Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.310
Peer-review started: May 7, 2015
First decision: June 24, 2015
Revised: September 4, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: December 24, 2015
AIM: To determine the clinical reasons for conversion to everolimus (EVL) and long-term outcomes in heart transplant (HT) recipients.
METHODS: A retrospective 12-mo study has been carried out in 14 Spanish centres to assess the efficacy and safety of conversion to EVL in maintenance HT recipients.
RESULTS: Two hundred and twenty-two patients were included (mean age: 53 ± 10.5 years; mean time from HT: 8.1 ± 4.5 years). The most common reasons for conversion were nephrotoxicity (30%), chronic allograft vasculopathy (20%) and neoplasms (17%). The doses and mean levels of EVL at baseline (conversion to EVL) and after one year were 1.3 ± 0.3 and 1.2 ± 0.6 mg/d and 6.4 ± 3.4 and 5.6 ± 2.5 ng/mL, respectively. The percentage of patients receiving calcineurin inhibitors (CNIs) at baseline and on the final visit was 95% and 65%, respectively. The doses and mean levels of CNIs decreased between baseline and month 12 from 142.2 ± 51.6 to 98.0 ± 39.4 mg/d (P < 0.001) and from 126.1 ± 50.9 to 89.2 ± 47.7 ng/mL (P < 0.001), respectively, for cyclosporine, and from 2.9 ± 1.8 to 2.6 ± 1.9 mg/d and from 8.3 ± 4.0 to 6.5 ± 2.7 ng/mL (P = 0.011) for tacrolimus. In the subgroup of patients converted because of nephrotoxicity, creatinine clearance increased from 34.9 ± 10.1 to 40.4 ± 14.4 mL/min (P < 0.001). There were 37 episodes of acute rejection in 24 patients (11%). The most frequent adverse events were oedemas (12%), infections (9%) and gastrointestinal problems (6%). EVL was suspended in 44 patients (20%). Since the database was closed at the end of the study, no further follow-up data is available.
CONCLUSION: Conversion to EVL in maintenance HT recipients allowed minimisation or suspension of the CNIs, with improved kidney function in the patients with nephrotoxicity, after 12 mo.
Core tip: This study is one of the largest multicentre Spanish series of heart transplant recipients converted to everolimus (EVL) reported to date. The results have helped to confirm the efficacy and safety profile of the drug under conditions of routine clinical practice. In the study, conversion to EVL in maintenance phase heart transplant recipients allowed a significant reduction in calcineurin inhibitor treatment with improved kidney function in patients with nephrotoxicity, after one year. Results regarding rejection episodes and EVL discontinuation, suggest that each patient should be individually evaluated for conversion to EVL based on their clinical profile and transplantation evolution.
- Citation: Manito N, Delgado JF, Crespo-Leiro MG, Arizón JM, Segovia J, González-Vílchez F, Mirabet S, Lage E, Pascual-Figal D, Díaz B, Palomo J, Rábago G, Sanz M, Blasco T, Roig E. Twelve-month efficacy and safety of the conversion to everolimus in maintenance heart transplant recipients. World J Transplant 2015; 5(4): 310-319
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/310.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.310
Since their introduction, calcineurin inhibitors (CNIs) have contributed enormously to reduce the incidence of rejection and to prolong heart transplant (HT) recipient survival[1]. However, long-term results are limited by the appearance of complications related to continued CNI-based immunosuppression[2,3], such as chronic renal dysfunction[4] or malignancies[5,6]. As a result, there has been growing interest in recent years in the development of immunosuppressive regimens with reduced CNI doses, or even without CNIs[7], for the prevention and management of such complications.
Everolimus [EVL (Certican®; Novartis Pharmaceuticals, Basel, Switzerland)] belongs to the family of mammalian target of rapamycin (mTOR) inhibitors, which are potent immune suppressors that act through inhibition of the intracellular signals regulating cell growth and division[8]. In de novo HT patients, EVL in combination with CNIs has demonstrated immunosuppressive efficacy in reducing the frequency of acute rejection and chronic allograft vasculopathy (CAV)[9-11]. In patients in the maintenance phase, some studies have suggested that the introduction of EVL makes it possible to reduce or even discontinue CNI therapy - generally in association with preservation or improvement of kidney function while maintaining immunosuppressive efficacy[12-16]. In addition, as a result of their antiproliferative properties, mTOR inhibitors offer additional benefits such as a demonstrated antitumor effect[17,18], the capacity to prevent or slow CAV progression[19] and they are associated with a lower incidence of cytomegalovirus (CMV) infection[20].
The present article reports the findings of an observational study in which HT recipients receiving maintenance immunosuppression converted to EVL in routine clinical practice and were followed for one year (Epi-transplant study, EVERODATA cardiac substudy). The study objectives were to determine the immunosuppressive regimens used with EVL, together with the clinical reasons for the use of the drug, and the long-term efficacy and safety of treatment conversion.
