Published online Feb 28, 2023. doi: 10.5499/wjr.v11.i1.1
Peer-review started: May 4, 2022
First decision: May 31, 2022
Revised: June 1, 2022
Accepted: February 1, 2023
Article in press: February 1, 2023
Published online: February 28, 2023
Processing time: 298 Days and 15.7 Hours
Systemic autoimmune rheumatic diseases (SARDs) are a group of diseases with multiorgan involvement and a high prevalence of chronic pain and fatigue. Patients with SARDs and post-coronavirus disease 2019 (COVID-19) syndrome experience aggravation of symptoms. In this context, it is essential to establish strategies to reduce chronic pain and fatigue and improve quality of life.
To assess the efficacy of transcranial direct current stimulation (tDCS) for the treatment of fatigue and pain-associated post-COVID-19 syndrome in patients with SARDs.
This study included nine patients with different types of SARDs. All patients had reverse transcription-polymerase chain reaction (RT-PCR) test confirmed COVID-19 as well as significant, persistent fatigue and pain that began to worsen after infection. Anodal tDCS was administered in five daily sessions (2mA, 20 min). Concomitantly, patients were involved in aerobic exercise program. All participants were evaluated using specific questionnaires and strength assessment by handgrip and physical function by timed-up-and-go test and sit-to-stand test at baseline (within one week before tDCS protocol), and one week after tDCS protocol. During all procedures, the patients’ treatments remained unchanged.
The sample comprised eight women and one man with a mean age of 48.7 ± 9.6 years. After the tDCS protocol, pain and fatigue significantly improved on the visual analog scale (P < 0.05). The physical function also improved 9.5 ± 2.7 vs 6.8 ± 0.8 (P = 0.001) for timed-up-go-test and 10.3 ± 3.7 vs 15.1 ± 4.0 (P = 0.037) for sit-to-stand test. None of the patients experienced any adverse events.
The present study showed that tDCS in combination with aerobic exercise was effective in improving physical function, and reducing fatigue/pain in SARDs patients with post-COVID-19 syndrome.
Core Tip: In the present study, we assessed the efficacy of five daily transcranial direct current stimulation (tDCS) sessions for the treatment of fatigue and pain-associated post-coronavirus disease 2019 (COVID-19) syndrome in nine patients with different systemic autoimmune rheumatic diseases (SARDs). After the tDCS protocol, pain and fatigue significantly improved on the visual analog scale. The physical function also improved 9.5 ± 2.7 vs 6.8 ± 0.8 (P = 0.001) for timed-up-go-test and 10.3 ± 3.7 vs 15.1 ± 4.0 (P = 0.037) for sit-to-stand test. None of the patients experienced any adverse events. In conclusion, tDCS in combination with aerobic exercise was effective in improving physical function, and reducing fatigue/pain in SARDs patients with post-COVID-19 syndrome.
- Citation: Missé RG, dos Santos AM, Borges IBP, Simões MSM, Silvério LR, Correia BL, Kim AWS, Caetano AM, Pasoto SG, Saad CGS, Domiciano DS, Tanaka C, Greve JMD, Baptista AF, Shinjo SK. Transcranial direct current electrical stimulation in combination with aerobic exercise: A pilot study in post-COVID-19 systemic autoimmune rheumatic patients. World J Rheumatol 2023; 11(1): 1-12
- URL: https://www.wjgnet.com/2220-3214/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5499/wjr.v11.i1.1
The World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, a pandemic in March 2020[1]. The rapid spread of COVID-19 worldwide has resulted in at least 230 million infections and 4.6 million deaths as of September 2021[2]. In addition, approximately one-third of recovered individuals have persistent symptoms called long-COVID-19 or post-COVID-19 syndrome[3]. The syndrome is characterized by signs or symptoms that develop during or after SARS-CoV-2 infection and persist for more than 12 wk after the COVID-19 recovery[3,4]. Fatigue and musculoskeletal pain have been observed in at least one-third and one-fifth of the patients with post-COVID-19 syndrome, respectively[3,4]. As a result, recent studies have shown the growing dependence on health systems in these patients because of the slow recovery of quality of life and functionality[3].
Systemic autoimmune rheumatic diseases (SARDs) comprise a broad group of diseases characterized by multiorgan and systemic involvement, including rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, spondyloarthritis, and systemic autoimmune myopathies. Frequently, patients with SARDs complain of fatigue and chronic pain, which negatively affect their quality of life[5-7]. In the context of COVID-19, these individuals may experience aggravated symptoms during and/or after the SARS-CoV-2 infection. Indeed, a recent study showed that patients with chronic pain (e.g., fibromyalgia) experience worsening fatigue and pain after COVID-19[8]. Moreover, these symptoms can persist chronically despite appropriate regular pharmacological treatments and physical exercise[5-7]. Therefore, it is essential to establish strategies that can reduce chronic pain and fatigue to minimize functional capacity impairment in these patients[9-11].
Transcranial direct current electrical stimulation (tDCS) is a noninvasive brain stimulation technique that has been applied to facilitate or inhibit brain areas, with promising results in pain modulation, fatigue reduction, and improvement in functional capacity in patients with regional complex pain[12,13], refractory myofascial syndrome, chronic fatigue[14], and fibromyalgia[12]. Recently, Pinto et al[15] showed that tDCS of the dorso-lateral pre-frontal cortex was largely effective in reducing fatigue in patients with Sjögren’s syndrome.
