Published online Jan 18, 2023. doi: 10.5500/wjt.v13.i1.1
Peer-review started: September 13, 2022
First decision: October 20, 2022
Revised: November 4, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 18, 2023
The coronavirus disease 2019 pandemic has significantly impacted liver tran
Core Tip: The coronavirus disease 2019 pandemic exerted significant challenges to the liver transplant structure worldwide, initially resulting in a decline in liver transplants but soon after rebounded. A better understanding of this infection together with robust guidance by the international transplant societies helped offset this decline. A multitude of considerations should be exercised throughout the liver transplant process to maintain acceptable safety and outcomes.
- Citation: Khazaaleh S, Alomari M, Sharma S, Kapila N, Zervos XB, Gonzalez AJ. COVID-19 in liver transplant patients: Impact and considerations. World J Transplant 2023; 13(1): 1-9
- URL: https://www.wjgnet.com/2220-3230/full/v13/i1/1.htm
- DOI: https://dx.doi.org/10.5500/wjt.v13.i1.1
The coronavirus disease 2019 (COVID-19) pandemic was declared an emergency by the World Health Organization in March 2020[1]. Since then it has had major impacts on many aspects of healthcare, including liver transplant in the United States. It greatly affected the pretransplant, perioperative, and post-transplant periods of liver transplantation.
It is widely accepted that patients with chronic liver disease, specifically those with cirrhosis, have a higher rate of hospitalization, length of hospital stay, morbidity, and mortality from COVID-19 infection compared to the general population[2]. In a large meta-analysis that included 40 studies primarily from the United States and China with more than 900000 participants, COVID-19 patients with chronic liver disease had higher odds of developing a severe infection [pooled odds ratio (OR) = 2.44; 95% confidence interval (CI): 1.89-3.16] and mortality (pooled OR = 2.35; 95%CI: 1.85-3.00) when compared to COVID-19 patients without chronic liver disease[3].
In contrast, literature evaluating COVID-19 effects on liver transplant recipients did not consistently demonstrate worse outcomes[4,5]. A systematic review of 1522 liver transplantation recipients who were infected with COVID-19 did not find a difference in cumulative incidence in mortality compared to patients who were not liver transplantation recipients. Additionally, the review did not find a difference in mortality between non-liver transplantation recipients vs liver transplantation recipients in patients who received a liver transplantation within 1 year vs 1-year post-transplant[4]. Still, the COVID-19 pandemic added significant challenges and restrictions to transplant policies and organ allocation. The healthcare structure was overwhelmed by critically ill patients with COVID-19 resulting in diversion of medical resources away from liver transplantation[6]. Furthermore, early concerns of patients contracting severe COVID-19 infection in light of immunosuppression discouraged their use. These uncertainties culminated in initial hardships in the overall management of patients with chronic liver disease thereby negatively affecting liver transplantation.
To revive the liver transplant process and provide organs for those in dire need, significant changes in liver transplant practice have been implemented per major transplant societies’ recommendations[7]. After an initial drop in the number of liver transplants performed in the United States in early 2020, a quick recovery in the latter half of 2020 and early 2021 followed[8]. This was likely due to better understanding of COVID-19, improved adherence to infection prevention recommendations, and replenished healthcare resources. Later, COVID-19 vaccination emerged as an efficient and cost-effective preventive strategy for patients with chronic liver disease, further helping to offset COVID-19-related shortcomings[9].
This comprehensive review discussed the major aspects and effects of the pandemic on the liver transplant process as a whole.
The liver is prone to direct COVID-19 infection because of expressed angiotensin-converting enzyme 2 receptor in the hepatobiliary epithelial cells. Although not fully understood, it is hypothesized that binding of the virus spike protein to angiotensin-converting enzyme 2 receptors allows viral entry and subsequent host cellular damage[10]. Indirect hepatotoxicity may occur due to hemodynamic instability, drug-induced liver damage, COVID-19-induced immune dysfunction, coagulopathy, and intestinal dysbiosis[11]. Moreover, since the angiotensin-converting enzyme 2 receptors are also expressed on cholangiocytes, some suggest that COVID-19 infection may worsen cholestasis in patients with primary biliary cholangitis and primary sclerosing cholangitis[12].
Similar to patients without underlying liver disease, patients with cirrhosis typically develop mildly elevated aminotransferase levels (< 5 times the upper limit of normal); nevertheless, severe acute hepatitis and even acute liver failure have also been reported[13]. Commonly, a pattern of aspartate transaminase greater than alanine transaminase is associated with disease severity[14]. Likewise, a low albumin level is linked to worse COVID-19 disease severity. It is unknown if this is just a marker of disease severity or merely a risk factor for severe disease.
In patients with cirrhosis, COVID-19 infection may result in hepatic decompensation, similar to other infections. In a retrospective, multicenter study from 13 Asian countries, 29% of COVID-19 patients with chronic liver disease presented with hepatic decompensation[15].
Liver biopsy in patients with COVID-19-induced liver injury is nonspecific. Histopathological changes include microvesicular steatosis, portal and lobular activity, and zone 3 focal necrosis[16,17]. In an autopsy-based series that included 48 cases, liver histologic findings included variable degrees of parenchymal lymphocytic infiltration in almost all patients and hepatic vascular alterations in some cases[18]. In our opinion, performing a liver biopsy does not add diagnostic benefit unless an alternative diagnosis is considered.
A significant body of research suggests increased mortality in COVID-19 patients with chronic liver disease. According to a multicenter, observational study from the United States, the presence of cirrhosis in those with COVID-19 infection was associated with higher mortality when compared to those without cirrhosis (relative risk: 4.6, 95%CI: 2.6-8.3)[19]. In a database study of COVID-19 patients with chronic liver disease, after adjusting for relevant confounders, the presence of cirrhosis was associated with higher 30-d mortality compared to those without cirrhosis (8.9% vs 1.7%; 95%CI: 2.91-3.77)[20]. A subsequent cohort study found that COVID-19-related mortality increased with cirrhosis progression; patients with Child-Pugh class B or C cirrhosis were found to have increased mortality (OR = 4.90, 95%CI: 1.16-20.61 and OR = 28.07, 95%CI: 4.42-178.46, respectively). Mortality was mostly attributed to pulmonary complications (79%), whereas liver-related mortality was seen in 12% of patients[21].
A rare but important long-term sequela of severe COVID-19 is cholangiopathy, at times resulting in progressive biliary destruction and liver failure requiring liver transplantation[22]. In a retrospective study by Faruqui et al[22] on patients hospitalized for severe COVID-19, 12 patients ultimately developed some degree of cholangiopathy defined by evidence of cholestasis (alkaline phosphatase ≥ 3 upper limit of normal) or radiologic biliary abnormalities. The majority were male (92%) with a mean time of cholangiopathy diagnosis of 118 d from COVID-19. One patient underwent liver transplantation.
COVID-19 management in patients with cirrhosis follows the same supportive routine measures for the general population, including the use of COVID-specific drug therapy. Deranged liver biochemistries are not an absolute contraindication to using therapy such as remdesivir. Remdesivir use alone can cause a further elevation in liver enzymes (up to 10 times the baseline)[23]. However, its use is discouraged if the alanine transaminase level is ≥ 5 upper limit of normal[24]. Although Paxlovid (combination nirmatrelvir and ritonavir) trials did not show any concerns about its use in cirrhotic patients, it is extensively metabolized by liver cytochrome P450 enzymes. Thus, this drug harbors the risk of accumulation and toxicity in patients with decompensated cirrhosis. We think this medication should be used judicially and in collaboration with infectious disease specialists.
The use of COVID-19 monoclonal antibodies is encouraged early in the infection course in cirrhosis. This is particularly important because cirrhotic patients tend to mount suboptimal humoral responses to COVID-19 vaccination and likely infection as well. Other immunomodulatory COVID-19 therapies include JAK inhibitors (baricitinib) and IL-6 receptor antagonists (tocilizumab)[25]. We learned from baricitinib use in rheumatological disorders that it may cause liver biochemistry abnormalities, and caution and regular monitoring should be exercised[26]. Additionally, the risk of hepatitis B virus reactivation has been documented with both baricitinib and tocilizumab; therefore, obtaining hepatitis B serology before treatment initiation is warranted to assess the need for prophylactic nucleoside analogue therapy[27].
Adherence to general preventive measures to avoid COVID-19 in patients with cirrhosis is paramount. These include social distancing, hand hygiene, proper use of personal protective equipment, and telemedicine clinic visits[28]. It is strongly recommended for patients with cirrhosis to receive the COVID-19 vaccine[9]. In a prospective, multicenter study aimed at comparing the humoral response to the COVID-19 vaccine between patients with chronic liver disease (437 individuals) and healthy controls (144 individuals), chronic liver disease was associated with lower rates of post-vaccination COVID-19 antibody positivity (77% vs 90%, P = 0.008). The rate of antibody positivity was similar among patients with chronic liver disease regardless of cirrhosis presence or even decompensation (P = 0.894)[9]. These findings suggest additional doses of COVID-19 vaccine might be warranted in this high-risk patient population to achieve adequate immunity[29]. In a propensity score-matched cohort study of United States veterans with cirrhosis, receiving only one dose of the COVID-19 vaccine (either Pfizer BNT162b2 mRNA or a Moderna mRNA-1273) resulted in a 64.8% reduction in COVID-19 infection and 100% prevention of hospitalization or mortality due to COVID-19 infection after 28 d[30].
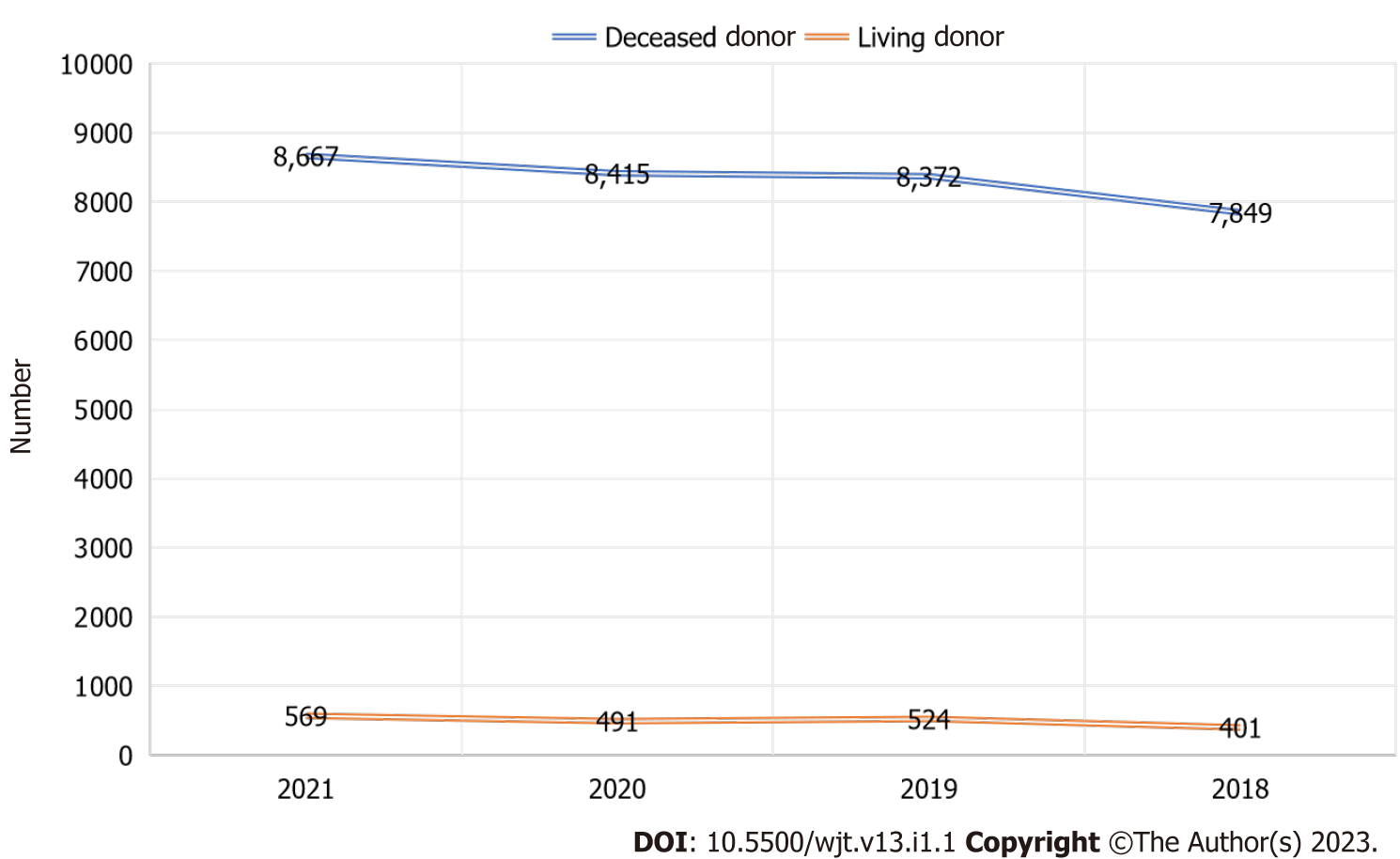
The United States performs the most liver transplants worldwide per year. The second-leading country in the number of liver transplants performed is China, followed closely by Brazil[31]. Currently, over 9000 liver transplants are performed every year in the United States. For the past 9 years, the number of annual liver transplants has increased steadily, setting annual records[32]. Despite the challenges of the COVID-19 pandemic, the year 2020 was no different, as we witnessed an increase of 10.1% in deceased donor liver transplantation. The major impact the pandemic had was on living donor liver transplants, which suffered a significant decline of 22% between February and April 2020. The liver transplants performed in the United States between 2018-2021 is depicted in Figure 1[33].
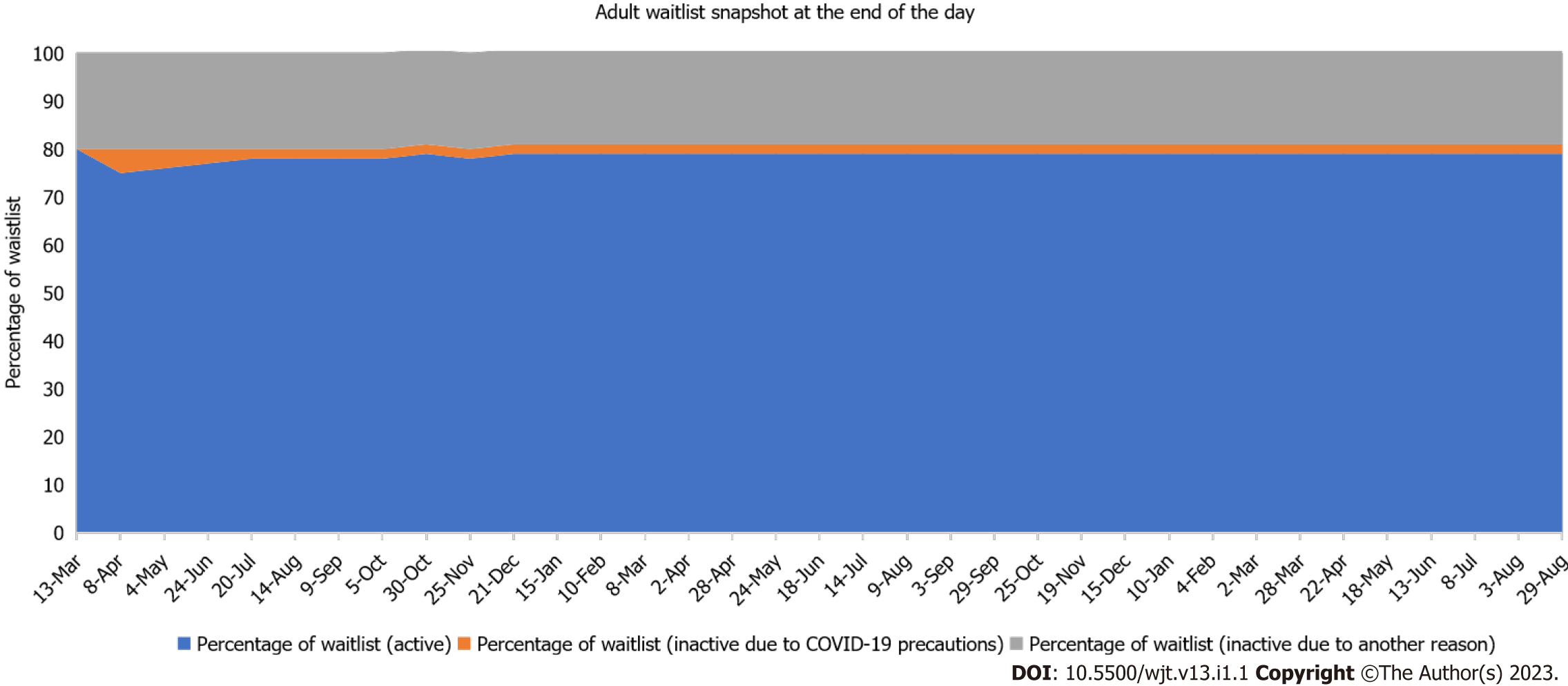
During the height of the pandemic, non-urgent liver transplantation was deferred to conserve hospital resources. Since the Centers for Medicare and Medicaid services have classified organ tran
On the other hand, the recent changes in the organ allocation system helped offset some of the COVID-19 challenges. As a replacement for geographic areas, nautical miles are now utilized. Priority for receiving organs is triaged by medical urgency within a concentric circle radius of 150, 250, and then 500 nautical miles. While this new policy is imperfect as it better serves well-occupied areas in the center of the United States when compared to other coastal areas, it indeed improved access to solid organs across the country[37].
The American Society of Transplantation guidelines and Organ Procurement and Transplantation Network formulated guidelines on using COVID-19-positive donors. The consensus early in the pandemic was to avoid liver transplants in active donor-positive situations due to the risk of developing acute respiratory distress syndrome or COVID-19-related thrombosis. However, given the high prevalence of the virus in the community, some transplant centers started transplanting patients with donor positivity in emergent situations.
In one Italian study, 17 liver transplant patients were studied for more than 1 year from their transplant with a COVID-19-positive donation. One patient tested positive 21 d after transplantation. However, no patients experienced severe complications from COVID-19[38]. Of note, post-transplant immunosuppression was not adjusted, and there was no use of anti-COVID-19 therapy after the transplant. It is important to mention that this study was limited by the small sample size but provided hope for patients receiving a liver transplant from COVID-19-positive donors.
Concern regarding the blood-borne transmission of COVID-19 during liver transplantation discouraged living donor liver transplants during the initial period of the pandemic. However, studies showed that, unlike lung transplant recipients, the risk of transmitting donor-derived COVID-19 infection was not likely in liver transplant patients[39]. Blood-borne transmission does not pose much risk as the degree of COVID-19 viremia is low[40].
Current literature also suggests a higher risk of contracting COVID-19 infection among healthcare providers compared to the general population[41]. Organ donation from a COVID-19-positive patient also has the risk of exposing all transplant team health professionals who typically work closely with other high-risk cirrhotic patients. On the occasion of transmitted COVID-19 infection to medical staff, self-isolation will exert further strain on healthcare staffing and resources. It is therefore imperative to assess the risks and benefits of using organs from a potential COVID-19-infected donor.
Liver transplant centers across the nation have developed their protocols and policies to manage listed patients having COVID-19 infection[42]. This is to ensure maximum benefits for their patients and to cause no harm. In our center, for example, listed patients who are infected with COVID-19 are temporarily inactivated until they are symptom-free and 3 wk have elapsed since their diagnosis. Moreover, we often perform a contrast-enhanced computed tomography of the chest and pulmonary function tests if the patient had respiratory symptoms prior to reactivation. On the contrary, if the patient did not develop any respiratory symptoms, they are reactivated without any further testing.
Fair allocation of liver grafts, possibly the scarcest organ of all, remains an ethical question in those with active COVID-19 infection[43]. The main principle of allocation is to achieve the greatest good for both the patient and the community. While benefiting those needing livers is likely to result in improved survival and health of patients and grafts, real risks of increased mortality or significant surgical complications exists in those with active COVID-19 infection. Considering the uncertainty regarding outcomes of liver transplant in candidates with active COVID-19 infection, these vital organs are better redirected to more suitable candidates with a higher chance of benefit pending infection resolution[44].
Additionally, it is important to note that exposure of health care providers to infected transplant patients continues to significantly burden hospital structures throughout the country. The ethical principles of justice and utility should dictate the just allocation of organs to those who would get the greatest benefit[45].
The post-transplant risk of COVID-19 is the risk of acquiring severe infection as a solid organ recipient on chronic immunosuppression with an inherent risk of prolonged viral shedding. The Spanish Society of Liver Transplantation found that liver transplant recipients may have double the risk of acquiring COVID-19 within an epidemic scenario (standardized incidence ratio: 191.2; 95%CI: 190.3-192.2) as compared to an age and sex-matched cohort[46]. A 2022 prospective double-center study from southern Italy followed 30 liver transplant recipients who were infected with COVID-19 and found that liver transplant recipients were more often symptomatic but did not have an increased risk for hospitalization or mortality[47].
The clinical presentation reported in observational studies included fever (61.4%), cough (58.6%), and dyspnea (36.2%)[48]. Webb et al[49] reported that gastrointestinal symptoms were common (27.9%). Interestingly, the liver transplant recipients had more gastrointestinal symptoms compared to the control group (30% vs 12%, P < 0.0001), whereas no significant difference was observed in respiratory symptoms. The same study compared outcomes of COVID-19 infection between those who underwent liver transplant (124 patients) and matched cohorts (474 patients). No difference in hospitalization (82% vs 76%, P = 0.106) or need for intensive care unit (31% vs 30%, P = 0.837) were observed. Overall, 28 (19%) patients in the liver transplant cohort died compared to 167 (27%) patients in the matched cohort (P = 0.046).
In a meta-analysis and systematic review by Kulkarni et al[4], which included 18 studies with a total of 1522 COVID-19-infected liver transplant recipients, there was no difference in mortality between liver transplant and non-liver transplant COVID-19 patients up to 1 year post-transplant. Approximately 23% of liver transplant patients had severe COVID-19 infection. Regarding immunosuppression, 71% and 49% of patients were on tacrolimus and mycophenolate mofetil, respectively. More than half of these patients required some adjustment of their immunosuppression medication. This analysis suggested that COVID-19-infected liver transplant recipients are not at an increased risk of poor outcomes.
The severity of COVID-19 infection often dictates the management of immunosuppressive agents. For example, those with a mild disease not requiring oxygen therapy may be managed as an outpatient without adjustment in their immunosuppressive agents. In contrast, liver recipient patients with moderate-to-severe COVID-19 infection are often managed in the hospital. Guidance for managing these patients stems largely from expert opinions. It is generally advised to lower the cumulative degree of immunosuppression, particularly mycophenolate. While steroid dose generally requires no modification during an active infection, calcineurin inhibitor drug monitoring is recommended to avoid acute kidney injury.
Other agents used in treating COVID-19 infection include oral antivirals such as molnupiravir and Paxlovid. The former is likely safe and effective in liver transplant recipient patients and considered a drug of choice by many hepatologists[50]. Paxlovid strongly interacts with calcineurin and mammalian target of rapamycin inhibitors. Therefore, concomitant use is prohibited[51]. In a single-center, retrospective study that included liver and kidney transplant recipients, COVID-19 monoclonal antibody treatment (casirivimab-imdevimab or bamlanivimab) reduced hospitalization from 32% to 15% (P = 0.045) with no mortality (13% vs 0%, P = 0.04)[52].
The Food and Drug Administration issued an Emergency Use Authorization in January of 2022 for Evusheld (tixagevimab and cilgavimab), a long-acting monoclonal antibody for pre-exposure prophylaxis of COVID-19, in patients with moderate-to-severe immune suppression including those who received a solid organ transplant[53]. This is an appealing preventive option for high-risk liver transplant recipients.
It is important to note that the quality of the literature presented in this review was affected by the evolving understanding of the COVID-19 virus and the ensuing rapid changes in liver society guidelines in response. Moreover, most of the discussed studies were limited by small sample size and retrospective, single center designs affecting the generalizability of their outcomes. In addition, the changes in liver allocation policies that occurred midway through the pandemic may have confounded the overall number of liver transplants performed in the United States.
While COVID-19 infection appears to be poorly tolerated in patients with chronic liver disease, liver transplant recipients, despite immunosuppression, have a similar rate of complications and mortality when compared to the general population. It is imperative to recognize important drug-drug interactions in liver transplant patients, notably Paxlovid interaction with calcineurin inhibitors to avoid drug toxicity. We also advocate for wider utilization of monoclonal antibody pre-exposure prophylaxis of COVID-19 infection in liver transplant patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tiejun W, China; Ulasoglu C, Turkey S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1867] [Reference Citation Analysis (0)] |
| 2. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 107] [Article Influence: 26.8] [Reference Citation Analysis (2)] |
| 3. | Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis. 2022;19:E53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 30] [Reference Citation Analysis (0)] |
| 4. | Kulkarni AV, Tevethia HV, Premkumar M, Arab JP, Candia R, Kumar K, Kumar P, Sharma M, Rao PN, Reddy DN. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. EClinicalMedicine. 2021;38:101025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 5. | Nickerson AM, Sobotka LA, Kelly SG. PRO: Liver Transplantation in the Times of COVID-19: Patients with COVID-19 Infection Should Undergo Liver Transplantation. Clin Liver Dis (Hoboken). 2021;18:230-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kumura K, Nishida S, Sogawa H, Veillette G, Bodin R, Wolf DC, Dhand A. Inferior Liver Transplant Outcomes during early COVID-19 pandemic in United States. J Liver Transplant. 2022;7:100099. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 394] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 8. | Organ transplants bounce back to near pre-COVID-19 Levels | UNOS. [Internet] [accessed 12 September 2022]. Available from: https://unos.org/news/transplants-bounce-back-to-near-pre-covid-19-levels/. [Cited in This Article: ] |
| 9. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 125] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 10. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14113] [Cited by in F6Publishing: 14170] [Article Influence: 3542.5] [Reference Citation Analysis (0)] |
| 11. | Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 226] [Article Influence: 75.3] [Reference Citation Analysis (2)] |
| 12. | Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021;56:218-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12405] [Article Influence: 3101.3] [Reference Citation Analysis (1)] |
| 14. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28564] [Article Influence: 7141.0] [Reference Citation Analysis (3)] |
| 15. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 184] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 16. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 473] [Reference Citation Analysis (0)] |
| 17. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5228] [Cited by in F6Publishing: 5537] [Article Influence: 1384.3] [Reference Citation Analysis (2)] |
| 18. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 19. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 20. | Ge J, Pletcher MJ, Lai JC; N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487-1501.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 21. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 181] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 22. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 23. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012 . [PubMed] [Cited in This Article: ] |
| 24. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827-1837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 987] [Cited by in F6Publishing: 915] [Article Influence: 228.8] [Reference Citation Analysis (0)] |
| 25. | Ngamprasertchai T, Kajeekul R, Sivakorn C, Ruenroegnboon N, Luvira V, Siripoon T, Luangasanatip N. Efficacy and Safety of Immunomodulators in Patients with COVID-19: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Infect Dis Ther. 2022;11:231-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, Beattie SD, Koch AE, Cardillo TE, Rooney TP, Macias WL, de Bono S, Schlichting DE, Smolen JS. Baricitinib in Patients with Refractory Rheumatoid Arthritis. N Engl J Med. 2016;374:1243-1252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 415] [Cited by in F6Publishing: 423] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 27. | Campbell C, Andersson MI, Ansari MA, Moswela O, Misbah SA, Klenerman P, Matthews PC. Risk of Reactivation of Hepatitis B Virus (HBV) and Tuberculosis (TB) and Complications of Hepatitis C Virus (HCV) Following Tocilizumab Therapy: A Systematic Review to Inform Risk Assessment in the COVID-19 Era. Front Med (Lausanne). 2021;8:706482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Güner R, Hasanoğlu I, Aktaş F. COVID-19: Prevention and control measures in community. Turk J Med Sci. 2020;50:571-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 281] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 29. | Ai J, Wang J, Liu D, Xiang H, Guo Y, Lv J, Zhang Q, Li J, Zhang X, Li Q, Liang J, Guo X, Feng Y, Liu L, Qin W, Wang X, Rao W, Tian Q, Zhang Y, Xie F, Jiang S, Yan Y, Qiu Y, Wu H, Hou Z, Zhang N, Zhang A, Ji J, Yang J, Huang J, Zhao Z, Gu Y, Bian L, Zhang Z, Zou S, Ji H, Ge G, Du X, Hou A, Zhu Y, Cong Q, Xu J, Zu H, Wang Y, Yan Z, Yan X, BianBa Y, Ci Q, Zhang L, Yang S, Gao X, Zhong L, He S, Liu C, Huang Y, Liu Y, Xu D, Zhu Q, Xu X, Lv M, Zhang W, Qi X. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin Gastroenterol Hepatol. 2022;20:1516-1524.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 30. | John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, Labrada M, Baracco G, Dahman B. Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis. JAMA Intern Med. 2021;181:1306-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Trotter JF, Cárdenas A. Liver transplantation around the world. Liver Transpl. 2016;22:1059-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | All-time records again set in 2021 for organ transplants, organ donation from deceased donors - UNOS. [Internet] [accessed 12 September 2022]. Available from: https://unos.org/news/2021-all-time-records-organ-transplants-deceased-donor-donation/. [Cited in This Article: ] |
| 33. | National data - OPTN. [Internet] [accessed 12 September 2022]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. [Cited in This Article: ] |
| 34. | The Centers for Medicare and Medicaid Services. Non-Emergent, Elective Medical Services, and Treatment Recommendations. [Internet] [accessed 12 September 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/index.html. [Cited in This Article: ] |
| 35. | Craig-Schapiro R, Salinas T, Lubetzky M, Abel BT, Sultan S, Lee JR, Kapur S, Aull MJ, Dadhania DM. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant. 2021;21:1576-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 36. | COVID-19 Resources for Organ Transplants and Donations | UNOS. [Internet] [accessed 12 September 2022]. Available from: https://unos.org/covid/. [Cited in This Article: ] |
| 37. | New national liver and intestinal organ transplant system in effect - OPTN. [Internet] [accessed 12 September 2022]. Available from: https://optn.transplant.hrsa.gov/news/new-national-liver-and-intestinal-organ-transplant-system-in-effect/. [Cited in This Article: ] |
| 38. | Romagnoli R, Gruttadauria S, Tisone G, Maria Ettorre G, De Carlis L, Martini S, Tandoi F, Trapani S, Saracco M, Luca A, Manzia TM, Visco Comandini U, De Carlis R, Ghisetti V, Cavallo R, Cardillo M, Grossi PA. Liver transplantation from active COVID-19 donors: A lifesaving opportunity worth grasping? Am J Transplant. 2021;21:3919-3925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Chang L, Yan Y, Wang L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus Med Rev. 2020;34:75-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 320] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 40. | Kute VB, Fleetwood VA, Meshram HS, Guenette A, Lentine KL. Use of Organs from SARS-CoV-2 Infected Donors: Is It Safe? Curr Transplant Rep. 2021;8:281-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Andersen MP, Østergaard L, Phelps M, Butt JH, Køber L, Gislason G, Christensen HC, Torp-Pedersen C, Schou M, Fosbøl EL, Kragholm K. Risk of coronavirus disease 2019 (Covid-19) contraction and severe infection in home- or healthcare professionals. J Infect. 2021;83:e12-e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Gonzalez AJ, Kapila N, Thomas E, Pinna A, Tzakis A, Zervos XB. Managing liver transplantation during the COVID-19 pandemic: A survey among transplant centers in the Southeast United States. World J Hepatol. 2021;13:2161-2167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 43. | Ibrahim B, Dawson R, Chandler JA, Goldberg A, Hartell D, Hornby L, Simpson C, Weiss MJ, Wilson LC, Wilson TM, Fortin MC. The COVID-19 pandemic and organ donation and transplantation: ethical issues. BMC Med Ethics. 2021;22:142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Ethics - Ethical Principles in the Allocation of Human Organs - OPTN. [Internet] [accessed 12 September 2022]. Available from: https://optn.transplant.hrsa.gov/professionals/by-topic/ethical-considerations/ethical-principles-in-the-allocation-of-human-organs/. [Cited in This Article: ] |
| 45. | Parent B, Caplan A, Mehta SA. Ethical considerations regarding COVID-19 vaccination for transplant candidates and recipients. Clin Transplant. 2021;35:e14421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 234] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 47. | Guarino M, Cossiga V, Loperto I, Esposito I, Ortolani R, Fiorentino A, Pontillo G, De Coppi L, Cozza V, Galeota Lanza A, Di Costanzo GG, Picciotto FP, Morisco F. COVID-19 in liver transplant recipients: incidence, hospitalization and outcome in an Italian prospective double-centre study. Sci Rep. 2022;12:4831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Becchetti C, Gschwend SG, Dufour JF, Banz V. COVID-19 in Liver Transplant Recipients: A Systematic Review. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 50. | Fischer WA 2nd, Eron JJ Jr, Holman W, Cohen MS, Fang L, Szewczyk LJ, Sheahan TP, Baric R, Mollan KR, Wolfe CR, Duke ER, Azizad MM, Borroto-Esoda K, Wohl DA, Coombs RW, James Loftis A, Alabanza P, Lipansky F, Painter WP. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14:eabl7430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 196] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 51. | Paxlovid Drug-Drug Interactions | COVID-19 Treatment Guidelines. [Internet] [accessed 12 September 2022]. Available from:[Internet] [accessed 12 September 2022]. Available from: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/. [Cited in This Article: ] |
| 52. | Ahearn AJ, Thin Maw T, Mehta R, Emamaullee J, Kim J, Blodget E, Kahn J, Sher L, Genyk Y. A Programmatic Response, Including Bamlanivimab or Casirivimab-imdevimab Administration, Reduces Hospitalization and Death in COVID-19 Positive Abdominal Transplant Recipients. Transplantation. 2022;106:e153-e157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 53. | Tixagevimab and Cilgavimab (Evusheld) for Pre-Exposure Prophylaxis of COVID-19. JAMA. 2022;327:384-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 85] [Article Influence: 42.5] [Reference Citation Analysis (0)] |