The Epi-transplant study was a retrospective observational study designed to define the use of immunosuppression in the clinical practice setting of solid organ transplants in Spain. The EVERODATA cardiac study in turn was the substudy conducted in HT recipients who started treatment with EVL in routine clinical practice at 14 Spanish centres between October 2006 and December 2007, with a follow-up period of 12 mo. The only inclusion criteria were a patient age of over 18 years and the absence of any experimental drug treatments. Since this was an observational study, there were no modifications to the patient’s current treatment regimen; therefore, introduction and use of EVL was carried out according to the specific protocol of each centre. Of the 256 patients in the database, only those who started EVL in the maintenance phase (over 30 d following HT) were included in the present analysis. After excluding 11 patients under 18 years of age and one patient in whom the type of transplant had not been specified, the final evaluable population consisted of 222 patients. All patients gave written informed consent for their participation in the study, as approved by the Investigation Review Board of the Vall d’Hebron Hospital (Barcelona, Spain).
According to the protocol, the patient’s demographic data were collected at the baseline visit (conversion to EVL), along with information on the underlying disease and transplant (first transplant, re-transplantation, multiorgan transplant, as well as time from the procedure and risk factors for the development of nephrotoxicity, CAV and neoplasms). Data were also collected on the immunosuppressive treatment used before conversion to EVL, and on the possible reasons for conversion: Nephrotoxicity [defined as serum creatinine (CrS) > 1.5 mg/dL or creatinine clearance (CrCl) < 50 mL/min in two measurements spaced at least one month apart, and in the absence of obstructive urological disease or nephropathy of other causes], CAV (diagnosed according to each centre’s protocol), neoplastic disease, neurotoxicity, intolerance of previous immunosuppressive treatment, or recurrent or refractory rejection (defined according to each centre’s protocol). After conversion, follow-up controls were carried out on days 7 and 14, and after 1, 3, 4, 6, 9 and 12 mo. At each timepoint, extensive laboratory tests were conducted, including kidney function parameters, complete blood count and lipid profile, as well as vital functions and physical examination. Immunosuppression doses were recorded and plasma EVL levels were determined according to the routine practice at each centre [Seradyn Innofluor® Certican® immunoassay kit (Seradyn, Inc., United States) or using liquid chromatography with mass spectrometry, GC-MS], with documentation of any adverse events related to the study medication. Kidney function was evaluated by means of CrCl (mL/min) measurements according to the Cockcroft-Gault equation: CrCl = [(140 - age) × weight in kg]/(72 × CrS in mg/dL), corrected × 0.85 in women. Any rejection was recorded and graded according to the classification of the International Society for Heart and Lung Transplantation. The obtention of surveillance endomyocardial biopsies or biopsies for the detection of rejection depended upon the routine protocol in each centre. Unfortunately, the database was closed at the end of the study; therefore, there are no data available regarding the follow-up of patients included in the study.
Descriptive statistics (mean, standard deviation, minimum and maximum for continuous variables and absolute numbers and percentages for categorical variables) were calculated for the study variables. Qualitative variables are expressed as total number of events and percentages; comparisons of percentages were performed with χ2 test. Quantitative variables are presented as means and standard deviation. The Student t-test was applied for comparative analyses with qualitative variables in case of normality; otherwise, it was applied the Mann-Whitney test. In comparisons of paired samples with normality it was performed the Student t-test if not it was used the Wilcoxon test. The hypothesis tests performed were two-tailed in all cases, and with a level of significance of 0.05. The SPSS version 13.0 statistical package was used for the analysis.
Table 1 summarises the baseline characteristics of the study population (n = 222) and the immunosuppressive regimen received by the patients before conversion to EVL. The mean age was 53 ± 10.5 years, with a clear male predominance (85%). The mean time elapsed from HT to the time of conversion was 8.1 ± 4.5 years. A total of 210 patients (95%) were receiving CNI treatment at baseline [cyclosporine (CsA): 72%], 189 (85%) patients were receiving an antimetabolite (mycophenolic acid derivatives: 80%), 154 (69%) patients were receiving corticosteroids, and 21 (9%) patients were receiving sirolimus (SRL; conversion to EVL in these patients was due to intolerance or clinical management difficulties, according to investigator criterion).