Nonetheless, moderate aerobic exercise training has been extensively prescribed as a remarkable tool in the management of chronic pain patients[16]. Despite this, recent studies also showed that the combined aerobic exercise and tDCS applied to the primary motor cortex leads to a larger effect size than aerobic exercise or tDCS alone in patients with fibromyalgia[17].
To date, there is a lack of information on tDCS in patients with other SARDs, as well as in those with post-COVID-19 syndrome. Based on the multisystemic components of SARDs and post-COVID syndrome, we hypothesize that tDCS of the primary motor cortex can lead to improvements in pain and fatigue related to post-COVID-19 syndrome symptoms in patients with SARDs.
This was a single-center, prospective, open-label, one-arm study that included adult SARDs patients with fatigue or pain associated with or potentiated by post-COVID-19 syndrome. The clinical trial was conducted from May to July 2021, and individuals who were regularly followed up in our tertiary outpatient rheumatology clinics were consecutively invited to participate in the study.Our center follows approximately 800 patients with rheumatoid arthritis, 1000 with systemic lupus erythematosus, 220 with systemic autoimmune myopathies, 280 with Sjögren’s syndrome, and 500 with spondy
The study was conducted in accordance with the Declaration of Helsinki and local regulations. This study was approved by the local ethics committee (CAAE 41916820.3.0000.0068) and registered at ClinicalTrials.gov (NCT04890483). Written informed consent was obtained from all the patients. We implemented the a-tDCS as a protocol as an aerobic exercise program due to evidence of priming effects in studies of patients with fibromyalgia and neurological conditions.
Fatigue or pain associated with or potentiated by COVID-19. Post-COVID-19 syndrome was defined as new or worsened fatigue or pain (local or diffuse) sustained for more than 12 wk after SARS-CoV-2 infection.
SARDs patients who fulfilled the following classification criteria were recruited: rheumatoid arthritis[18], systemic lupus erythematosus[19], systemic autoimmune myopathies[20], Sjögren’s syndrome[21], and spondyloarthritis[22]; COVID-19 confirmed by real-time reverse transcription-polymerase chain reaction (RT-PCR) or serology for IgG and IgM[23] from March 2020 to February 2021; persistent fatigue or pain associated with or potentiated by COVID-19 (post-COVID-19 syndrome), defined by new or worsened fatigue, or pain (local or diffuse) sustained for more than 12 wk after the SARS-CoV-2 infection; and relatively stable use of medications in the previous months.
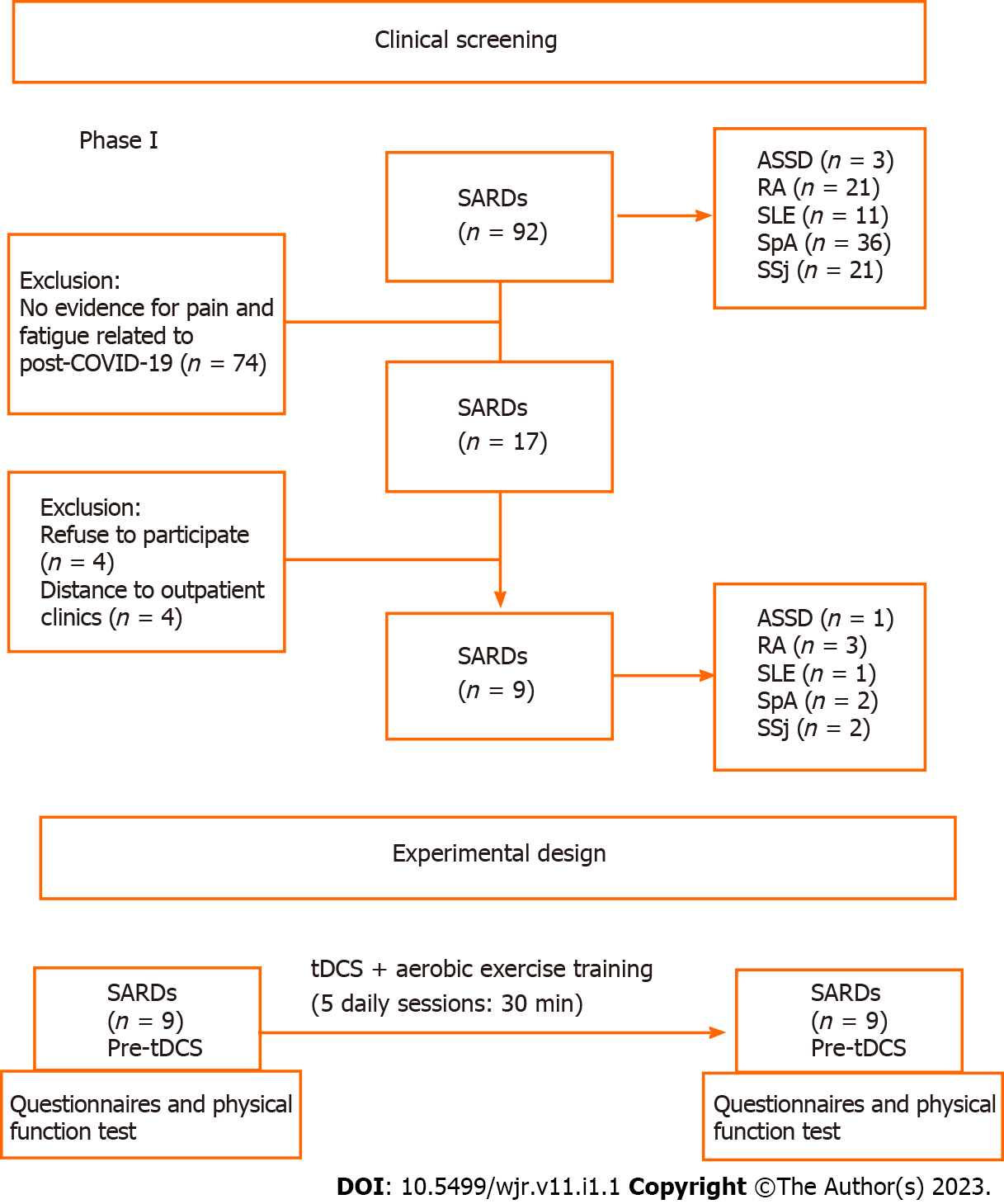
Patients with neoplasms, cardiac pacemaker users, users of cranial metal clips or prostheses, pregnancy, personal history of seizures or epilepsy (related or not to COVID-19), use of drugs that lower seizure threshold, and cutaneous lesions (scalp, in the area of application of the electrodes) (Figure 1) were excluded.
