Published online Jul 12, 2015. doi: 10.5499/wjr.v5.i2.59
Peer-review started: July 1, 2014
First decision: July 18, 2014
Revised: March 24, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: July 12, 2015
Antiphospholiipid syndrome (APS) is an autoimmune disease characterized by the pathological action of antiphospholipid antibodies (aPL), that leads to recurrent pregnancy loss and thrombosis. Despite limited evidence, it is clear that there are both inherited and acquired components of the ontogeny of these antibodies. Animal genetic studies and human familial and population studies highlight the influence of genetic factors in APS, particularly human leukocyte antigen associations. Similarly, both animal and human studies have reported the importance of acquired factors in APS development and infectious agents in particular have a great impact on aPL production. Bacterial and viral agents have been implicated in the induction of autoimmune responses by various mechanisms including molecular mimicry, cryptic autoantigens exposure and apoptosis. In this review we highlight the latest updates with regards to inherited and acquired factors leading to the manufacturing of pathogenic antibodies and APS.
Core tip: This article reviews the most up to date theories regarding the production of pathogenic antiphospholipid antibodies (aPL) in antiphospholipid syndrome. It focuses on both the genetic and environmental aspects related to aPL production. The genetic factors highlighted include human leukocyte antigen (HLA) and non-HLA associations and where available, data linking genes to clinical manifestations is presented. The key infectious agents linked to the formation of pathogenic aPL and those mechanisms by which these agents induce a break in immune tolerance are also discussed.
- Citation: Willis R, Gonzalez EB. Pathogenetic mechanisms of antiphospholipid antibody production in antiphospholipid syndrome. World J Rheumatol 2015; 5(2): 59-68
- URL: https://www.wjgnet.com/2220-3214/full/v5/i2/59.htm
- DOI: https://dx.doi.org/10.5499/wjr.v5.i2.59
Antiphospholipid syndrome (APS) is a mutisystemic autoimmune disease, whose pathology is driven by the action of antiphospholipid antibodies (aPL), and is characterized by recurrent thrombosis and pregnancy morbidity[1]. These antibodies are heterogenous, have numerous antigenic targets and interact with numerous negatively charged phospholipids (PLs) and protein complexes. However, during the 1990s, several groups showed simultaneously that β2-glycoprotein I (β2GPI), and prothrombin, are the major antigenic targets of aPL. More than 90% of binding activity in APS patients target these 2 antigens[2-4].
The decades since APS was first described have seen an increased understanding of the pathological mechanisms of aPL that lead to the various clinical manifestations in the disease[5]. In contrast, relatively little has been uncovered regarding the ontogeny of pathogenic aPL. However, various pathogenetic processes have been proposed based on the available evidence and what seems clear is that both inherited and acquired factors play roles in the initial induction of pathogenic aPL in APS patients.
Correlations of several genetic markers with the production of aPL and APS characteristic manifestations, such as thrombosis, have been highlighted using several APS human and animal studies. Major Histocompatibility Complex (MHC) genes could potentially affect both pathological aPL development and also the expression of disease in APS patients[6]. Other studies highlight the effect of classical thrombogenic genetic risk factors on disease phenotype in APS patients[7]. These various genetic markers are likely to confer a baseline risk with regards to aPL production and APS development, while exposure to various environmental factors augment and intensify this risk, in essence inducing the break in tolerance needed for autoantibody production[8]. One of these key environmental factors seems to be infectious agents and indeed, most of the work done to elucidate the effect of environmental factors on aPL production has centered on viral and bacterial infectous agents[8]. As was stated above, aPL represent a varied group of antibodies that target various antigens and clinical reports indicate that not all these antibodies cause disease. It is therefore very likely that only a select group of environmental agents, most likely infectious agents and even then only a select few viral or bacterial entities, are important in disease development[8,9]. However, there is some limited evidence for other environmental factors such as malignancies, vaccinations and drugs being associated with aPL production[8]. In this brief review, we outline the latest updates regarding proposed inherited and acquired factors contributing to the formation of pathogenic aPL.
The first evidence of a genetic component to the production of pathogenic aPL in APS was provided by studies in mice. In NZW x BXSB F1 (W/B F1) male mice, the spontaneous production of pathogenic aPL, namely IgG aCL that display β2GPI-dependent binding to cardiolipin, has been reported[10]. Indeed, these W/B F1 male mice are SLE-prone mice that, in addition to aCL, develop autoantibodies to negatively charged PLs such as phosphatidylserine (PS) and phosphatidylinositol, circulating immune complexes, and nephritis. Thrombocy topenia and myocardial infarction on the background of degenerative coronary vascular disease is often found in these mice, which is akin to features of SLE and APS[10-12]. The failure of either central or peripheral T-cell tolerance mechanisms is an important aspect of the production of self-reactive autoantibodies in autoimmune diseases. Gene analysis showed that the genes responsible for the development of pathogenic aCL in these mice used certain VH and VK genes preferentially, while those for non-pathogenic aCL utilized random V gene combinations. This indicates antigen-driven rather than germ-line encoded antibody production[13]. In this study, the pathogenic aCL showed a 91.5% homology to a known germ-line VH gene, suggesting that the pathogenic aPL were generated by somatic mutations. Similarly, in MRL-lpr/lpr mice (lupus prone mice), numerous somatic mutations in the VH region of a gene encoding a monoclonal aCL compared to the related germ-line VH gene were noted, indicating antigen-driven stimulation and a possible failure in peripheral tolerance mechanisms[14]. ACL are also produced in normal C57BL/6J mice, with estrogen treatment increasing the incidence and levels of these antibodies, underscoring the role that environmental factors such as hormones may play in modifying genetic susceptibility in APS patients[15]. However, the aCL that are produced in these mice are not β2GPI dependent but instead show diminished binding to cardiolipin in the presence of the β2GPI cofactor[16]. Interestingly, an additional lupus murine model (NZW x NZB F1 mice) failed to produce aCL despite the production of other autoantibodies such as anti-dsDNA[17].