| n (%) | Mean ± SD | |
| Recipient age (yr) | - | 53 ± 10.5 |
| Sex | ||
| Male | 189 (85%) | - |
| Female | 33 (15%) | - |
| Mean time from transplant to conversion (yr) | - | 8.1 ± 4.5 |
| Type of transplant | ||
| First transplant | 215 (96.7%) | - |
| Re-transplant | 6 (2.8%) | - |
| Multiorgan transplant | 1 (0.4%) | - |
| Reasons for transplanta | ||
| Ischaemic myocardiopathy | 114 (51%) | - |
| Dilated myocardiopathy | 74 (33%) | - |
| Valve disease | 14 (6%) | - |
| Othersb | 16 (7%) | - |
| Donor age (yr) | - | 32.2 ± 12.7 |
| Donor positive for CMV serologyd | 106 (48%) | - |
| Recipient positive for CMV serologye | 155 (70%) | - |
| Pre-transplant risk factorsc | 139 (63%) | - |
| Arterial hypertension | 55 (25%) | - |
| Diabetes | 36 (16%) | - |
| Renal failure | 4 (2%) | - |
| Osteoporosis | 3 (1%) | - |
| Hypercholesterolaemia | 29 (13%) | - |
| Dyslipidaemia | 29 (13%) | - |
| Smoking | 23 (10%) | - |
| Baseline immunosuppression | ||
| CNI | 210 (95%) | - |
| CsA | 152 (72%) | - |
| Dose, mg/d | - | 142.3 ± 51.6 |
| Blood levels, ng/mL | - | 126.1 ± 50.9 |
| Tacrolimus | 58 (28%) | - |
| Dose, mg/d | - | 2.9 ± 1.8 |
| Blood levels, ng/mL | - | 8.3 ± 4.0 |
| Antimetabolite | 189 (85%) | - |
| MMF | 143 (76%) | - |
| Dose, mg/d | - | 1.446.1 ± 499.0 |
| Blood levels, ng/mL | - | 2.9 ± 1.7 |
| MFS | 8 (4%) | - |
| Dose, mg/d | - | 742.5 ± 413.1 |
| Blood levels, ng/mL | - | 3.8 ± 1.7 |
| Azathioprine | 38 (20%) | - |
| Dose, mg/d | - | 84.9 ± 46.5 |
| Blood levels, ng/mL | - | - |
| Corticosteroids | 154 (69%) | - |
| Dose, mg/d | - | 5.2 ± 4.2 |
| SRL | 21 (9%) | - |
| Dose, mg/d | - | 5.8 ± 2.5 |
The most frequent reason for conversion to EVL was nephrotoxicity with the previous immunosuppressive treatment (Table 2). This was the reason reported in 30% of patients. CAV and malignancies were respectively the reason in 20% and 17% of the patients. Other reasons included intolerance to mycophenolate mofetil (MMF)/mycophenolic acid, intolerance to sirolimus, neurotoxicity, recurrent/refractory rejection, aesthetic problems, repeated CMV infection or severe arterial hypertension.
| Reason for conversion | Percentage | 95%CI |
| Nephrotoxicity | 30.00% | 24.0-36.0 |
| CAV | 20.50% | 15.2-25.8 |
| Malignancy | 17.20% | 12.1-21.9 |
| Nephrotoxicity + CAV | 9.80% | 5.9-13.7 |
| Nephrotoxicity + neoplasms | 7.00% | 3.6-10.4 |
| CAV + malignancy | 2.00% | 0.2-3.8 |
| Others | 13.00% | 8.6-17.4 |
The mean EVL dose at baseline and after one year was 1.3 ± 0.3 and 1.2 ± 0.6 mg/d, respectively. The mean EVL concentration after 7 d was 6.4 ± 3.4 ng/mL. From this point and up to the last visit (5.6 ± 2.5 ng/mL), the levels remained stable between 5.6 and 5.9 ng/mL.
Twelve months after conversion to EVL, 65% of the patients were receiving a CNI (CsA: 77%; Table 3). The most frequent regimens were the combination of EVL and CsA (with or without corticosteroids; 46%), and the combination of EVL and MMF (± corticosteroids; 30%). In the subgroup of patients who converted because of nephrotoxicity, 51% were receiving treatment with CNIs (CsA: 82%), though the most frequently used regimen was a CNI-free regimen based on EVL and MMF (± corticosteroids; 41%). In those patients in whom CNI treatment was maintained, the mean CsA dose was significantly reduced from 142 ± 51.6 mg/d at baseline to 98.0 ± 39.5 mg/d after 12 mo (P < 0.001). Serum levels of CsA decreased significantly from 126.1 ± 50.9 ng/mL before conversion to 89.2 ± 47.7 ng/mL after one year (P < 0.001). The tacrolimus concentration at baseline and after 12 mo was 2.9 ± 1.8 and 2.6 ± 1.9 mg/d, respectively - the level at the end of follow-up was significantly lower than at baseline (6.5 ± 2.7 ng/mL vs 8.3 ± 4.0 ng/mL; P = 0.011).