Two rheumatologists performed the initial patient’ screening and a final review of frontline rheumatology with extensive experience with patients with COVID-19 performed the patient eligibility procedure.
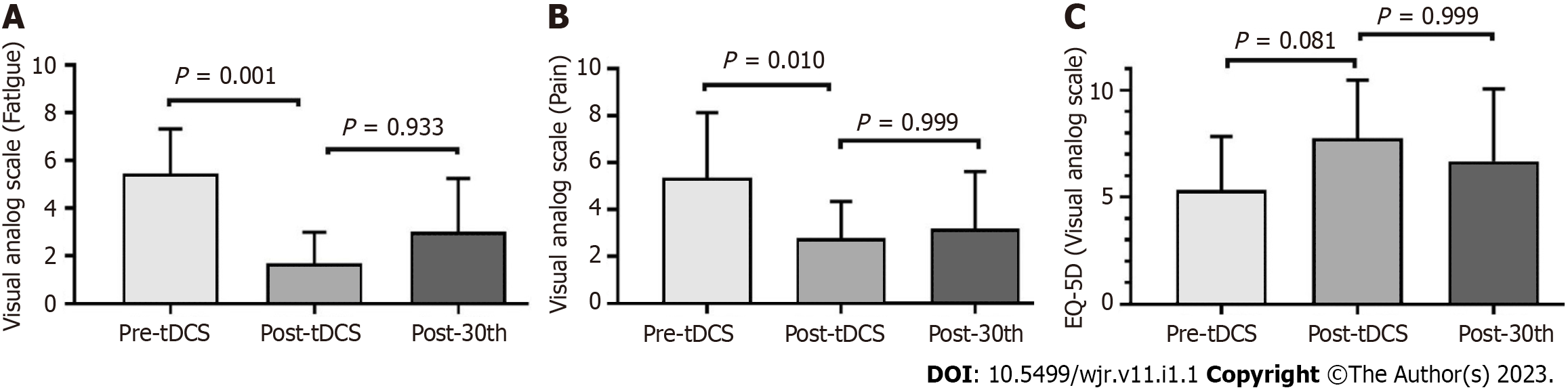
The following data were collected within one week before the tDCS intervention (pre-tDCS): age and sex; body mass index; the International Physical Activity Questionnaire, which classifies the level of physical activity and the weekly metabolic equivalent (METs), such as METs as high physical activity score, patients that comply with vigorous physical activity (≥ 5 d/wk and ≥ 30 min per session or vigorous activities during (≥ 3 d/wk and ≥ 20 min) and concomitant moderate physical activities (≥ 5 d/wk and ≥ 30 min per session); moderate activity score - vigorous activities (≥ 3 d/wk and ≥ 20 min per session) or moderate physical activities (≥ 5 d/wk and ≥ 30 min per session), or the sum of any activities performed ≥ 5 d/wk, and ≥ 150 min/wk[24,25]. Comorbidities included systemic arterial hypertension, dyslipidemia, diabetes mellitus, depression, anxiety, and fibromyalgia. Fibromyalgia was defined according to the American College of Rheumatology classification criteria for fibromyalgia[26]. Depression and anxiety were based on the criteria defined by the American Psychiatric Association[27]. Pharmacological treatment includes glucocorticoids and immunosuppressive drugs and associated medications. SARDs activity status was assessed using the indices of activity validated for each disease (Clinical Disease Activity Index/Disease Activity Score-28[28], Sjögren’s Syndrome Disease Activity Index (ESSDAI)[29], Bath Ankylosing Spondylitis Activity Index[30], Manual Muscle Testing-8 (MMT-8)[31], Systemic Lupus Erythematosus Disease Activity Index[32]. Fatigue status and severity were assessed using the visual analog scale (VAS), Fatigue Assessment of Chronic Illness Therapy[33], Modified Fatigue Impact Scale (MFIS)[34], and Fatigue Severity Scale (FSS)[35]. Pain and fatigue diary was recorded during the tDCS and training sessions for aerobic exercise, at baseline and session by session by VAS before each tDCS/aerobic exercise protocol. Physical function was examined with timed-up-and-go test[36] and stand-to-sit test[37]. Muscle strength was examined with Handgrip test[38]; and quality of life was determined by EQ-5D[39].
These data were also collected within one week (Figure 2). During all protocols, the patient’ treatments remained unchanged.