A subsequent analysis of the clinical features present in NZW and BXSB mice and their offspring revealed that similar disease phenotypes were seen in both male BXSB parental mice and the male F1 progeny BXSB x NZW but these features were less frequent and intense in the parental mice. In stark contrast, the typical clinical features were not expressed in NZW parent female mice or female F1 BXSB x NZW female progeny[18]. These results possibly indicated that BXSB genetic markers determine the disease expression while genes found in the NZW mice served to upregulate or modify the expression of manifestations of APS in their offspring. An additional consideration is that modifying alleles such as BXSB Y-linked autoimmune accelerator gene may be an important factor in disease expression[18-20]. A mapping of the BXSB alleles that contributed to the development of aCL, anti-platelet antibodies, thrombocytopenia, and mycocardial infarction was subsequently achieved by analysis of the genome, focusing on microsatellite markers in NZW x (NZWxBXSB) male F1 backcross offspring[18]. This genetic evaluation demonstrated that the complete expression of each feature was determined by the complementary activity of two independently segregating major dominant alleles. Full genetic concordance existed for antiplatelet antibodies and thrombocytopenia but different combinations of two dominant alleles acting independently were responsible for other features, suggesting that no single genetic factor can explain the pathogenesis of APS[18].
The first direct evidence of certain MHC II alleles being involved in the induction of pathogenic aPL and development of APS clinical manifestations came from Papalardo et al[21] Utilizing a β2GPI–induced aPL production in mice, this group showed that thrombogenic aPL production and tissue factor upregulation occur in wild type mice after immunization with human β2GPI but do not occur in MHC-II knockout [MHC(-/-)] mice. Furthermore, the production of pathogenic aPL after inoculation with β2GPI was restored in MHC(-/-) that were modified to express human DQ6, DR4 or DQ8 genes. Interestingly, the quantity of pathogenic aPL that was produced varied among these 3 transgenic mouse groups. These studies confirm the involvement of certain haplotypes in the induction of aPL as well as their varied importance[21].
Associations between several human leukocyte antigen (HLA)-DR and DQ haplotypes and aPL development have been reported but frequent logistical issues such as inappropriately matched control populations and small sample populations make interpretation problematic[6,7]. The underlying problem is the difficulty in defining disease phenotypes appropriately due to variable clinical expression, the coexistence of clinical entities and variability in the progression of disease. Indeed, disease phenotypes may vary over time even in a single patient with APS, especially at advanced ages[6]. Furthermore, these issues have made defining HLA associations with individual clinical features of APS extremely difficult. However, we discuss below the HLA genes which are associated with an increased susceptibility to the development of APS and the production of aPL antibodies.
Familial APS was initially described in a group of related individuals who consistently tested positive for syphilis in the absence of the infection and developed overt autoimmune disease years later[22]. Since then, many studies have reported the high prevalence of PAPS correlated with aPL such as lupus anticoagulant (LA) and aCL, and other autoantibodies in families[23,24]. The frequent finding of aCL in first-degree relatives of patients with PAPS or secondary antiphospholipid syndrome (SAPS) has also been demonstrated[25,26]. A dominant or co-dominant model for the inheritance of APS was suggested by segregation analysis studies in a group of seven families with a 30% prevalence of primary APS among them[27]. However, the study failed to find any HLA associations or correlation with other putative genes including β2GPI and Fas. In an English-Canadian family, the paternal haplotype A30; Cw3; B60; DR4; DRw53; DQw3 was associated with aCL production in secondary APS patients and individuals without disease[28]. DR4 and DR7 have also been reported to be associated with the presence of LA in families[29,30]. Another study evaluated family members, all who had SLE and a myriad of APS clinical manifestations, and revealed that DR4, DRw53 and DQw7 composed a haplotype found in twins and their mother[31].
Many HLA associations with APS have also been found in population studies of unrelated individuals. HLA-DQw7 (HLA-DQB1*0301) linked to HLA-DR4 and/or –DR5 was found to be associated with LA in a group of SLE patients[32]. DR4 and DRw53 were found to occur more frequently in primary APS[33]. Other primary APS associated HLA include DQB1*0301/4, DQB1*0604/5/6/7/9, DQA1*0102, DQA1*0301/2, DRB1*04 and DR7[34-36]. Similar results were found in a large study of Italian SLE patients, in which HLA-DRB1*04, -DRB1*07, -DQA1*0201, -DQA1*0301,-DQB1*0302,-DRB3*0301 were associated with aCL and DQB1*0302 with anti-β2GPI[37]. In Japanese patients, DRB1*09 has been reported to be associated with aCL production in patients with lupus-associated APS[38] A strong association exists between anti-β2GPI and HLA-DR4 haplotypes, particularly when linked to HLA-DQ8 (DQB1*0302) in Caucasian and Mexican Americans, while the association with anti-β2GPI was attributed to the HLA-DRB1*1302;DQB1*0604/0605 haplotype in African American and Caucasian British patients with primary APS[34,39]. In black American populations, there is evidence that C4A or C4B null alleles are associated with the presence of aCL. It is interesting to note however, that in the Hopkins Lupus Cohort, composed of a significant number of African Americans, patients who were homozygous for C4A deficiency had a lower frequency of aCL and LA than patients without this deficiency[40-42].
Mutations in genes not associated with the MHC region, such as a substitution of valine for leucine at amino acide residue 247 in domain V of β2GPI, can contribute to APS development. This polymorphism is more prevalent in APS patients, especially those with arterial thrombosis, compared to matched controls and is linked to anti-β2GPI production in these patients[43-45]. Other thrombophilia-related genetic factors like factor V Leiden (FVL), prothrombin mutations and deficiencies of antithrombin III, protein C and protein S have also been linked to APS disease manifestations[46].
The prevalence of the FVL G1691A mutation in Caucasian populations has been reported to range from 1% to as high as 15%[47,48]. Studies have shown that persons homozygous for FVL have an approximately 80-fold increase and heterozygous individuals a seven-fold increase in the lifetime risk for a thrombotic event compared to the general population. However, FVL seems to have a milder effect on the development of thrombosis in APS patients than in the general population due to the effect of aPL, but this mutation may increase the thrombogenic effect of aPL in several patients[49-51]. The G20210A prothrombin mutation (F2 G20210A) does confer an elevated risk of deep venous thromboembolism in the general population, although to a lesser degree than FVL, but in APS patients the effect seems to be less consistent. While it was first reported that the gene did not increase risk in Caucasian and Mexican mestizo APS patients[52-54], studies that followed demonstrated that an elevated rate of thrombotic disease in patients with APS could be attributed to the presence of the gene. The initial report was of a young female patient with the homozygous G20210A mutation and lupus associated APS[55-57]. However, reports that followed could not demonstrate an association between this mutation and thrombosis in APS[51,58].