| Immunosuppressor regimen | n (%) | 95%CI |
| Overall study population | n = 1471 | |
| EVL + tacrolimus + MMF ± corticosteroids | 11 (7.5%) | 3.2-11.7 |
| EVL + CsA + MMF ± corticosteroids | 7 (4.8%) | 1.3-8.2 |
| EVL + tacrolimus ± corticosteroids | 11 (7.5%) | 3.2-11.7 |
| EVL + CsA ± corticosteroids | 67 (45.6%) | 37.5-53.6 |
| Total with CNIs | 96 (65.3%) | 57.6-73.0 |
| EVL + MMF ± corticosteroids | 44 (29.9%) | 22.5-37.3 |
| EVL + corticosteroids | 7 (4.8%) | 1.3-8.2 |
| Total without CNIs | 51 (34.7%) | 27.0-42.4 |
| Patients converted due to nephrotoxicity | n = 66 | |
| EVL + tacrolimus + MMF ± corticosteroids | 3 (4.5%) | 0.1-9.6 |
| EVL + CsA + MMF ± corticosteroids | 5 (7.6%) | 1.2-14.0 |
| EVL + tacrolimus ± corticosteroids | 3 (4.5%) | 0.1-9.6 |
| EVL + CsA ± corticosteroids | 23 (34.9%) | 23.4-46.3 |
| Total with CNIs | 34 (51.5%) | 39.5-63.6 |
| EVL + MMF ± corticosteroids | 27 (40.9%) | 29.0-52.8 |
| EVL + corticosteroids | 5 (7.6%) | 1.2-14.0 |
| Total without CNIs | 32 (48.5%) | 36.4-60.5 |
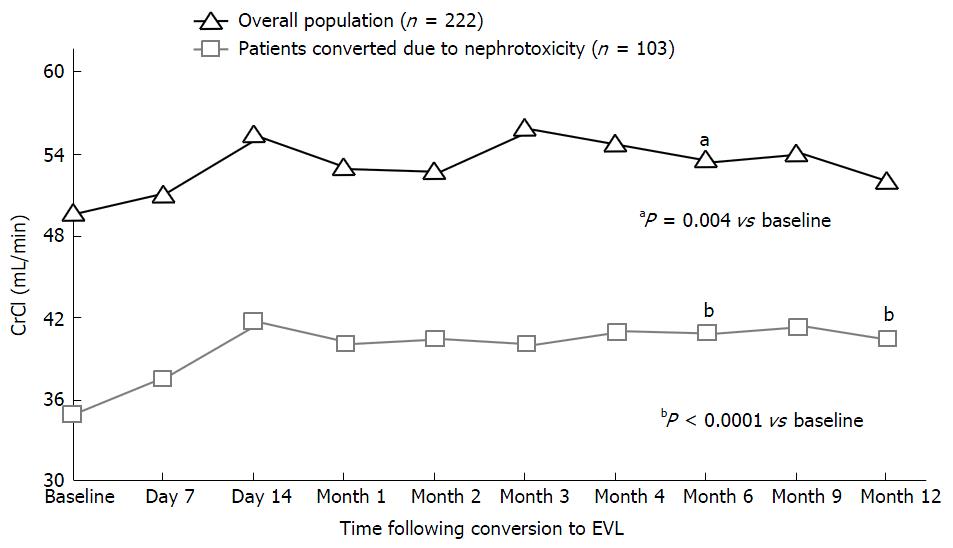
Figure 1 shows the evolution of kidney function in the overall study population and in the subgroup of patients converted to EVL due to nephrotoxicity. Twelve months after conversion, CrS had decreased from 1.7 ± 0.7 mg/dL at baseline to 1.6 ± 0.7 mg/dL, though the difference was not significant. In turn, CrCl increased from 49.6 ± 21.2 mL/min at baseline to 51.9 ± 21.1 mL/min one year after conversion (P = ns). In the subgroup of patients converted to EVL because of nephrotoxicity (n = 103), the baseline values of CrS and CrCl were 2.2 ± 0.7 mg/dL and 34.9 ± 10.1 mL/min, respectively. Twelve months after conversion, statistically significant improvements were observed: CrS 2.0 ± 0.8 mg/mL (P < 0.05) and CrCl 40.4 ± 14.4 mL/min (P < 0.001). No data on proteinuria are available, as this parameter is not usually monitored in HT clinical practice.
There were 37 episodes of acute rejection in 24 patients (11%). Sixteen of these episodes were grade ≥ 3A (4 episodes in patients receiving the combination of EVL, MMF and corticosteroids, 3 episodes in patients receiving EVL, CNIs and corticosteroids, 3 episodes in patients receiving EVL, MMF, CNIs and corticosteroids, one episode in a patient receiving EVL and corticosteroids, and 5 episodes with other unspecified combinations) and 13 episodes were grade < 3A (the histological grade was not known in 8 episodes). All grade ≥ 3A rejections were treated according to the protocol applied in the centre, while none of the grade < 3A rejections required treatment. The acute rejection episodes were distributed as follows: 14 episodes up to 3 mo after conversion to EVL (< 3A: 7; ≥ 3A: 6; unknown: 1), 14 episodes in the period 3-6 mo after conversion (< 3A: 3; ≥ 3A: 7; unknown: 4), and 9 episodes in the period 6-12 mo after conversion (< 3A: 3; ≥ 3A: 3; unknown: 3).
Twenty-six patients (12%) died during follow-up. The causes of death were: Sudden death (6 cases), neoplasms (6 cases), CAV (5 cases), and one case each of infection, primary graft failure, respiratory depression, digestive bleeding, pulmonary thromboembolism, cerebrovascular stroke and unknown cause.
A total of 152 adverse events were registered in 97 patients (44%) - the most frequent problems being oedema (12%), infections of any kind (9%), and gastrointestinal disorders (6%) (Table 4). Forty-four patients (20%) had to discontinue EVL treatment during the study. The most important reasons for discontinuation were oedemas (29%), gastrointestinal disorders (18%), bone marrow suppression (9%), and the development of pneumonitis (9%) (Table 5).