For tDCS, the anode was sourced from a battery-powered direct current generator (Activadose II, United States) and was exerted by two electrodes measuring 5 cm × 7 cm (35 cm²) (Ibramed, Brazil) covered by a sponge with saline solution and fixed to the head using Velcro straps. Electrodes were used in accordance with the International System 10/20. Targeting the primary motor cortex, positioned in C3 or C4 10/20 EEG positions, with the cathode placed in the supraorbital region (Fp1 and Fp2, respectively). The active current of tDCS was applied with an electrical current intensity of 2 mA and a density of 0.057 mA/cm² for 20 min, with an up and down ramp of 10 s.
Aerobic exercise was characterized by walking on a treadmill for 30 min with intensity adjusted through measurement of effort during and after 20 min of tDCS sessions. In addition, the perception of recovery was collected between sessions[40].
Adverse events were registered during and after each application through questionnaire related to sensations such as burning, tingling, itching, burning (head), headache, nausea, fatigue, emotional lability (tendency to shift rapidly and dramatically between different emotional states), difficulty in concentrating and nervousness.
Patients’ aherence to the protocol was assessed by the researchers in all stages of the study.
The data distribution was set at a = 0.5. With purpose to analyze differences in the clinical characteristics between groups student t test or U man-Whitney test. P value was considered statistically significant when < 0.05. The individual analysis for the pre-tDCS and post-tDCS values was expressed as percentage change using the formula [(post - pre)/pre] × 100. The effect size (ES), a measure of the magnitude of change, was calculated using Cohen’s d for the FACIT and MFIS scores and their domains. The software used was SPSS version 25 (Chicago, IL, United States).
Of 2800 SARDs patients followed at our clinics, from April 2020 to February 2021, 92 had COVID-19. Seventeen patients showed new worsening or pain and fatigue for more than 12 wk following SARS-CoV-2 infection. Eight patients refused to participate in the present study (Figure 1). Therefore, nine patients were included in the present study: three with rheumatoid arthritis, two with spondyloarthritis, two with primary Sjögren’s syndrome, one with antisynthetase syndrome, and one with systemic lupus erythematosus. These nine patients, whose general features of the participants are shown in Table 1. Mean VAS scores for pain and fatigue were 5.3 ± 2.7 and 5.4 ± 1.8, respectively. Concerning the quality-of-life parameters, the patients had a current EQ-5D VAS of 5.3 ± 2.3 (Figure 2).
| Patient | Sex | Age (yr) | BMI (kg/m²) | Disease | Disease duration (yr) | Prednisone (mg/d) | IS | Other medications | Comorbidities | Physical activity levels | Initial disease status |
| #1 | F | 56 | 31.7 | ASSD | 6 | 0 | MTX, LFN | Amitriptyline | SAH | Moderate | MMT-8 = 80; MYOACT = 0 |
| #2 | F | 39 | 37.4 | RA | 15 | 5 | TOF, MTX | Gabapentin, dipyrone | DLP, FM | Low | DAS28 = 5.4; CDAI = 28 |
| #3 | F | 43 | 34.3 | RA | 21 | 5 | TOF, MTX | Dipyrone | SAH, DLP | Low | DAS28 = 5.7; CDAI = 45 |
| #4 | F | 55 | 30.4 | RA | 4 | 15 | TOF | Dipyrone | - | Moderate | DAS28 = 3.2; CDAI = 3 |
| #5 | F | 29 | 29.0 | SLE | 12 | 5 | MMF | Dipyrone | - | Low | SLEDAI = 4 |
| #6 | M | 56 | 23.7 | SpA | 22 | 0 | - | Meloxicam | - | Low | BASDAI = 3.5; ASDAS = 2.5 |
| #7 | F | 46 | 35.6 | SpA | 26 | 5 | MTX, CZP | Tramadol | SAH | Low | BASDAI = 5.6; ASDAS = 3.0 |
| #8 | F | 53 | 26.8 | SSj | 6 | 0 | HCQ | Dipyrone | SAH | Low | ESSDAI = 2 |
| #9 | F | 57 | 26.2 | SSj | 13 | 0 | HCQ | Sertraline | SAH, FM | Low | ESSDAI = 2 |
At their assessment, we classified two patients (#1 and #4) as being in clinical remission, three patients (#5, #8, and #9) with mild disease activity, and three patients (patients #2, #3 and #6) with high underlying disease activity. All patients, regardless of their underlying disease status, reported worsening fatigue or pain after infection with COVID-19 (Table 1).