As a result of the rarity of deficiencies in antithrombin III and protein C and S it has proven difficult to accurately assess the role played by these mutations in increasing thrombotic risk in patients with aPL. However, studies have linked an elevated incidence of thrombotic disease with deficiencies of both protein C and S in APS[59,60]. Polymorphisms in other relevant genes including thrombomodulin, annexin A5, methylenetetrahydrofolate reductase, plasminogen activator inhibitor-1, tumor necrosis factor α, platelet glycoproteins GP Ia/IIa and GP IIb/IIIa, tissue factor pathway inhibitor, can also possibly increase the risk of thrombotic disease in APS but data is limited[61].
Early efforts to induce aPL production in animal models focused on immunization of animals with theorized antigenic targets. Initial experiments utilized cardiolipin antigens but these failed to allow for production of aPL in animal models[62]. After the discovery that the main antigenic target of pathogenic aPL was in fact β2GPI, subsequent experiments utilized immunization with heterologous β2GPI rather than pure PLs. This led to the successful induction of aPL production in mice and these antibodies were able to induce pathogenic effects[2,62]. Researchers then hypothesized that perhaps molecular mimicry played a key role in pathogenic aPL production. In essence, foreign PL-binding proteins that shared structural similarities to β2GPI could bind to self PLs in APS patients, and in so doing allow for the assembly of immunogenic complexes that stimulate aPL production.
Subsequent studies made use of a synthesized 15 amino acid peptide, GDKV, which spanned an area of the fifth domain of β2GPI known to be a major PL-binding site of the molecule. This peptide was able to induce pathogenic aPL and anti-β2GPI production in immunized mice[63]. A monoclonal antibody with aPL and anti-β2GPI activity generated from these GDKV-immunized mice was shown to be pathogenic using in vivo models for thrombus enhancement and microcirculation[64]. A search for candidate peptides with structural similarities to GDKV among libraries of peptides from viral and bacterial agents produced several candidates (Table 1). Similar results in experimental animal models were then reported using these candidate peptides[65]. When compared to GDKV, peptides from cytomegalovirus (TIFI and VITT), from adenovirus (TADL) and from Bacillus subtilis (SGDF) all bound to PLs with greater affinity and induced higher anti-β2GPI levels in experimental animals. The thrombogenic and proinflammatory capacity of induced antibodies in mice immunized with TIFI was subsequently confirmed[65,66].
| Peptide | Source | Amino acid sequence | Inhibition of β2GPI binding to CL (%)1 |
| GDKV | Gly274-Cys288 in domain V of human B2GPI | GDKVSFFCKNKKC | 43 |
| GDKV2 | Modified GDKV with all six residues between Lys282-Lys287 replaced with Lys | GDKVSFFCKKKKKKC | 56 |
| TADL | Thr77-Glu96 of Adv type2 DNA binding protein | TADLAIASKKKKKRPSPKPE | 68 |
| TIFI | Thr101-Thr120 of ULB0-HCMVA from human CMV | TIFILFCCSKEKRKKKQAAT | 75 |
| VITT | Val51-Ile70 of US27-HCMVA from human CMV | VITTILYYRRKKKSPSDT | 83 |
| SGDF | Ser237-Ser256 of TLP-BACSU from Bacillus subtilis | SGDFEYTYKGKKKKMAFATS | NA |
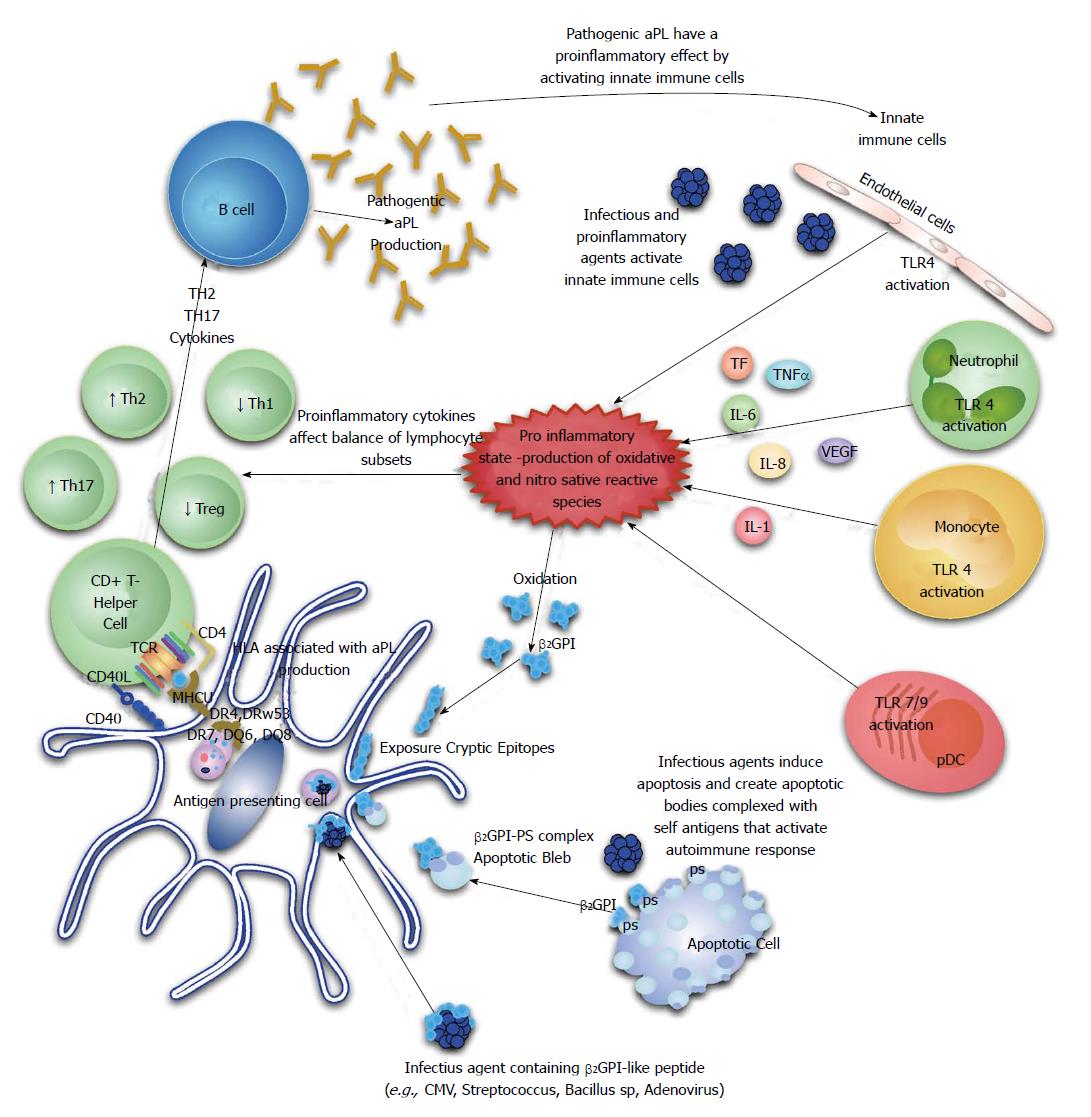
An interesting set of experiments that focused on a hexapeptide, TLRVYK, which is a known antigen of pathogenic monoclonal anti-β2GPI, found in micro-organisms provided further evidence of molecular mimicry being involved in aPL production[67]. BALB/c mice immunized with Haemophilus influenzae, Neisseria gonorrhoeae or tetanus toxoid produced high anti-TLRVYK and anti-β2GPI antibodies which were then isolated and passively transferred to naive mice at day 0 of pregnancy. These antibodies induced a higher frequency of fetal loss, thrombocytopenia and prolonged activated partial thromboplastin times at day 15 after inoculation. Even further evidence comes from a study utilizing protein H found in Streptococcus pyogenes isolates. Protein H was able to bind to β2GPI, induce changes in the conformation of the protein, expose cryptic epitopes and consequently allow for the development of anti-β2GPI antibodies[68].
Several infectious agents have been linked to aPL production and APS manifestations[63]. Human immunodeficiency virus, Human T-cell lymphoma/leukemia virus, CMV, hepatitis B and C viruses, parvovirus B19 and Varicella Zoster Virus are a few for which these associations have been reported[69]. It is clear that infectious agents play a major role in pathogenic aPL production but what remains uncertain is the mechanism which underlies the break in tolerance allowing for these autoantibodies to be produced. Additional methods of autoimmune induction by infectious agents include the release of cytokines and chemkines, selective activation or destruction of unique lymphocyte subsets or hidden epitope exposure during cell necrosis or apoptosis[70-72].