| Adverse event | n | % total events (95%CI) (n = 152) | % total evaluable patients (95%CI) (n = 222) |
| Oedemas | 27 | 17.8% (11.7-23.8) | 12.2% (7.9-16.5) |
| Infections | 20 | 13.2% (7.8-18.5) | 9.0% (5.2-12.8) |
| Gastrointestinal disorders | 13 | 8.6% (4.1-13.0) | 5.9% (2.8-8.9) |
| Skin disorders | 12 | 7.9% (3.6-12.2) | 5.4% (2.4-8.4) |
| Haematological disorders | 10 | 6.6% (2.6-10.5) | 4.5% (1.8-7.2) |
| Pericardial effusion | 6 | 3.9% (0.9-7.0) | 2.7% (0.6-4.8) |
| Pneumonitis | 5 | 3.3% (0.5-6.1) | 2.3% (0.3-4.2) |
| Oral aphthae | 3 | 2.0% (0.2-4.2) | 1.4% (0.1-2.9) |
| Pleural effusion | 2 | 1.3% (0.1-3.1) | 0.9% (0.1-2.1) |
| Healing disorders | 2 | 1.3% (0.1-3.1) | 0.9% (0.1-2.1) |
| Others | 52 | 34.2% (26.7-41.8) | 23.4% (17.9-29.0) |
| Drug withdrawal | n | % total patients that discontinue treatment (95%CI) (n = 44) | % total evaluable patients (95%CI) (n = 222) |
| Oedemas | 13 | 29.5% (16.1-43.0) | 5.9% (2.8-9.8) |
| Gastrointestinal disorders | 8 | 18.2% (6.8-29.6) | 3.6% (1.2-6.1) |
| Bone marrow suppression | 4 | 9.1% (0.6-17.6) | 1.8% (0.1-3.6) |
| Pneumonitis | 4 | 9.1% (0.6-17.6) | 1.8% (0.1-3.6) |
| Skin disorders | 2 | 4.6% (0.1-10.7) | 0.9% (0.1-9.5) |
| Others | 14 | 31.8% (18.1-45.6) | 6.3% (3.1-9.5) |
Between baseline and 12 mo after conversion to EVL, significant increases were observed in total cholesterol (175.4 ± 40.3 mg/dL vs 189.3 ± 41.9 mg/dL; P = 0.002), HDL-cholesterol (52.0 ± 18.5 mg/dL vs 57.5 ± 18.9 mg/dL; P < 0.05) and LDL-cholesterol (94.2 ± 31.01 mg/dL vs 105.6 ± 74.6; P < 0.001), but not in triglyceride concentration (147.3 ± 83.1 mg/dL vs 148.5 ± 74.6 mg/dL; P = ns).
The results of this observational study show that in clinical practice of HT in Spain, chronic nephrotoxicity due to CNIs accounts for practically one third of all indications of EVL treatment - while CAV or malignancies are also a frequent reason for conversion. CNI-related nephrotoxicity is associated with prolonged exposure to CsA or tacrolimus and is characterised by progressive deterioration of kidney function, often accompanied by arterial hypertension and occasionally proteinuria[2]. CNIs induce tubular atrophy and interstitial fibrosis through the induction of ischaemia secondary to microvascular damage of the afferent renal arteries[21], activation of the renin-angiotensin-aldosterone system, or TGF-β1 stimulation[22]. In reference to post-transplantation neoplasms, CNIs have been associated with pro-oncogenic mechanisms related to an increase in the expression of growth factors such as TGF-β or VEGF, the inhibition of DNA repair, and alterations of the apoptosis signalling pathways[23-25]. As a result, in recent years there has been growing interest in the development of new immunosuppressive regimens aimed at reducing the use of CNIs in maintenance immunosuppression[7]. The most common strategies consist of introducing or escalating the presence of drugs such as MMF[26] or mTOR inhibitors[27-29].
In our study, the introduction of EVL in HT recipients in the maintenance phase allowed a global reduction in the use of CNIs and the establishment of a variety of immunosuppressive regimens (a fact that reflects the existence of highly tailored therapy in the clinical practice of HT). Twelve months after conversion more than one-third of the patients were receiving a CNI-free regimen based on EVL, while in the rest of the cases CNIs were maintained. These findings reflect the existing clinical inertia in HT at the time that the study was performed, with clinicians being reluctant to withdraw CNIs in order to avoid potential rejections. Despite this, a significant reduction was achieved in the dose (30%) and levels (21%) of the CNIs in this group of patients. After one year, all these changes in immunosuppression were associated with a preservation of kidney function and those patients specifically converted to EVL because of nephrotoxicity reported a significant increase in CrCl of +5.5 mL/min.
As mentioned, minimisation of CNIs by adding treatment with EVL remains the most common strategy in clinical practice in patients with impaired renal function. In earlier publications involving fewer patients, the use of EVL allowed reductions of approximately 35%-50% in the dose of CsA, with no concomitant increase in rejection risk[12,13]. The recent Scandinavian NOCTET study is the only randomised clinical trial published on the use of EVL in HT recipients with renal impairment. In this study, a total of 282 maintenance phase transplant recipients (190 HT and 92 lung transplants) with different degrees of kidney dysfunction (glomerular filtration rate: 20-90 mL/min per 1.73 m2) were randomised either to continue standard immunosuppression or to start EVL plus CNI minimisation[30]. After one year, the mean change in CrCl in both groups was +0.5 mL/min and +4.6 mL/min, respectively (P < 0.0001), vs -2.4 mL/min and +3.2 mL/min after two years (P < 0.001)[31]. The reduction in exposure to CsA was 56%, and patients that converted earlier to EVL after HT showed higher CrCl increments. The acute rejection rate was similar in both groups, though there was a significant increase in adverse effects with EVL. In this regard, the EVERODATA study has confirmed the results from the NOCTET trial in a larger number of patients treated under conditions of clinical practice, establishing the usefulness of EVL for the minimisation of the CNIs in HT recipients with renal impairment.