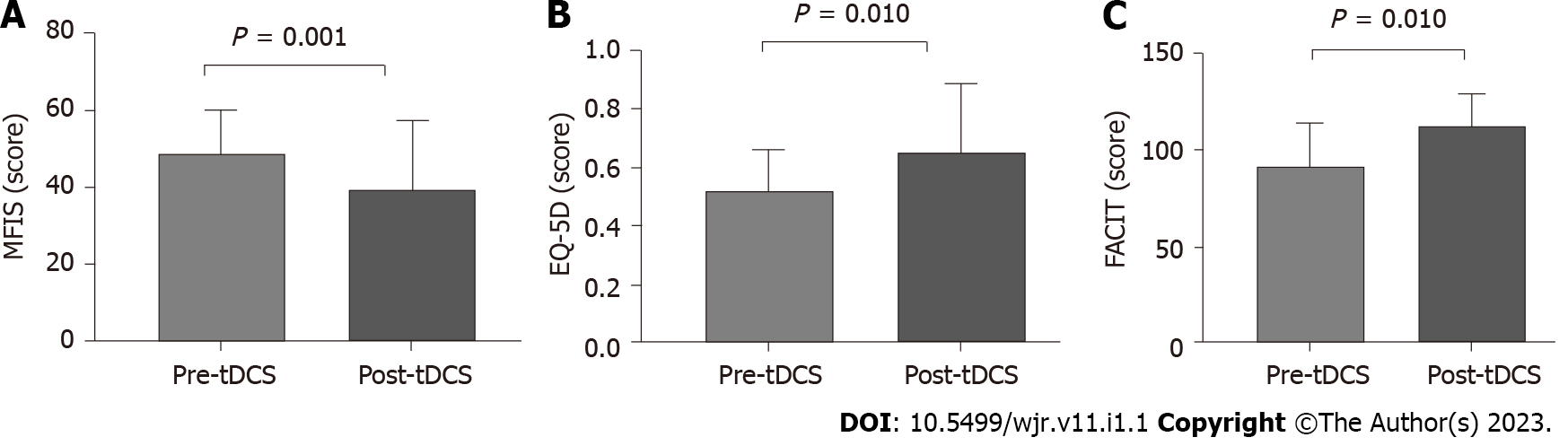
After tDCS intervention in combination with aerobic exercise training sessions, we observed significant decreases in fatigue and pain VAS scores (P < 0.05) (Figure 2). No changes were observed related to fatigue scores and domains assessed by FACIT and MFIS) (Figure 3). Nonetheless, we observed a high ES of 1.00 (95%CI: 0.80-1.90) in the general FACIT scores. Findings were similar for the physical domains in the same questionnaire: ES of 0.80 (95%CI: -0.16 to 1.70). In contrast, the other FACIT domains the ES for emotional well-being was low: ES of 0.66 (95%CI: -0.28 to 1.50); the ES for functional well-being was very low, 0.38 (95%CI: -0.55 to 1.30), and a very low ES was seen for family well-being, 0.16 (95%CI: -0.77 to 1.12). Similar findings were observed by individual data analysis (Table 2).
| Variables | Patients | |||||||||
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | ||
| Pre-tDCS | Fatigue, VAS, (0.0-10) | 8 | 5 | 7 | 10 | 3 | 3 | 7 | 2 | 3 |
| Post-tDCS | 3 | 3 | 3 | 6 | 2 | 2 | 2.5 | 0 | 3 | |
| Δ (%) | -62.5 | -40 | -57.1 | -40 | -33.3 | -33.3 | -64.2 | -100 | 0 | |
| Pre-tDCS | Pain, VAS, (0.0-10) | 5 | 5 | 6 | 8 | 2 | 8 | 6 | 4 | 5 |
| Post-tDCS | 0 | 2 | 2 | 4 | 1 | 2 | 1.5 | 0 | 3 | |
| Δ (%) | -100 | -60 | -66.6 | -50 | -50 | -75 | -75 | -100 | -40 | |
| Pre-tDCS | FSS, (0.0-7.0) | 6 | 5 | 5 | 5 | 5 | 5 | 4 | 3 | 4 |
| Post-tDCS | 3 | 3 | 3 | 3 | 2 | 4 | 0 | 3 | 1 | |
| Δ (%) | -50 | -40 | -40 | -40 | -60 | -20 | -100 | 0 | -75 | |
Concerning the general MFIS, the ES was large -0.81 (95%CI: 1.10 to 0.29), as well as the physical domains, ES of -0.81 (95%CI: -1.77 to 0.28). The ES was low for cognitive domains, 0.11 (95%CI: -1.1 to 0.80), and a very low ES was observed for psychosocial domains, -0.19 (95%CI: -0.10 to 0.80). Concerning the physical function a significant improvement was evidenced on timedupand-go test and sittostand test (P < 0.05) (Table 3). During all tDCS interventions, the intensity of the aerobic exercise did not change (mean exercise intensity was 5.5 ± 0.8), and the patients’ perception of recovery through exercise remained unchanged during the protocol. Furthermore, there were no adverse effects of tDCS. In all protocols, patient adherence was 100%.
| Pre-tDCS | Post-tDCS | P value | |
| MFIS (domains) | |||
| Physical (0-36) | 23.2 ± 2.2 | 19.1 ± 1.8 | 0.332 |
| Cognitive (0-40) | 21.0 ± 5.6 | 21.6 ± 6.3 | 0.974 |
| Psychosocial (0-8) | 4.3 ± 2.9 | 3.7 ± 1.3 | 0.864 |
| FACIT (domains) | |||
| Physical well-being (0-28) | 15.4 ± 5.0 | 20.1 ± 5.0 | 0.987 |
| Family and social well-being (0-28) | 16.4 ± 3.6 | 17.0 ± 3.7 | 0.964 |
| Emotional well-being (0-24) | 16.3 ± 4.4 | 19.3 ± 4.4 | 0.521 |
| Functional well-being (0-28) | 15.3 ± 6.1 | 17.6 ± 3.9 | 0.683 |
| Physical function | |||
| Timed-Up-Go test (s) | 9.5 ± 2.7 | 6.8 ± 0.8 | 0.001 |
| Sit-To-Stand test (repetitions) | 10.3 ± 3.7 | 15.1 ± 4.0 | 0.037 |
| Strength | |||
| Handgrip (kg) | 21.1 ± 5.7 | 24.5 ± 8.3 | 0.522 |
To the best of our knowledge, this is the first study to demonstrate the efficacy of tDCS combined with aerobic exercise training in reducing pain and fatigue after COVID-19 in patients with SARDs.
We conducted a prospective analysis with a well-defined study design that included selected SARDs patients. To mitigate the risk of bias, the patients’ pharmacological therapy was unchanged and the patients did not engage in other non-pharmacological interventions outside of the aerobic exercise training program. In addition, we followed up with all patients instant messaging and regularly scheduled face-to-face interviews.
In the analyzed patients who had been classified as having clinical remission, the underlying disease (patients #1 and #4) remained inactive, with no observed recurrence of the disease. Two patients had mild disease activity (patients #5 and #8), with no clinical worsening. A patient (patient #2) who was classified as having high disease activity, maintained a high activity rate, with a slight improvement from the initial value. Patients #3 and #6, both classified as having high disease activity in the initial assessment, radically changed their indices, reaching metric values of remission in their final assessment, even though treatment of the underlying diseases did not change during the execution of the study. As the perception of pain is extremely subjective and there were no variations in the laboratory data used, we believe that the worsening of this variable after COVID-19 negatively affected the disease activity indices. These two cases demonstrated that after treatment with tDCS, improvement in pain was reflected in the improvement in disease activity data. Only patient #9 reported worsening of clinical joint symptoms after tDCS, and her ESSDAI changed from mild to moderate activity. Patient #8 did not have her final data evaluated because she was in social isolation due to contact with a family member with a recent diagnosis of COVID-19.
It is important to emphasize that no changes were made to the patients’ disease-modifying drug protocols throughout the course of tDCS treatment. Because the application of neurostimulation with tDCS took place for only five days, we chose not to change the treatments for autoimmune diseases, so that possible positive findings of improvement in relation to pain and/or fatigue would not be affected by other treatment changes. Therefore, we believe that the improvement in the pain and fatigue VAS indices was correlated with the use of neurostimulation (Table 2).
tDCS has been used as a non-pharmacological intervention with notable results in the management of several chronic pain syndromes, such as fibromyalgia[41,42]. Beyond its effects on pain, a significant amount of evidence has demonstrated improvements in physical function, mood and health-related quality of life[42,43]. Concerning SARDs, a recent study showed notable improvements in fatigue related to primary Sjögren’s syndrome[15], with no adverse effects related to tDCS and the disease status parameters. This result suggests that tDCS is a potentially safe and efficient way to improve fatigue in patients with Sjögren’s syndrome[15]. However, the authors applied tDCS without concomitant intervention[15]. Previous studies have shown that tDCS with a concomitant non-pharmacological strategy (e.g., cognitive, motor task or exercise training) facilitates the identification of tDCS target levels that most effectively produce priming effects. Priming effects are the result of increased functional connectivity due to neurotransmitter release, which leads to increased cortical excitability in the primary cortex and influences pain processing[42-49]. Moreover, evidence has shown that aerobic exercise training in conjunction with tDCS affects the motor cortex and can result in a major hypoalgesia response to pain related to fibromyalgia[42]. These previous studies support our findings related to improvements in overall pain and fatigue, as measured by the VAS. Interestingly, significant improvements observed in physical function. Similar results have been reported in patients with stroke[46] and Parkinson’s disease[47], suggesting that tDCS potentially affects physical function scores[48].
The limitations of the present study include its limited sample size and the lack of a control group. An observation worthy of attention is that only one patient reported new symptoms of persistent fatigue and pain, whereas all the other patients reported worsening of these symptoms after SARS-CoV-2 infection. Although no studies have reported that COVID-19 exacerbates pain and fatigue in patients with SARDs, the hypothesis is plausible, given that these individuals are more likely to have chronic pain and related symptoms. SARS-CoV-2 infection may be related to one of the components of a vicious cycle of pain, fatigue, decreased physical function, and decreased quality of life that has been observed in various SARDs patients, leading to worse overall symptoms. However, no study has assessed these relationships. Future multicenter studies and representative sample sizes are needed to investigate the potential relationship between the disease parameters of post-COVID-19 and potential predictive tDCS response in patients with SARDs and post-COVID-19 and the differences between the adds-on therapy tDCS and aerobic exercise vs sham-tDCS and aerobic exercise.
tDCS may be an effective strategy to reduce fatigue and pain triggered or potentiated by COVID-19 and improve global function and quality of life. Moreover, tDCS combined with aerobic exercise training led to improvements in physical function. Additional studies with larger sample sizes and randomized designs with objective measures of these outcomes are required to confirm our findings.
Patients with systemic autoimmune rheumatic disease (SARDs) with post- coronavirus disease 2019 (COVID-19) syndrome experience aggravated symptoms.
Given this context, it is essential to establish strategies that can reduce chronic pain and fatigue, and improve equality of life.
To assess the efficacy of transcranial direct current stimulation in treatment of fatigue and pain associated with post-COVID-19 syndrome in patients with SARDs.
This is a quantitative pilot study that included nine patients with different SARDs. All patients had reverse transcription-polymerase chain reaction test confirmed COVID-19 as well as significant, persistent fatigue and pain that began to worsen after infection. Anodal transcranial direct current stimulation (tDCS) was administered in five daily sessions (2mA, 20 min). Concomitantly, patients were involved in aerobic exercise program.
The sample was composed of eight women and one man with a mean age of 48.7 ± 9.6 years. After the tDCS protocol, the pain and fatigue significantly improved as shown by a visual analog scale (P < 0.05). The physical function also improved 9.5 ± 2.7 vs. 6.8 ± 0.8 (P = 0.001) for timed-up-go-test and 10.3 ± 3.7 vs 15.1 ± 4.0 (P = 0.037) for sit-to-stand test. No patient experienced adverse events.
tDCS may be an effective strategy to treat fatigue and pain due to COVID-19 in patients with SARDs and improve the physical function.
The present study contributes to new treatment options to treat pain and fatigue in patients with SARDs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rehabilitation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kirkik D, Turkey; Papadopoulos K, Thailand S-Editor: Liu JH L-Editor: Ma JY P-Editor: Liu JH
| 1. | Ochani R, Asad A, Yasmin F, Shaikh S, Khalid H, Batra S, Sohail MR, Mahmood SF, Ochani R, Hussham Arshad M, Kumar A, Surani S. The COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29:20-36. [PubMed] |
| 2. | WHO. World alliance for patient safety: WHO draft guidelines for adverse event reporting and learning systems: from information to action 2021. Available from: https://www.who.int/covid-19/information. |
| 3. | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 2982] [Article Influence: 745.5] [Reference Citation Analysis (0)] |
| 4. | Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3187] [Cited by in RCA: 2878] [Article Influence: 719.5] [Reference Citation Analysis (1)] |
| 5. | Seifert O, Baerwald C. Impact of fatigue on rheumatic diseases. Best Pract Res Clin Rheumatol. 2019;33:101435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lampa J. Pain without inflammation in rheumatic diseases. Best Pract Res Clin Rheumatol. 2019;33:101439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. 2021;397:2098-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 589] [Article Influence: 147.3] [Reference Citation Analysis (0)] |
| 8. | Cankurtaran D, Tezel N, Ercan B, Yildiz SY, Akyuz EU. The effects of COVID-19 fear and anxiety on symptom severity, sleep quality, and mood in patients with fibromyalgia: a pilot study. Adv Rheumatol. 2021;61:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Misse RG, Borges IBP, Dos Santos AM, Gupta L, Shinjo SK. Effect of exercise training on fatigue and pain in patients with systemic autoimmune myopathies: A systematic review. Autoimmun Rev. 2021;20:102897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Baptista AF, Baltar A, Okano AH, Moreira A, Campos ACP, Fernandes AM, Brunoni AR, Badran BW, Tanaka C, de Andrade DC, da Silva Machado DG, Morya E, Trujillo E, Swami JK, Camprodon JA, Monte-Silva K, Sá KN, Nunes I, Goulardins JB, Bikson M, Sudbrack-Oliveira P, de Carvalho P, Duarte-Moreira RJ, Pagano RL, Shinjo SK, Zana Y. Applications of Non-invasive Neuromodulation for the Management of Disorders Related to COVID-19. Front Neurol. 2020;11:573718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Silva Filho E, Moura S, Santos ADC, Brasileiro-Santos MDS, Albuquerque JA. Transcranial direct current stimulation as a strategy to manage COVID-19 pain and fatigue. Rev Assoc Med Bras (1992). 2021;67:26-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Khedr EM, Omran EAH, Ismail NM, El-Hammady DH, Goma SH, Kotb H, Galal H, Osman AM, Farghaly HSM, Karim AA, Ahmed GA. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimul. 2017;10:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Lagueux É, Bernier M, Bourgault P, Whittingstall K, Mercier C, Léonard G, Laroche S, Tousignant-Laflamme Y. The Effectiveness of Transcranial Direct Current Stimulation as an Add-on Modality to Graded Motor Imagery for Treatment of Complex Regional Pain Syndrome: A Randomized Proof of Concept Study. Clin J Pain. 2018;34:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Misse RG, Dos Santos AM, De Souza JM, Shinjo SK. Transcranial direct current stimulation improves myofascial pain syndrome and chronic fatigue. Reumatismo. 2020;72:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Pinto ACPN, Piva SR, Vieira AGDS, Gomes SGCN, Rocha AP, Tavares DRB, Santana MVA, Carlesso C, Andriolo A, Santos FC, Fregni F, Trevisani VFM. Transcranial direct current stimulation for fatigue in patients with Sjogren's syndrome: A randomized, double-blind pilot study. Brain Stimul. 2021;14:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Borisovskaya A, Chmelik E, Karnik A. Exercise and Chronic Pain. Adv Exp Med Biol. 2020;1228:233-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Front Hum Neurosci. 2016;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6830] [Cited by in RCA: 6182] [Article Influence: 412.1] [Reference Citation Analysis (0)] |
| 19. | Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2816] [Cited by in RCA: 3507] [Article Influence: 269.8] [Reference Citation Analysis (0)] |
| 20. | Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, Alfredsson L, Amato AA, Barohn RJ, Liang MH, Singh JA, Aggarwal R, Arnardottir S, Chinoy H, Cooper RG, Dankó K, Dimachkie MM, Feldman BM, Garcia-De La Torre I, Gordon P, Hayashi T, Katz JD, Kohsaka H, Lachenbruch PA, Lang BA, Li Y, Oddis CV, Olesinska M, Reed AM, Rutkowska-Sak L, Sanner H, Selva-O'Callaghan A, Song YW, Vencovsky J, Ytterberg SR, Miller FW, Rider LG; International Myositis Classification Criteria Project Consortium, the Euromyositis Register, and the Juvenile Dermatomyositis Cohort Biomarker Study and Repository (UK and Ireland). 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 2017;69:2271-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 420] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 21. | Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH; European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4161] [Cited by in RCA: 3916] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 22. | Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, Huang F, Gu J, Khan MA, Kirazli Y, Maksymowych WP, Mielants H, Sørensen IJ, Ozgocmen S, Roussou E, Valle-Oñate R, Weber U, Wei J, Sieper J. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2694] [Cited by in RCA: 2464] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 23. | Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5481] [Cited by in RCA: 4909] [Article Influence: 981.8] [Reference Citation Analysis (1)] |
| 24. | Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11293] [Cited by in RCA: 13614] [Article Influence: 618.8] [Reference Citation Analysis (0)] |
| 25. | Singh R, Pattisapu A, Emery MS. US Physical Activity Guidelines: Current state, impact and future directions. Trends Cardiovasc Med. 2020;30:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2564] [Article Influence: 170.9] [Reference Citation Analysis (0)] |
| 27. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Arlington, VA, USA: American Psychiatric Publishing, 2013. [DOI] [Full Text] |
| 28. | Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4129] [Cited by in RCA: 4601] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 29. | Serrano EV, Valim V, Miyamoto ST, Giovelli RA, Paganotti MA, Cadê NV. Transcultural adaptation of the "EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI)" into Brazilian Portuguese. Rev Bras Reumatol. 2013;53:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281-2285. [PubMed] |
| 31. | Rider LG, Koziol D, Giannini EH, Jain MS, Smith MR, Whitney-Mahoney K, Feldman BM, Wright SJ, Lindsley CB, Pachman LM, Villalba ML, Lovell DJ, Bowyer SL, Plotz PH, Miller FW, Hicks JE. Validation of manual muscle testing and a subset of eight muscles for adult and juvenile idiopathic inflammatory myopathies. Arthritis Care Res (Hoboken). 2010;62:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 32. | Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288-291. [PubMed] |
| 33. | Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof. 2005;28:192-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 413] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 34. | Larson RD. Psychometric properties of the modified fatigue impact scale. Int J MS Care. 2013;15:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 35. | Nadarajah M, Mazlan M, Abdul-Latif L, Goh HT. Test-retest reliability, internal consistency and concurrent validity of Fatigue Severity Scale in measuring post-stroke fatigue. Eur J Phys Rehabil Med. 2017;53:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8741] [Cited by in RCA: 9324] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 37. | Newcomer KL, Krug HE, Mahowald ML. Validity and reliability of the timed-stands test for patients with rheumatoid arthritis and other chronic diseases. J Rheumatol. 1993;20:21-27. [PubMed] |
| 38. | Innes EV. Handgrip strength testing: a review of the literature. Australian Occup Ther J. 1999;46:120-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 275] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 39. | Greiner W, Weijnen T, Nieuwenhuizen M, Oppe S, Badia X, Busschbach J, Buxton M, Dolan P, Kind P, Krabbe P, Ohinmaa A, Parkin D, Roset M, Sintonen H, Tsuchiya A, de Charro F. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ. 2003;4:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 40. | Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377-381. [PubMed] |
| 41. | Knotkova H, Hamani C, Sivanesan E, Le Beuffe MFE, Moon JY, Cohen SP, Huntoon MA. Neuromodulation for chronic pain. Lancet. 2021;397:2111-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 42. | Baptista AF, Fernandes AMBL, Sá KN, Okano AH, Brunoni AR, Lara-Solares A, Jreige Iskandar A, Guerrero C, Amescua-García C, Kraychete DC, Caparelli-Daquer E, Atencio E, Piedimonte F, Colimon F, Hazime FA, Garcia JBS, Hernández-Castro JJ, Cantisani JAF, Karina do Monte-Silva K, Lemos Correia LC, Gallegos MS, Marcolin MA, Ricco MA, Cook MB, Bonilla P, Schestatsky P, Galhardoni R, Silva V, Delgado Barrera W, Caumo W, Bouhassira D, Chipchase LS, Lefaucheur JP, Teixeira MJ, de Andrade DC. Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC2-NIN-CP). Pain Rep. 2019;4:e692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Ferro Moura Franco K, Lenoir D, Dos Santos Franco YR, Jandre Reis FJ, Nunes Cabral CM, Meeus M. Prescription of exercises for the treatment of chronic pain along the continuum of nociplastic pain: A systematic review with meta-analysis. Eur J Pain. 2021;25:51-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 44. | Rice D, Nijs J, Kosek E, Wideman T, Hasenbring MI, Koltyn K, Graven-Nielsen T, Polli A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J Pain. 2019;20:1249-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 45. | Elsner B, Kwakkel G, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J Neuroeng Rehabil. 2017;14:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 46. | Chen KS, Chen R. Invasive and Noninvasive Brain Stimulation in Parkinson's Disease: Clinical Effects and Future Perspectives. Clin Pharmacol Ther. 2019;106:763-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 47. | Dagan M, Herman T, Harrison R, Zhou J, Giladi N, Ruffini G, Manor B, Hausdorff JM. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson's disease. Mov Disord. 2018;33:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 48. | Machado DGDS, Unal G, Andrade SM, Moreira A, Altimari LR, Brunoni AR, Perrey S, Mauger AR, Bikson M, Okano AH. Effect of transcranial direct current stimulation on exercise performance: A systematic review and meta-analysis. Brain Stimul. 2019;12:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 49. | Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988-3998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |