. Clinical presentation can include pain and swelling in the transplant area, rising creatinine, oliguria and/or signs of systemic infection[27]. In the immediate post-transplant period, urine leaks can manifest via the drains or through the wound, leading to delayed healing and increased risk of infection[7,28]. In addition, leaking urine can translocate into the retroperitoneal space, pelvis and occasionally in the pre-sacral and scrotal area[29]. The leaking of infected urine could lead to peri-nephric infections and abscess formation. This is important considering that urinary tract infections occur in about 23% of patients receiving a kidney transplant[30].
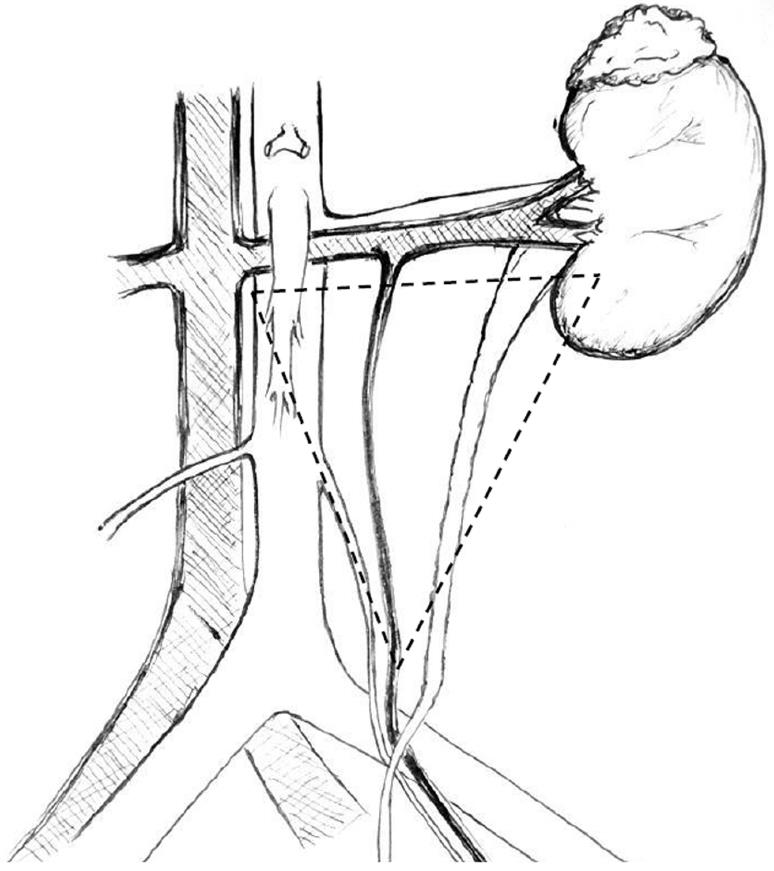
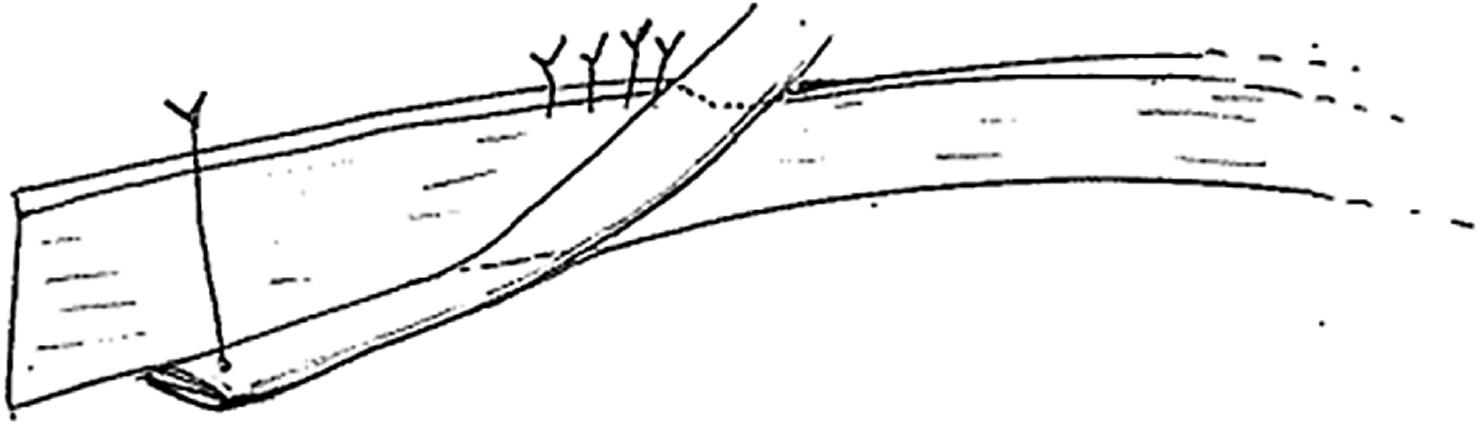
Most urological complications can be traced back to technical errors during retrieval, bench dissection or implantation[28]. The vast majority of leaks occur at the distal portion of the ureter, most commonly at the site of the ureteroneocystostomy[26]. Distal ureteral ischemia and necrosis secondary to compromised blood supply is thought to be the main culprit for early ureteral complications in most patients in the absence of technical difficulties during the transplant operation[31]. In contrast to the native ureters, which derive their blood supply via both renal arteries and pelvic collaterals, the transplanted ureter depends solely on the blood supplied by the branches of the renal artery that traverse in peri-ureteric tissues. This area, also known as the “golden triangle” (Figure 2), contains important arterial branches, such as the lower polar artery, which supplies the distal ureter. Indeed, the importance of preserving the peri-ureteral connective tissue in order to prevent disastrous urinary complications is well documented in the literature[14,32-35]. Male donors, male recipients, African American recipients, Taguchi technique, graft arterial reconstruction, multiple renal arteries and recipient diabetes were established as independent risk factors for urinary complications[36-39]. We believe that gentle handling of the ureter and peri-ureteric tissue, and keeping the length of the ureter as short as possible without tension is of key importance. A ureter that appears ischemic after reperfusion should be resected proximally until an adequately perfused area is reached. In this situation, achieving a tension-free urinary anastomosis may require special techniques, such as ipsilateral uretero-ureterostomy (joining the transplant ureter to the native ureter of that side), pyelovesicostomy, psoas hitch, Boari flap or fashioning of an ileal ureter, in that order of priority. In general, the risk of urinary complications following laparoscopic donor nephrectomy has decreased substantially over time, now comparable to open nephrectomy[40].
Figure 2 The golden triangle.
Bordered by the lower pole of the kidney on the left, the junction between the renal vein and the inferior vena cava on the right and gonadal vein.
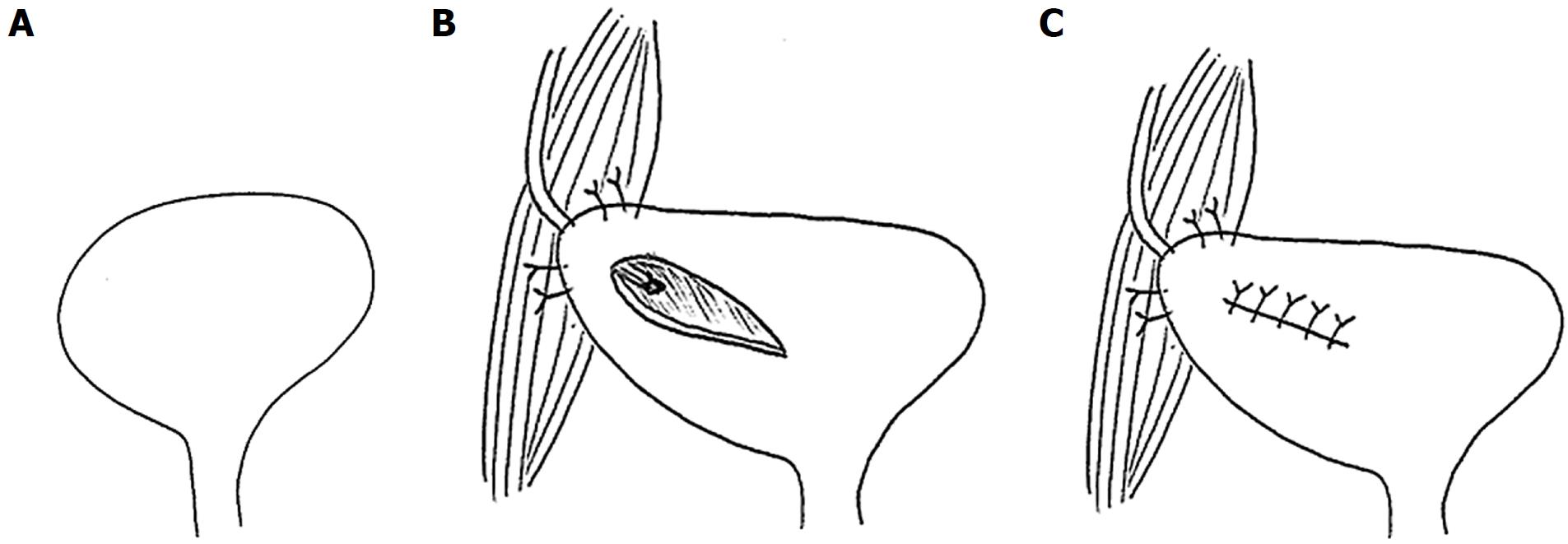
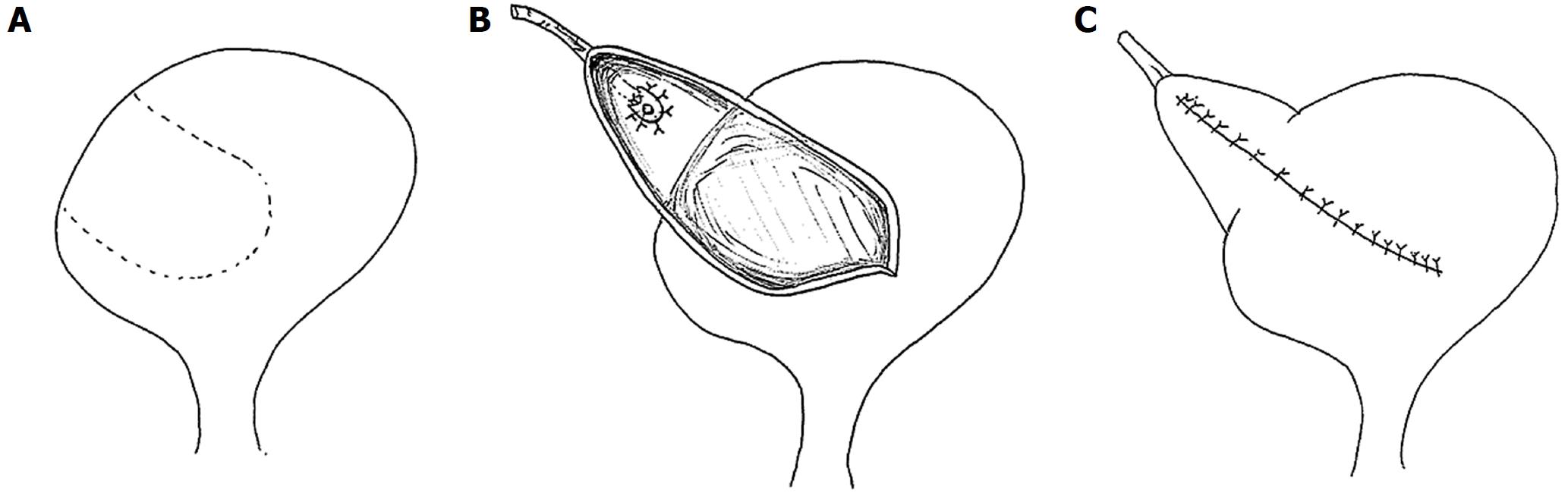
The ureterovesical anastomosis associated with the lowest rate of complications continues to be a subject of debate. The Leadbetter-Politano technique (Figure 3) was primarily used in the early days of kidney transplantation[41]. This has been largely superseded by the less technically demanding Lich-Gregoir technique (Figure 4)[42]. The Taguchi technique (Figure 5) has been associated with unacceptably higher incidence of complications compared to the Lich-Gregoir technique[43,44]. In a recent meta-analysis, which included two randomized controlled studies and 24 observational studies, the Lich-Gregoir technique was found to significantly reduce the incidence of ureteral leaks when compared to the Leadbetter-Politano and Taguchi techniques[45]. The incidence of ureteral stricture and reflux, however, did not differ significantly. The use of a shorter ureter and the avoidance of a separate cystostomy are two hypothetical advantages over the Leadbetter-Politano technique[46]. A modification of the Lich-Gregoir technique, using a short muscular tunnel over the distal ureter, has been shown to reduce complications in two separate retrospective studies[46,47]. In one Chinese study, primary termino-terminal ipsilateral ureteroureterostomy, was associated with significantly less urinary fistulas when compared to the established Lich-Gregoir technique[23].
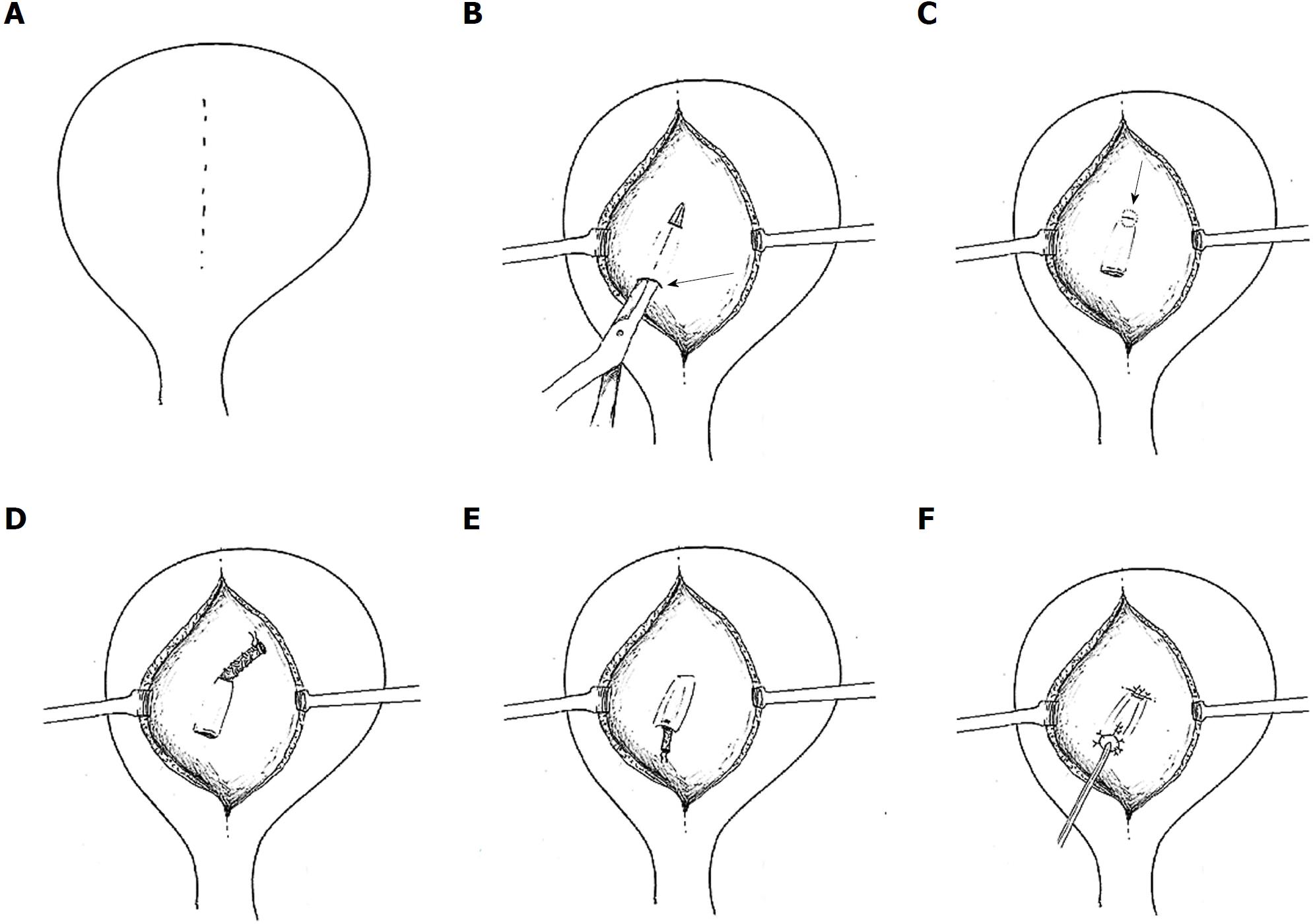
Figure 3 Leadbetter-Politano technique.
A: A longitudinal bladder incision is performed to gain access to the interior of the bladder; B: A second cystotomy is done to introduce the neo-ureter in the bladder. Subsequently, an Overholt is inserted from the second cystotomy and tunnelled close to the bladder wall for about 3 cm; C: A new hiatus is created at the end of the tunnel; D: The neo-ureter is pulled through the mucosal tunnel and the new mucosal hiatus using a free suture as a guide rail; E: Closure of the second cystotomy and then sub-mucosal transposition of distal neo-ureter; F: Fixation of the neo-ureter orifice and closure of the bladder mucosa.
Figure 4 Lich-Gregoir technique.
A: Bladder wall incision through the detrusor muscle is performed, leaving a very thin layer of muscle and uroepithelium unbreached; B: The distal part is completely incised to create a neo-ureter-bladder anastomosis; C: Suturing of the neo-ureter is performed via the same access used to introduce it into the bladder; D: The ureter is positioned in the groove and in direct contact to the uroepithelium, followed by closure of the muscle over the ureter while carefully avoiding constriction of the neo-ureter.
Figure 5 Taguchi technique.
A suture is positioned at the distal end of the neo-ureter and subsequently introduced in the bladder via a cystotomy. The neo-ureter is later fixed to the bladder wall by bringing the suture out through the bladder wall and closed.
Currently, many centers have adopted the routine use of ureteric stent during kidney transplantation. A meta-analysis, which included seven randomized controlled studies, confirmed that routine prophylactic stenting is generally well tolerated and significantly reduces major urological complications[48]. In a recently published Cochrane database systematic review, it was established that 13 transplant recipients need to be treated (with using JJ stent) in order to prevent one major urological complication[48]. Despite some opposition due to the higher incidence of urinary tract infections, current evidence recommends the routine use of prophylactic stenting.
MANAGEMENT OF URINARY LEAKS
In general, one can select between two main approaches (conservative vs reconstructive surgery) depending on the site, cause and extent of the leak. One has to keep in mind that these treatment strategies are not based on robust scientific evidence and tend to vary between centers based on anecdotal experiences. The current best available evidence is merely based on retrospective studies.
A conservative approach typically involves insertion of a percutaneous nephrostomy followed by antegrade stenting of the collecting system (unless already performed during the transplant operation), together with a Foley catheter replacement. Retrograde stenting of a transplant ureter is technically demanding and often impossible, even by the most skilled urologists, because of the atypical position of the ureteric orifice. Antegrade stenting, although generally easier, can still pose technical challenge in the absence of pelvi-caliceal dilatation. Interventional radiologists and transplant surgeons can work together to manage difficult cases[49]. This procedure diverts the urinary flow away from the leaking site and, thereby, fully decompresses the collecting system in order to allow for healing to take place. The Foley catheter is usually removed once the leak has resolved. Many centers report stent deployment for a period of 6-12 wk[14,33,35,46]. The presence of recurrent urinary tract infection may hasten the time for stent removal.
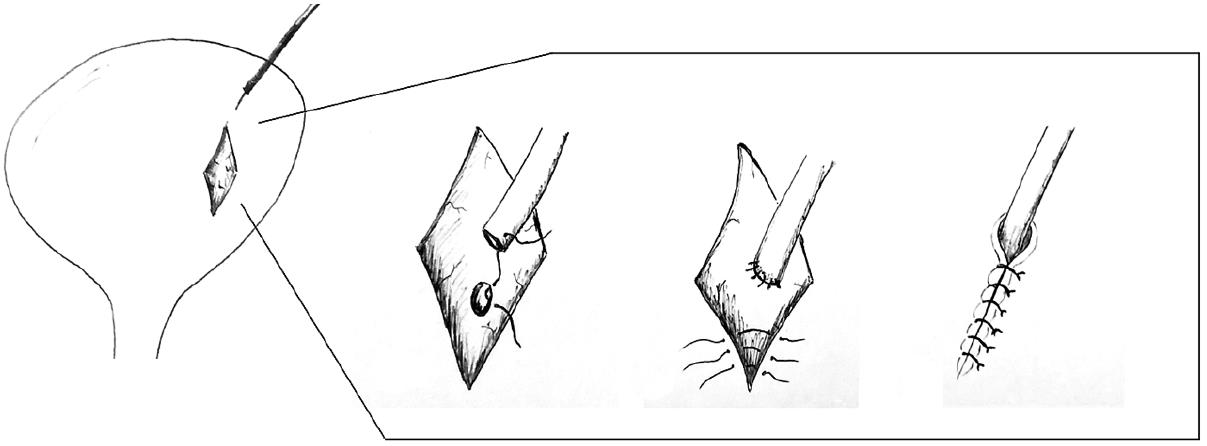
Surgical exploration is required if the urine leak fails to resolve following maximal decompression, especially when dealing with major urine extravasations or necrotic ureters. During the surgical procedure, the necrotic ureter should be resected proximally until healthy tissue is reached, followed by re-implantation. If the remaining viable ureter is short, an ipsilateral uretero-ureterostomy, pyelovesicostomy, psoas hitch, Boari flap or fashioning of an ileal ureter are alternative techniques that could be employed for tension-free ureteric anastomosis[50]. A psoas hitch (Figure 6) involves extensive dissection and mobilization of the urinary bladder to allow mobilization towards the transplant ureter, usually up to 5 cm. Subsequently, the bladder is anchored to the ipsilateral psoas muscle. Alternatively, a Boari flap (Figure 7) can be fashioned to attain an additional 10 cm. If required, this can be used in conjunction with the psoas hitch technique to bridge larger gaps between the short transplant ureter and the bladder. Contracted or atrophic urinary bladders in anuric patients seriously limit these options. In this circumstance, an ipsilateral uretero-ureterostomy can be an alternative option if the cause of native kidney failure was not reflux disease. A pyelovesicostomy or an ileal ureter can be fashioned, the latter being preferred for larger gaps, in situations where no donor or recipient ureter can be salvaged[51]. Both these techniques are devoid of an anti-reflux mechanism. In all cases, serial ultrasound examinations together with close monitoring of the transplant excretory function is of chief importance to anticipate any secondary ureteral strictures.
Figure 6 Psoas hitch.
A: A psoas hitch procedure is used to bridge the gap between the urinary bladder and a short ureter; B: Mobilization of the urinary bladder is achieved by dissecting the attachments of the urinary bladder, which is subsequently hitched to the Psoas muscle; C: Ureter implantation is performed via a transverse incision, which is later closed.
Figure 7 Boari flap.
A: A Boari flap is used when a Psoas hitch is not enough to bridge the gap between the bladder and a short ureter to allow for a tension-free anastomosis. A U-shaped flap composed of all tissue layers is created. The base should be proportional to the length of the flap to avoid ischemia; B: The ureter is implanted to the apex of the flap via end-to-end anastomosis or a sub-mucosal tunnel; C: The bladder incision together with the flap are subsequently closed.
Traditionally, urine leaks have been corrected by open reconstruction. Over the last two decades, advances in interventional radiology have allowed several patients to be effectively managed percutaneously, avoiding the morbidity associated with open surgery[49,52]. This conservative approach has been shown to be successful in a number of retrospective studies, with a success rate varying between 30% and 87%[19,21,53-55]. This considerable inter-center variability is probably related to different baseline characteristics. We believe that the outcome largely depends on the etiology, site and extent of the urine leak. In general, small leaks at the ureter implantation site tend to do well with conservative management, while extensive leaks, especially if related to ureter necrosis, do better with open surgery. When in doubt, we treat conservatively in the first instance and then proceed to surgical reconstruction only if the patient fails to respond. The type of surgery is frequently dictated by the intra-operative findings and the overall state of the patient. Surgical reconstruction is usually successful in the majority of cases[19,21,23,55]. Nonetheless, some patients required more than one surgical procedure for complete resolution[23].
LIMITATION
This narrative review is intended to provide a general overview of the early urological complications after kidney transplantation. Although we performed an extensive literature search, this review lacks the scientific rigor of article selection found in a systematic review, and is therefore susceptible to selection bias. In addition, the selected articles have not been subjected to quality evaluation.
CONCLUSION
Urological complications, especially urine leaks, remain the most common type of surgical complication following kidney transplantation. The preservation of peri-ureteric tissue during kidney retrieval, employing the Lich-Gregoir ureteroneocystostomy technique and routine prophylactic ureteral stenting, have been associated with lower incidence of such complications. Serial ultrasound examination of the transplanted graft in the early post-operative period is of key importance for early detection of these potential complications. The first line management of urine leaks is usually percutaneous urinary decompression. Failing this approach, surgical intervention is usually required, especially if dealing with major leaks or necrotic ureters. Although urological complications are associated with significant morbidity and occasionally mortality, the prognosis is generally excellent if recognized and treated successfully in a timely manner.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Manuscript source: Unsolicited manuscript
P- Reviewer: Boteon YL, Kim JS S- Editor: Ji FF L- Editor: Filipodia E- Editor: Yin SY