The majority of circulating β2GPI exists in a reduced form containing unpaired cysteines (free thiols), which are involved in the interaction with platelets and endothelial cells. This abundant pool of free thiols may serve as an antioxidant reservoir protecting cells or critical molecules from oxidative stress and oxidation of β2GPI has been shown to confer an increase in its immunogenicity through a Th1 immunological mechanism. It is therefore possible that the generation of reactive oxidative and nitrosative species by certain infectious agents could allow for generation of an abundance of oxidized β2GPI and foster autoantibody production. Indeed, serum from patients with APS assessed by a novel enzyme linked immunoassay (ELISA) assay, have a significant increase in oxidized β2GPI[73] (Figure 1).
The break in tolerance in APS patients is also likely to involve regulatory T-cell (Treg) function based on recent evidence. Peripheral blood mononuclear cells isolated from healthy donors were subjected to increasing concentrations of aPL and there was evidence of significant changes in T-cell subsets compared to controls[74]. T-helper2 (Th2) and Th17 cell frequencies were increased, while Th1 and Treg cells were decreased. Subsequently, a study done in primary APS patients reported a reduced frequency of CD4+ CD25+ foxp3+ T-regulatory cells in these patients compared to controls[75]. Taken together, these studies indicate that Th1/Th2 imbalance, Th17 upregulation and Treg dysfunction play potential roles in aPL production and APS development (Figure 1).
Rauch et al[76] have recently put forward a hypothesis that highlights the central part played by toll-like receptors (TLRs), especially TLR4, in inducing a break in tolerance, aPL production and epitope spread to several autoantigens based on their work[76]. Quite recently, Aguilar-Valenzuela et al[77] demonstrated for the first time that both TLR7 and TLR9 are involved in pathogenic aPL production by utilizing lupus prone mice treated with CMV derived peptides in the presence of TLR7 or TLR9 agonists and other lupus prone mice deficient in TLR7 or both TLR7 and TLR9.
Although there is only limited and often inconclusive data linking environmental agents such as vaccines, drugs and cancer to APS, these associations have been reported[78,79]. Associations with acrylamide, silicone and vaccines have been outlined in case reports but remain unproven[80,81].
Drugs are able to bind self-antigens, alter their processing and presentation to immune cells and in essence creating neopeptides or expose cryptic epitopes, facilitating autoimmune induction[82]. Similar to other non-infectious environmental agents, several drugs have been reported to be associated with aPL production but conclusive evidence has not been presented. These drugs include antibiotics, propranolol, chlorpromazine, antiarrhytmic agents, quinine, amoxicillin, phenytoin, chlorothiazide, oral contraceptives, anti-hypertensive medications, alpha-interferon, and infliximab[82-85].
Solid and hematologic cancers have been linked to aPL, which is perhaps most significant as it relates to an increased risk for thrombosis in patients with an already elevated risk and the potential for development of catastrophic antiphospholipid syndrome (CAPS). The underlying pathogenetic mechanisms of to this association are as yet unclarified but may be related to an anti-tumor immune response or neoantigen formation during immunomodulatory drug therapy with agents like interferon-α[86].
Apoptosis is a normal regulatory process of tissue turnover in response to different homeostatic stimuli. However, as a result of this process there is continuous exposure of self-antigens to the immune system and so the key to prevention of autoimmune induction is efficient clearing of apoptotic debris. In the thymus and bone marrow, these clearance mechanisms are extremely efficient and since there is also a lack of co-stimulatory signals in these central lymphoid organs, no induction of autoantibodies occurs under normal circumstances. However, apoptosis results in disruption of intracellular boundaries and the clustering and structural modification of nuclear, cytoplasmic and membrane antigens. In the absence of efficient clearance mechanisms, normally unexposed antigens are subject to immune recognition, resulting in autoantibody production[87].
During apoptosis, a negatively charged PL, PS, which is normally found almost entirely on the inner cytoplasmic leaflet, is transferred to the outer leaflet[88,89]. This is important in APS as it provides an antigen for aPL binding and such autoantibodies that bind apoptotic cells via interaction with PL-β2GPI complexes have been identified[90-92]. Indeed, the antigenic reactivity of several aPL with a complex formed between anionic phospholipid (e.g., PS) and β2GPI or β2GPI in isolation[93]. During the apoptotic process in autoimmune patients, the sequestration of PS induces specific recognition by macrophages and subsequent removal[94], and the PS/β2GPI complex recruits anti-β2GPI, which then facilitates apoptotic cell clearance and preserves tissue homeostasis[95].
The concept that apoptosis plays a role in the production of aPL was first proposed by Piroux et al[92]. Subsequent studies have provided evidence that apoptotic cells/β2GPI complexes can act as a source of anti-β2GPI antibodies. Levine et al[96] reported that β2GPI do not readily bind to the surface of viable cells but rather to the surface of apoptotic cells. Once bound, the exposure of an essential epitope facilitates recognition by aPL from patients with primary APS and SLE. Interestingly, increased aPL production can be induced in mice immunized with apoptotic cells alone or complexed to β2GPI. A recent study highlighted the importance of the Ro60 receptor in β2GPI precipitation in apoptotic bodies[95-99].
The relative degree to which inherited and acquired factors determine the risk for developing aPL and APS has not been fully elucidated. The most likely scenario is a complex interplay of a multitude of environmental factors in a genetically susceptible patient, which then induces autoantibody development and consequently typical disease manifestations. Once there is a more complete comprehension of the relative contributions of these varied factors, researchers and clinicians alike will be able to implement more effective preventive and therapeutic management guidelines for these patients. Future studies should focus on the elucidation of those specific immune factors leading to a break in tolerance and subsequent aPL production, as a stepping stone to the development of appropriate preventive and therapeutic modalities.
P- Reviewer: Blanco LP, Toubi E S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4924] [Cited by in F6Publishing: 4364] [Article Influence: 242.4] [Reference Citation Analysis (0)] |
| 2. | McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci USA. 1990;87:4120-4124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1130] [Cited by in F6Publishing: 1170] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 3. | Galli M, Comfurius P, Maassen C, Hemker HC, de Baets MH, van Breda-Vriesman PJ, Barbui T, Zwaal RF, Bevers EM. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544-1547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 984] [Cited by in F6Publishing: 992] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 4. | Amengual O, Atsumi T, Koike T. Antiprothombin antibodies and the diagnosis of antiphospholipid syndrome. Clin Immunol. 2004;112:144-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Willis R, Harris EN, Pierangeli SS. Pathogenesis of the antiphospholipid syndrome. Semin Thromb Hemost. 2012;38:305-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Sebastiani GDGM. Genetic aspects of the antiphospholipid syndrome: HLA associations. Ed by Cervera, Reverter and Khamashta. Oxford, UK: Elsevier BC 2009; 81-89. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Castro-Marrero J, Balada E, Vilardell-Tarrés M, Ordi-Ros J. Genetic risk factors of thrombosis in the antiphospholipid syndrome. Br J Haematol. 2009;147:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Gharavi AE, Pierangeli SS, Harris EN. Origin of antiphospholipid antibodies. Rheum Dis Clin North Am. 2001;27:551-563. [PubMed] [Cited in This Article: ] |
| 9. | Gharavi AE, Pierangeli SS, Harris EN. Viral origin of antiphospholipid antibodies: endothelial cell activation and thrombus enhancement by CMV peptide-induced APL antibodies. Immunobiology. 2003;207:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Hashimoto Y, Kawamura M, Ichikawa K, Suzuki T, Sumida T, Yoshida S, Matsuura E, Ikehara S, Koike T. Anticardiolipin antibodies in NZW x BXSB F1 mice. A model of antiphospholipid syndrome. J Immunol. 1992;149:1063-1068. [PubMed] [Cited in This Article: ] |
| 11. | Hang LM, Izui S, Dixon FJ. (NZW x BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med. 1981;154:216-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Oyaizu N, Yasumizu R, Miyama-Inaba M, Nomura S, Yoshida H, Miyawaki S, Shibata Y, Mitsuoka S, Yasunaga K, Morii S. (NZW x BXSB)F1 mouse. A new animal model of idiopathic thrombocytopenic purpura. J Exp Med. 1988;167:2017-2022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Kita Y, Sumida T, Iwamoto I, Yoshida S, Koike T. V gene analysis of anti-cardiolipin antibodies from (NZW x BXSB) F1 mice. Immunology. 1994;82:494-501. [PubMed] [Cited in This Article: ] |
| 14. | Kita Y, Sumida T, Ichikawa K, Maeda T, Yonaha F, Iwamoto I, Yoshida S, Koike T. V gene analysis of anticardiolipin antibodies from MRL-lpr/lpr mice. J Immunol. 1993;151:849-856. [PubMed] [Cited in This Article: ] |
| 15. | Ahmed SA, Verthelyi D. Antibodies to cardiolipin in normal C57BL/6J mice: induction by estrogen but not dihydrotestosterone. J Autoimmun. 1993;6:265-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Verthelyi D, Ansar Ahmed S. Characterization of estrogen-induced autoantibodies to cardiolipin in non-autoimmune mice. J Autoimmun. 1997;10:115-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Gharavi AE, Mellors RC, Elkon KB. IgG anti-cardiolipin antibodies in murine lupus. Clin Exp Immunol. 1989;78:233-238. [PubMed] [Cited in This Article: ] |
| 18. | Ida A, Hirose S, Hamano Y, Kodera S, Jiang Y, Abe M, Zhang D, Nishimura H, Shirai T. Multigenic control of lupus-associated antiphospholipid syndrome in a model of (NZW x BXSB) F1 mice. Eur J Immunol. 1998;28:2694-2703. [PubMed] [Cited in This Article: ] |
| 19. | Izui S, Masuda K, Yoshida H. Acute SLE in F1 hybrids between SB/Le and NZW mice; prominently enhanced formation of gp70 immune complexes by a Y chromosome-associated factor from SB/Le mice. J Immunol. 1984;132:701-704. [PubMed] [Cited in This Article: ] |
| 20. | Izui S, Higaki M, Morrow D, Merino R. The Y chromosome from autoimmune BXSB/MpJ mice induces a lupus-like syndrome in (NZW x C57BL/6)F1 male mice, but not in C57BL/6 male mice. Eur J Immunol. 1988;18:911-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Papalardo ERPZ, Christadoss P, Pierangeli S. Induction of pathogenic antiphospholipid antibodies in vivo are dependent on expression of MHC-II genes. International Congress on Antiphospholipid Antibodies. Vol 19. Galveston, Texas: Lupus 2010; 496. [Cited in This Article: ] |
| 22. | Harvey AM, Shulman LE. Connective tissue disease and the chronic biologic false-positive test for syphilis (BFP reaction). Med Clin North Am. 1966;50:1271-1279. [PubMed] [Cited in This Article: ] |
| 23. | Exner T, Barber S, Kronenberg H, Rickard KA. Familial association of the lupus anticoagulant. Br J Haematol. 1980;45:89-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Jolidon RM, Knecht H, Humair L, de Torrente A. Different clinical presentations of a lupus anticoagulant in the same family. Klin Wochenschr. 1991;69:340-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Mackworth-Young C, Chan J, Harris N, Walport M, Bernstein R, Batchelor R, Hughes G, Gharavi A. High incidence of anticardiolipin antibodies in relatives of patients with systemic lupus erythematosus. J Rheumatol. 1987;14:723-726. [PubMed] [Cited in This Article: ] |
| 26. | Goldberg SN, Conti-Kelly AM, Greco TP. A family study of anticardiolipin antibodies and associated clinical conditions. Am J Med. 1995;99:473-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Goel N, Ortel TL, Bali D, Anderson JP, Gourley IS, Smith H, Morris CA, DeSimone M, Branch DW, Ford P. Familial antiphospholipid antibody syndrome: criteria for disease and evidence for autosomal dominant inheritance. Arthritis Rheum. 1999;42:318-327. [PubMed] [Cited in This Article: ] |
| 28. | Dagenais P, Urowitz MB, Gladman DD, Norman CS. A family study of the antiphospholipid syndrome associated with other autoimmune diseases. J Rheumatol. 1992;19:1393-1396. [PubMed] [Cited in This Article: ] |
| 29. | Rouget JP, Goudemand J, Montreuil G, Cosson A, Jaillard J. Lupus anticoagulant: a familial observation. Lancet. 1982;2:105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Mackie IJ, Colaco CB, Machin SJ. Familial lupus anticoagulants. Br J Haematol. 1987;67:359-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | May KP, West SG, Moulds J, Kotzin BL. Different manifestations of the antiphospholipid antibody syndrome in a family with systemic lupus erythematosus. Arthritis Rheum. 1993;36:528-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Arnett FC, Olsen ML, Anderson KL, Reveille JD. Molecular analysis of major histocompatibility complex alleles associated with the lupus anticoagulant. J Clin Invest. 1991;87:1490-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Asherson RA, Doherty DG, Vergani D, Khamashta MA, Hughes GR. Major histocompatibility complex associations with primary antiphospholipid syndrome. Arthritis Rheum. 1992;35:124-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Caliz R, Atsumi T, Kondeatis E, Amengual O, Khamashta MA, Vaughan RW, Lanchbury JS, Hughes GR. HLA class II gene polymorphisms in antiphospholipid syndrome: haplotype analysis in 83 Caucasoid patients. Rheumatology (Oxford). 2001;40:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Bertolaccini ML, Atsumi T, Caliz AR, Amengual O, Khamashta MA, Hughes GR, Koike T. Association of antiphosphatidylserine/prothrombin autoantibodies with HLA class II genes. Arthritis Rheum. 2000;43:683-688. [PubMed] [Cited in This Article: ] |
| 36. | Vargas-Alarcon G, Granados J, Bekker C, Alcocer-Varela J, Alarcón-Segovia D. Association of HLA-DR5 (possibly DRB1*1201) with the primary antiphospholipid syndrome in Mexican patients. Arthritis Rheum. 1995;38:1340-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Galeazzi M, Sebastiani GD, Tincani A, Piette JC, Allegri F, Morozzi G, Bellisai F, Scorza R, Ferrara GB, Carcassi C. HLA class II alleles associations of anticardiolipin and anti-beta2GPI antibodies in a large series of European patients with systemic lupus erythematosus. Lupus. 2000;9:47-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Hashimoto H, Yamanaka K, Tokano Y, Iida N, Takasaki Y, Kabasawa K, Nishimura Y. HLA-DRB1 alleles and beta 2 glycoprotein I-dependent anticardiolipin antibodies in Japanese patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:423-427. [PubMed] [Cited in This Article: ] |
| 39. | Arnett FC, Thiagarajan P, Ahn C, Reveille JD. Associations of anti-beta2-glycoprotein I autoantibodies with HLA class II alleles in three ethnic groups. Arthritis Rheum. 1999;42:268-274. [PubMed] [Cited in This Article: ] |
| 40. | Wilson WA, Perez MC, Michalski JP, Armatis PE. Cardiolipin antibodies and null alleles of C4 in black Americans with systemic lupus erythematosus. J Rheumatol. 1988;15:1768-1772. [PubMed] [Cited in This Article: ] |
| 41. | Wilson WA, Scopelitis E, Michalski JP, Pierangeli SS, Silveira LH, Elston RC, Harris EN. Familial anticardiolipin antibodies and C4 deficiency genotypes that coexist with MHC DQB1 risk factors. J Rheumatol. 1995;22:227-235. [PubMed] [Cited in This Article: ] |
| 42. | Petri M, Watson R, Winkelstein JA, McLean RH. Clinical expression of systemic lupus erythematosus in patients with C4A deficiency. Medicine (Baltimore). 1993;72:236-244. [PubMed] [Cited in This Article: ] |
| 43. | Hirose N, Williams R, Alberts AR, Furie RA, Chartash EK, Jain RI, Sison C, Lahita RG, Merrill JT, Cucurull E. A role for the polymorphism at position 247 of the beta2-glycoprotein I gene in the generation of anti-beta2-glycoprotein I antibodies in the antiphospholipid syndrome. Arthritis Rheum. 1999;42:1655-1661. [PubMed] [Cited in This Article: ] |