In relation to CNI withdrawal after conversion to EVL, a study of 45 HT recipients with progressive deterioration of kidney function reported a 17% improvement in CrCl one year after conversion to EVL[14]. Recently, Engelen et al[32] published a prospective, two-year follow-up study of 58 HT recipients with renal failure converted to EVL from initial CNI treatment (mean time after HT: 5.6 years). CrCl increased from 43.6 to 49.5 mL/min (P = 0.02), though in 14% of the patients CNI treatment was reintroduced because of adverse effects. In 2009, Groetzner et al[33], in a study of 63 HT recipients (0.5-18.4 years from transplantation) with kidney dysfunction (CrCl < 60 mL/min), compared CNI withdrawal plus the introduction of SRL and MMF vs reduction (40%) in the levels of CNI treatment[33]. After one year, CrCl improved significantly as a result of CNI withdrawal (53 mL/min vs 38 mL/min; P = 0.01), although the rate of adverse events was higher with the mTOR inhibitor.
At present, there is no clear evidence that CNI withdrawal is a better strategy for responding to nephrotoxicity. In this regard González-Vílchez et al[16] compared, in a retrospective multi-centre cohort of 394 maintenance cardiac recipients with renal failure (GFR < 60 mL/min per 1.73 m2), 235 patients in whom CNI was replaced with an mTOR-i (sirolimus or EVL) with 159 patients in whom mTOR-i was used to minimise CNIs. They concluded that in terms of renal benefits, irrespective of the strategy (minimisation vs withdrawal) the results support an earlier use of mTOR-i. The selection of either a conversion or a CNI minimisation protocol should be based on the clinical characteristics of the patients, particularly their rejection risk[16].
Controversy remains regarding the indicated type of CNI withdrawal - abrupt (overnight) or gradual - following the introduction of EVL. Recent data from the Spanish HT registry suggest that kidney function only improves if CNI treatment is withdrawn during the first three months after conversion to therapy with an mTOR inhibitor[34]. Some authors recommend an abrupt conversion during the first post-HT year (mean 5.5 mo) in patients with advanced renal failure (stage 4 of the KDOQI guides) or on dialysis[35]. In 16 patients that met these criteria, the mean glomerular filtration rate increased from 29 mL/min per 1.73 m2 to 62 mL/min per 1.73 m2 (P < 0.001) with this treatment strategy, while in the control group (15 patients with chronic renal failure converted 96 mo after HT) the observed increase in the mean glomerular filtration rate failed to reach statistical significance (from 26 mL/min per 1.73 m2 to 28 mL/min per 1.73 m2; P = 0.225).
Similar to the observations of smaller HT series reporting on the conversion to EVL or concomitant minimisation of CNI treatment[12-14], the EVERODATA study reported an acute rejection in 11% of the patients after introduction of EVL, although grade ≥ 3A rejections were seen in less than 4% of the cases. No rejection was associated with symptoms or haemodynamic compromise. Rejections were observed predominantly in the first 6 mo after conversion and to a similar degree in patients with or without CNI treatment. Recently González-Vílchez et al[36] have shown, in 284 long-term HT recipients, a high rate of acute rejection after conversion from a CNI to mTOR-i in maintenance HT. By multivariate analysis, rejection risk was associated with a history of late AR prior to PSI conversion, early conversion (< 5 year) after transplantation and age < 50 year at the time of conversion. Use of mycophenolate mofetil was a protective factor.
In our study adverse events were recorded in 44% of the patients, and EVL was discontinued in 20%. Oedemas were the only problem with an incidence of > 10% and represented the main reason for drug discontinuation (approximately one out of every three patients). It is difficult to establish comparisons for this observation, since in most HT studies oedemas due to mTOR inhibitors are usually not homogeneously documented. Recently, a study of 56 HT recipients converted to EVL or SRL (plus withdrawal/reduction of CNI treatment) has suggested that EVL offers a better tolerability profile, with fewer infections and oedemas than SRL, with frequencies similar to those recorded in our series (approximately 14% for both adverse events with EVL vs approximately 70% and 65% with SRL, respectively; P < 0.05)[37]. On the other hand, proteinuria, a frequently reported adverse event with mTOR inhibitors, was not routinely assessed in our first patients due to the ignorance about how clinically relevant proteinuria was in HT patients. Recently, a randomised study evaluating HT patients with Cyclosporine nephrotoxicity showed a better improvement in CrCL in patients without baseline proteinuria, whereas CrCl significantly worsened in patients with baseline proteinuria (-20%; P = 0.04)[38].
Since EVERODATA is an observational study, there is no control group to inform of safety and efficacy of EVL vs other treatments with CNI reduction/withdrawal. In addition, the 12-mo follow-up does not allow the drawing of firm conclusions regarding the long-term potential benefits of mTOR inhibitors on the outcomes of CAV or malignancies. On the other hand, a study of this kind allows us to evaluate an important number of patients with different profiles - something that cannot be done in controlled clinical trials, due to their restrictive inclusion criteria. The EVERODATA study is the largest multicentre series of HT recipients converted to EVL published to date, and the results obtained have contributed to define the efficacy and safety profile of the drug under conditions of routine clinical practice.
In conclusion, conversion to EVL in maintenance phase HT recipients allowed a significant reduction in CNI treatment, with stable kidney function and, specifically, significant improvement in patients with nephrotoxicity, one year after conversion. The number of rejections observed and the rate of EVL discontinuations, suggest that each patient should be individually evaluated for conversion to EVL, based on their clinical profile and transplantation evolution.
Calcineurin inhibitors (CNIs) have contributed to reduce the incidence of rejection and to prolong heart transplant (HT) recipient survival. However, CNI-based immunosuppression is associated with complications such as chronic renal dysfunction and malignancies. Immunosuppressive regimens reducing CNI doses or even withdrawing CNIs with the introduction of other immunosuppressive agents, such as mammalian target of rapamycin (mTOR) inhibitors, could prevent these complications.
The present paper describes one of the largest multicentre Spanish series of HT recipients converted to everolimus (EVL) reported to date. The results helped to confirm the efficacy and safety profile of the drug under conditions of routine clinical practice.
The study results suggest that conversion to EVL (mTOR inhibitor) in HT recipients in the maintenance phase allowed a significant reduction of the CNIs. One year after conversion, such reduction was globally associated with stable kidney function and with a significant improvement in patients with nephrotoxicity.
Conversion to EVL in HT recipients may be an alternative option in order to reduce the use of CNIs and prevent kidney failure.
Creatinine clearance rate is the volume of serum or plasma that is cleared of creatinine by one minute’s excretion of urine (mL/min). Chronic allograft vasculopathy is the long-term loss of function in transplanted organs due to the fibrosis of the transplanted tissue’s blood vessels.
This is a well-done retrospective case series of heart transplant patients who received everolimus during maintenance phase. The current paper would have been more interesting with an historical control cohort, for instant the 1-year experience prior to introduction of everolimus.
P- Reviewer: Deshpande SR, Lin J, Puddu PE
S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:996-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 2. | Griffiths MH, Crowe AV, Papadaki L, Banner NR, Yacoub MH, Thompson FD, Neild GH. Cyclosporin nephrotoxicity in heart and lung transplant patients. QJM. 1996;89:751-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Ippoliti G, Rinaldi M, Pellegrini C, Viganò M. Incidence of cancer after immunosuppressive treatment for heart transplantation. Crit Rev Oncol Hematol. 2005;56:101-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1703] [Cited by in F6Publishing: 1588] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 5. | Epailly E, Albanell J, Andreassen A, Bara C, Campistol JM, Delgado JF, Eisen H, Fiane AE, Mohacsi P, Schubert S. Proliferation signal inhibitors and post-transplant malignancies in heart transplantation: practical clinical management questions. Clin Transplant. 2011;25:E475-E486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Crespo-Leiro MG, Alonso-Pulpón L, Vázquez de Prada JA, Almenar L, Arizón JM, Brossa V, Delgado JF, Fernandez-Yañez J, Manito N, Rábago G. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transplant. 2008;8:1031-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Schuler W, Sedrani R, Cottens S, Häberlin B, Schulz M, Schuurman HJ, Zenke G, Zerwes HG, Schreier MH. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 434] [Cited by in F6Publishing: 454] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sørensen K, Hummel M, Lind JM. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 936] [Cited by in F6Publishing: 859] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 10. | Eisen HJ, Kobashigawa J, Starling RC, Pauly DF, Kfoury A, Ross H, Wang SS, Cantin B, Van Bakel A, Ewald G. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13:1203-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Arora S, Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Bøtker HE, Rådegran G, Gude E, Ioanes D, Solbu D. The Effect of Everolimus Initiation and Calcineurin Inhibitor Elimination on Cardiac Allograft Vasculopathy in De Novo Recipients: One-Year Results of a Scandinavian Randomized Trial. Am J Transplant. 2015;15:1967-1975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Ross H, Pflugfelder P, Haddad H, Cantarovich M, White M, Ignaszewski A, Howlett J, Vaillancourt M, Dorent R, Burton JR. Reduction of cyclosporine following the introduction of everolimus in maintenance heart transplant recipients: a pilot study. Transpl Int. 2010;23:31-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Schweiger M, Wasler A, Prenner G, Stiegler P, Stadlbauer V, Schwarz M, Tscheliessnigg K. Everolimus and reduced cyclosporine trough levels in maintenance heart transplant recipients. Transpl Immunol. 2006;16:46-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Moro López JA, Almenar L, Martínez-Dolz L, Sánchez-Lázaro I, Agüero J, Buendía F, Ortiz V, Salvador A. Progression of renal dysfunction in cardiac transplantation after the introduction of everolimus in the immunosuppressive regime. Transplantation. 2009;87:538-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zuckermann A. Clinical experience with Certican (everolimus) in maintenance heart transplant patients at the Medical University of Vienna. J Heart Lung Transplant. 2005;24:S206-S209; discussion S210-S211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | González-Vílchez F, Vazquez de Prada JA, Paniagua MJ, Gomez-Bueno M, Arizon JM, Almenar L, Roig E, Delgado J, Lambert JL, Perez-Villa F. Use of mTOR inhibitors in chronic heart transplant recipients with renal failure: calcineurin-inhibitors conversion or minimization? Int J Cardiol. 2014;171:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Campistol JM. Minimizing the risk of posttransplant malignancy. Transplantation. 2009;87:S19-S22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2365] [Cited by in F6Publishing: 2321] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 19. | Delgado JF, Manito N, Segovia J, Almenar L, Arizón JM, Campreciós M, Crespo-Leiro MG, Díaz B, González-Vílchez F, Mirabet S. The use of proliferation signal inhibitors in the prevention and treatment of allograft vasculopathy in heart transplantation. Transplant Rev (Orlando). 2009;23:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Kobashigawa J, Ross H, Bara C, Delgado JF, Dengler T, Lehmkuhl HB, Wang SS, Dong G, Witte S, Junge G. Everolimus is associated with a reduced incidence of cytomegalovirus infection following de novo cardiac transplantation. Transpl Infect Dis. 2013;15:150-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 897] [Cited by in F6Publishing: 940] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 22. | Cattaneo D, Perico N, Gaspari F, Remuzzi G. Nephrotoxic aspects of cyclosporine. Transplant Proc. 2004;36:234S-239S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005;352:1371-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 852] [Cited by in F6Publishing: 810] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 25. | Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67:1167-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Angermann CE, Störk S, Costard-Jäckle A, Dengler TJ, Siebert U, Tenderich G, Rahmel A, Schwarz ER, Nägele H, Wagner FM. Reduction of cyclosporine after introduction of mycophenolate mofetil improves chronic renal dysfunction in heart transplant recipients--the IMPROVED multi-centre study. Eur Heart J. 2004;25:1626-1634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Rothenburger M, Zuckermann A, Bara C, Hummel M, Strüber M, Hirt S, Lehmkuhl H. Recommendations for the use of everolimus (Certican) in heart transplantation: results from the second German-Austrian Certican Consensus Conference. J Heart Lung Transplant. 2007;26:305-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Zuckermann A, Manito N, Epailly E, Fiane A, Bara C, Delgado JF, Lehmkuhl H, Ross H, Eisen H, Chapman J. Multidisciplinary insights on clinical guidance for the use of proliferation signal inhibitors in heart transplantation. J Heart Lung Transplant. 2008;27:141-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Manito N, Delgado JF, Crespo-Leiro MG, González-Vílchez F, Almenar L, Arizón JM, Díaz B, Fernández-Yáñez J, Mirabet S, Palomo J. Clinical recommendations for the use of everolimus in heart transplantation. Transplant Rev (Orlando). 2010;24:129-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Gullestad L, Iversen M, Mortensen SA, Eiskjaer H, Riise GC, Mared L, Bjørtuft O, Ekmehag B, Jansson K, Simonsen S. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation. 2010;89:864-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Gullestad L, Mortensen SA, Eiskjær H, Riise GC, Mared L, Bjørtuft O, Ekmehag B, Jansson K, Simonsen S, Gude E. Two-year outcomes in thoracic transplant recipients after conversion to everolimus with reduced calcineurin inhibitor within a multicenter, open-label, randomized trial. Transplantation. 2010;90:1581-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Engelen MA, Amler S, Welp H, Vahlhaus C, Gunia S, Sindermann JR, Rothenburger M, Stypmann J. Prospective study of everolimus with calcineurin inhibitor-free immunosuppression in maintenance heart transplant patients: results at 2 years. Transplantation. 2011;91:1159-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Groetzner J, Kaczmarek I, Schulz U, Stegemann E, Kaiser K, Wittwer T, Schirmer J, Voss M, Strauch J, Wahlers T. Mycophenolate and sirolimus as calcineurin inhibitor-free immunosuppression improves renal function better than calcineurin inhibitor-reduction in late cardiac transplant recipients with chronic renal failure. Transplantation. 2009;87:726-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Delgado JF, Crespo MG, Manito N, Camprecios M, Rábago G, Lage E, Arizón JM, Roig E. Usefulness of sirolimus as rescue therapy in heart transplant recipients with renal failure: analysis of the Spanish Multicenter Observational Study (RAPACOR). Transplant Proc. 2009;41:3835-3837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Gude E, Gullestad L, Arora S, Simonsen S, Hoel I, Hartmann A, Holdaas H, Fiane AE, Geiran OR, Andreassen AK. Benefit of early conversion from CNI-based to everolimus-based immunosuppression in heart transplantation. J Heart Lung Transplant. 2010;29:641-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | González-Vílchez F, Vázquez de Prada JA, Paniagua MJ, Almenar L, Mirabet S, Gómez-Bueno M, Díaz-Molina B, Arizón JM, Delgado J, Pérez-Villa F. Rejection after conversion to a proliferation signal inhibitor in chronic heart transplantation. Clin Transplant. 2013;27:E649-E658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Moro JA, Almenar L, Martínez-Dolz L, Sánchez-Lázaro I, Agüero J, Salvador A. Tolerance profile of the proliferation signal inhibitors everolimus and sirolimus in heart transplantation. Transplant Proc. 2008;40:3034-3036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Potena L, Prestinenzi P, Bianchi IG, Masetti M, Romani P, Magnani G, Fallani F, Coccolo F, Russo A, Ponticelli C. Cyclosporine lowering with everolimus versus mycophenolate mofetil in heart transplant recipients: long-term follow-up of the SHIRAKISS randomized, prospective study. J Heart Lung Transplant. 2012;31:565-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |