Copyright
©The Author(s) 2017.
World J Transplant. Jun 24, 2017; 7(3): 179-192
Published online Jun 24, 2017. doi: 10.5500/wjt.v7.i3.179
Published online Jun 24, 2017. doi: 10.5500/wjt.v7.i3.179
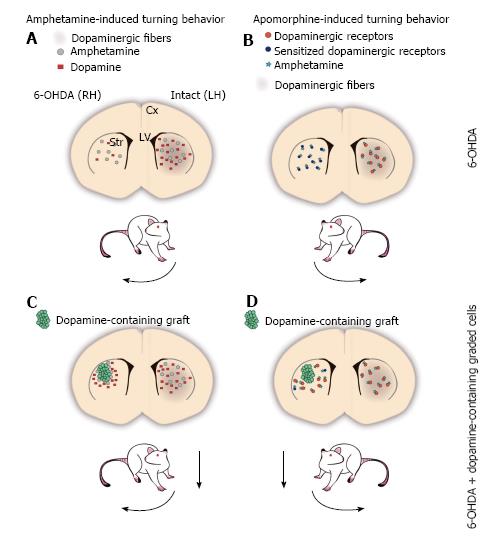
Figure 1 The 6-OHDA rat model of Parkinson’s disease.
A-D: Schemes of a coronal representation of the rat brain. Dopaminergic fibers are depicted with brown shadowing, which is lacking in the 6-OHDA-lesioned hemisphere; A: Amphetamine (grey circles) administration promotes the release of dopamine (red squares) from the intact dopaminergic terminals of the striatum, disproportionally increasing dopamine concentration in the non-lesioned side relative to the lesioned side, as the latter contains fewer (or none at all) dopaminergic terminals. The asymmetry in extracellular dopamine levels between both hemispheres induces the stereotypical behavior known as circling or turning behavior, ipsilateral to the lesioned side (curved arrow next to the rat); B: Apomorphine is a dopaminergic receptor agonist that can activate postsynaptic dopamine receptors in the striatum (orange circles). 6-OHDA-induced dopaminergic denervation in one hemisphere of the striatum, results in postsynaptic supersensitivity to dopamine in the lesioned side (sensitized dopamine receptors are represented as dark blue circles), such that apomorphine (teal stars) stimulation increases the activity in the lesioned side to a greater extent than in the non-lesioned side. The supersensitivity effect promotes that lesioned animals turn contralateral to the lesioned side after apomorphine administration (curved arrow); C: Amphetamine stimulates dopamine-containing cells (green circles) grafted into the denervated striatum increasing extracellular dopamine concentration in the lesioned side, which leads to a decrement in motor asymmetry (dashed arrow); D: Grafted cells that release dopamine decrease the supersensitivity effect on the lesioned hemisphere, normalizing the response to dopamine or agonists relative to the non-lesioned side. Thus, after apomorphine administration, grafted animals decrease their turn number (dashed arrow). Cx: Cortex; LH: Left hemisphere; LV: Lateral ventricles; RH: Right hemisphere; Str: Striatum.
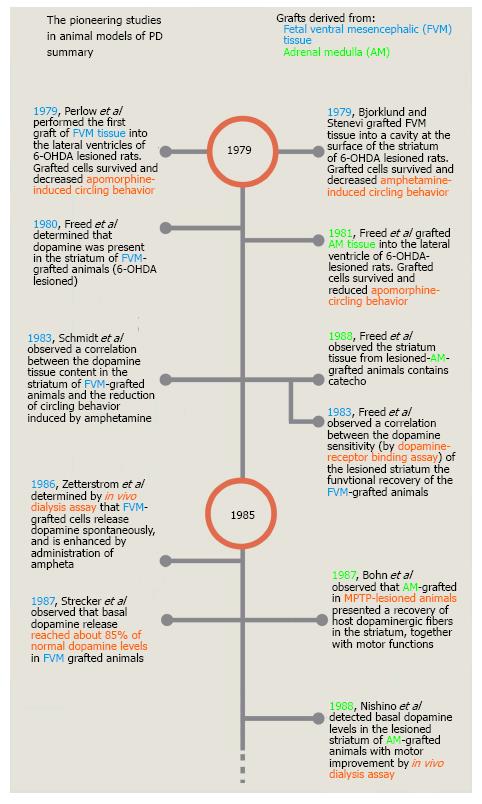
Figure 2 Timeline of the pioneering studies on cell transplantation in animal models of Parkinson’s disease.
This timeline shows only a few of the studies performed during the first 10 years of cell grafting in animal models of PD. Most of these studies were selected because they were the first published reports of either the use of a new animal model of PD, a site of grafting, a type of cell or a specific technique. PD: Parkinson’s disease; FVM: Fetal ventral mesencephalic cells; AM: Adrenal medulla.
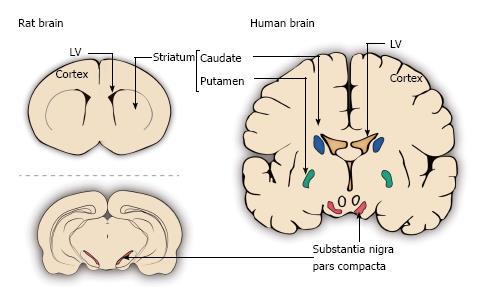
Figure 3 Schematic representation of different sites in the rat and human brains used for grafting in Parkinson’s disease.
The depicted grafting sites include the lateral ventricles (LV), the striatum (in rat) or caudate nucleus and putamen (in human) and the substantia nigra pars compacta. The above schemes are coronal sections of the rat striatum and human caudate (blue) and putamen (green) together with the substantia nigra pars compacta (red). The scheme below is a coronal section at the level of the rat substantia nigra pars compacta (red).
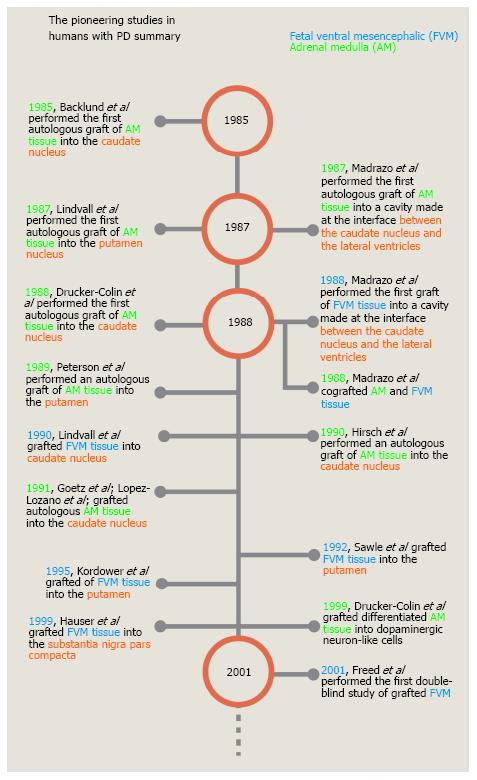
Figure 4 Timeline of the pioneering studies on cell transplantation in human patients with Parkinson’s disease.
This timeline shows only a few of the studies performed during the first 15 years of cell grafting in patients with PD. Most of them are the first published reports in which, a new site of grafting or a new type of cell were used. PD: Parkinson’s disease.
- Citation: Boronat-García A, Guerra-Crespo M, Drucker-Colín R. Historical perspective of cell transplantation in Parkinson’s disease. World J Transplant 2017; 7(3): 179-192
- URL: https://www.wjgnet.com/2220-3230/full/v7/i3/179.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i3.179