| 44. | Atsumi T, Tsutsumi A, Amengual O, Khamashta MA, Hughes GR, Miyoshi Y, Ichikawa K, Koike T. Correlation between beta2-glycoprotein I valine/leucine247 polymorphism and anti-beta2-glycoprotein I antibodies in patients with primary antiphospholipid syndrome. Rheumatology (Oxford). 1999;38:721-723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Prieto GA, Cabral AR, Zapata-Zuñiga M, Simón AJ, Villa AR, Alarcón-Segovia D, Cabiedes J. Valine/valine genotype at position 247 of the beta2-glycoprotein I gene in Mexican patients with primary antiphospholipid syndrome: association with anti-beta2-glycoprotein I antibodies. Arthritis Rheum. 2003;48:471-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Reverter JCTM. Genetic aspects of the antiphospholipid syndrome: associations with clinical manifestations. Antiphospholipid Syndrome in Systemic Autoimmune Disease Vol 10. Johannesburg, South Africa: Elsevier 2009; 91-10. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet. 1995;346:1133-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 708] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 48. | Franco RF, Elion J, Tavella MH, Santos SE, Zago MA. The prevalence of factor V Arg306--> Thr (factor V Cambridge) and factor V Arg306--> Gly mutations in different human populations. Thromb Haemost. 1999;81:312-313. [PubMed] [Cited in This Article: ] |
| 49. | Schütt M, Klüter H, Hagedorn-Greiwe M, Fehm HL, Wiedemann GJ. Familial coexistence of primary antiphospholipid syndrome and factor VLeiden. Lupus. 1998;7:176-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Brenner B, Vulfsons SL, Lanir N, Nahir M. Coexistence of familial antiphospholipid syndrome and factor V Leiden: impact on thrombotic diathesis. Br J Haematol. 1996;94:166-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Chopra N, Koren S, Greer WL, Fortin PR, Rauch J, Fortin I, Senécal JL, Docherty P, Hanly JG. Factor V Leiden, prothrombin gene mutation, and thrombosis risk in patients with antiphospholipid antibodies. J Rheumatol. 2002;29:1683-1688. [PubMed] [Cited in This Article: ] |
| 52. | Bentolila S, Ripoll L, Drouet L, Crassard I, Tournier-Lasserve E, Piette JC. Lack of association between thrombosis in primary antiphospholipid syndrome and the recently described thrombophilic 3’-untranslated prothrombin gene polymorphism. Thromb Haemost. 1997;78:1415. [PubMed] [Cited in This Article: ] |
| 53. | Bertolaccini ML, Atsumi T, Hunt BJ, Amengual O, Khamashta MA, Hughes GR. Prothrombin mutation is not associated with thrombosis in patients with antiphospholipid syndrome. Thromb Haemost. 1998;80:202-203. [PubMed] [Cited in This Article: ] |
| 54. | Ruiz-Argüelles GJ, Garcés-Eisele J, Ruiz-Delgado GJ, Alarcón-Segovia D. The G20210A polymorphism in the 3’-untranslated region of the prothrombin gene in Mexican mestizo patients with primary antiphospholipid syndrome. Clin Appl Thromb Hemost. 1999;5:158-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Sivera P, Bosio S, Bertero MT, Demaestri M, Mazza U, Camaschella C. G20210A homozygosity in antiphospholipid syndrome secondary to systemic lupus erythematosus. Haematologica. 2000;85:109-110. [PubMed] [Cited in This Article: ] |
| 56. | Torresan M, Machado TF, Siqueira LH, Ozelo MC, Arruda VR, Annichino-Bizzacchi JM. The impact of the search for thrombophilia risk factors among antiphospholipid syndrome patients with thrombosis. Blood Coagul Fibrinolysis. 2000;11:679-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Forastiero R, Martinuzzo M, Adamczuk Y, Varela ML, Pombo G, Carreras LO. The combination of thrombophilic genotypes is associated with definite antiphospholipid syndrome. Haematologica. 2001;86:735-741. [PubMed] [Cited in This Article: ] |
| 58. | Galli M, Finazzi G, Duca F, Norbis F, Moia M. The G1691 --> A mutation of factor V, but not the G20210 --> A mutation of factor II or the C677 --> T mutation of methylenetetrahydrofolate reductase genes, is associated with venous thrombosis in patients with lupus anticoagulants. Br J Haematol. 2000;108:865-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood. 1999;93:1271-1276. [PubMed] [Cited in This Article: ] |
| 60. | Erkan D, Zhang HW, Shriky RC, Merrill JT. Dual antibody reactivity to beta2-glycoprotein I and protein S: increased association with thrombotic events in the antiphospholipid syndrome. Lupus. 2002;11:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Castro-Marrero J, Balada E, Ordi-Rios J, Vilardell-Tarrés M. Genetics of Antiphospholipid Syndrome. In: Antiphospholipid Syndrome; Chapter 3. Ed by Buliková A. InTech, Croatia 2012; 35-66. [DOI] [Cited in This Article: ] |
| 62. | Gharavi AE, Sammaritano LR, Wen J, Elkon KB. Induction of antiphospholipid autoantibodies by immunization with beta 2 glycoprotein I (apolipoprotein H). J Clin Invest. 1992;90:1105-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 168] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Gharavi AE, Pierangeli SS, Colden-Stanfield M, Liu XW, Espinola RG, Harris EN. GDKV-induced antiphospholipid antibodies enhance thrombosis and activate endothelial cells in vivo and in vitro. J Immunol. 1999;163:2922-2927. [PubMed] [Cited in This Article: ] |
| 64. | Gharavi AE, Pierangeli SS, Gharavi EE, Hua T, Liu XW, Barker JH, Anderson GH, Harris EN. Thrombogenic properties of antiphospholipid antibodies do not depend on their binding to beta2 glycoprotein 1 (beta2GP1) alone. Lupus. 1998;7:341-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Gharavi AE, Pierangeli SS, Espinola RG, Liu X, Colden-Stanfield M, Harris EN. Antiphospholipid antibodies induced in mice by immunization with a cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002;46:545-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Gharavi AE, Vega-Ostertag M, Espinola RG, Liu X, Cole L, Cox NT, Romagnoli P, Labat K, Pierangeli SS. Intrauterine fetal death in mice caused by cytomegalovirus-derived peptide induced aPL antibodies. Lupus. 2004;13:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, Tobar A, Shoenfeld Y. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109:797-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | van Os GM, Meijers JC, Agar Ç, Seron MV, Marquart JA, Åkesson P, Urbanus RT, Derksen RH, Herwald H, Mörgelin M. Induction of anti-β2 -glycoprotein I autoantibodies in mice by protein H of Streptococcus pyogenes. J Thromb Haemost. 2011;9:2447-2456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Sène D, Piette JC, Cacoub P. [Antiphospholipid antibodies, antiphospholipid syndrome and viral infections]. Rev Med Interne. 2009;30:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | van de Berg PJ, Heutinck KM, Raabe R, Minnee RC, Young SL, van Donselaar-van der Pant KA, Bemelman FJ, van Lier RA, ten Berge IJ. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis. 2010;202:690-699. [PubMed] [Cited in This Article: ] |
| 71. | Prandota J. Possible pathomechanism of autoimmune hepatitis. Am J Ther. 2003;10:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Nakagawa K, Harrison LC. The potential roles of endogenous retroviruses in autoimmunity. Immunol Rev. 1996;152:193-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 73. | Passam FH, Giannakopoulos B, Mirarabshahi P, Krilis SA. Molecular pathophysiology of the antiphospholipid syndrome: the role of oxidative post-translational modification of beta 2 glycoprotein I. J Thromb Haemost. 2011;9 Suppl 1:275-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Xiao J, Zhu F, Liu X, Xiong J. Th1/Th2/Th17/Treg expression in cultured PBMCs with antiphospholipid antibodies. Mol Med Rep. 2012;6:1035-1039. [PubMed] [Cited in This Article: ] |
| 75. | Dal Ben ER, do Prado CH, Baptista TS, Bauer ME, Staub HL. Decreased levels of circulating CD4+CD25+Foxp3+ regulatory T cells in patients with primary antiphospholipid syndrome. J Clin Immunol. 2013;33:876-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 76. | Rauch J, Dieudé M, Subang R, Levine JS. The dual role of innate immunity in the antiphospholipid syndrome. Lupus. 2010;19:347-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Aguilar-Valenzuela R, Nickerson K, Romay-Penabad Z, Shlomchik MJ, Vargas G, Shilagard T, Pierangeli S. Involvement of TLR7 and TLR9 in the production of antiphospholipid antibodies. Arthritis Rheum. 2011;63:s281 (abstract 723) Available from: http://www.blackwellpublishing.com/acrmeeting/abstract.asp?MeetingID=781&id=95469. [Cited in This Article: ] |
| 78. | Molina V, Shoenfeld Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity. 2005;38:235-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 79. | Martinuc Porobic J, Avcin T, Bozic B, Kuhar M, Cucnik S, Zupancic M, Prosenc K, Kveder T, Rozman B. Anti-phospholipid antibodies following vaccination with recombinant hepatitis B vaccine. Clin Exp Immunol. 2005;142:377-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Alusík S, Jandová R, Gebauerová M, Tesárek B, Fabián J. [The anticardiolipin syndrome after breast reconstruction]. Rozhl Chir. 1990;69:298-301. [PubMed] [Cited in This Article: ] |
| 81. | Rothschild B. Acrylamine-induced autoimmune phenomena. Clin Rheumatol. 2010;29:999-1005. [PubMed] [Cited in This Article: ] |
| 82. | Uetrecht J. Current trends in drug-induced autoimmunity. Autoimmun Rev. 2005;4:309-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | El-Rayes BF, Edelstein M. Unusual case of antiphospholipid antibody syndrome presenting with extensive cutaneous infarcts in a patient on long-term procainamide therapy. Am J Hematol. 2003;72:154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Sherer Y, Blank M, Shoenfeld Y. Antiphospholipid syndrome (APS): where does it come from? Best Pract Res Clin Rheumatol. 2007;21:1071-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 85. | Merrill JT, Shen C, Gugnani M, Lahita RG, Mongey AB. High prevalence of antiphospholipid antibodies in patients taking procainamide. J Rheumatol. 1997;24:1083-1088. [PubMed] [Cited in This Article: ] |
| 86. | Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31:256-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum. 2004;34:501-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 88. | Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3792] [Cited by in F6Publishing: 3914] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 89. | Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182:1597-1601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 501] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 90. | Price BE, Rauch J, Shia MA, Walsh MT, Lieberthal W, Gilligan HM, O’Laughlin T, Koh JS, Levine JS. Anti-phospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a beta 2-glycoprotein I-dependent manner. J Immunol. 1996;157:2201-2208. [PubMed] [Cited in This Article: ] |
| 91. | Casciola-Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1996;93:1624-1629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 339] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 92. | Piroux V, Eschwège V, Freyssinet JM. Cell damage at the origin of antiphospholipid antibodies and their pathogenic potential in recurrent pregnancy loss. Infect Dis Obstet Gynecol. 1997;5:176-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 93. | Koike T, Bohgaki M, Amengual O, Atsumi T. Antiphospholipid antibodies: lessons from the bench. J Autoimmun. 2007;28:129-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 94. | Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207-2216. [PubMed] [Cited in This Article: ] |
| 95. | Manfredi AA, Rovere P, Heltai S, Galati G, Nebbia G, Tincani A, Balestrieri G, Sabbadini MG. Apoptotic cell clearance in systemic lupus erythematosus. II. Role of beta2-glycoprotein I. Arthritis Rheum. 1998;41:215-223. [PubMed] [Cited in This Article: ] |
| 96. | Levine JS, Subang R, Nasr SH, Fournier S, Lajoie G, Wither J, Rauch J. Immunization with an apoptotic cell-binding protein recapitulates the nephritis and sequential autoantibody emergence of systemic lupus erythematosus. J Immunol. 2006;177:6504-6516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 459] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 98. | Levine JS, Subang R, Koh JS, Rauch J. Induction of anti-phospholipid autoantibodies by beta2-glycoprotein I bound to apoptotic thymocytes. J Autoimmun. 1998;11:413-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Rauch J, Subang R, D’Agnillo P, Koh JS, Levine JS. Apoptosis and the antiphospholipid syndrome. J Autoimmun. 2000;15:231-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |