Published online Dec 22, 2016. doi: 10.5498/wjp.v6.i4.442
Peer-review started: July 2, 2016
First decision: August 5, 2016
Revised: September 9, 2016
Accepted: October 5, 2016
Article in press: October 9, 2016
Published online: December 22, 2016
To investigate whether differential influence on the QTc interval exists among four second generation antipsychotics (SGAs) in psychosis.
Data were drawn from a pragmatic, randomized head-to-head trial of the SGAs risperidone, olanzapine, quetiapine, and ziprasidone in acute admissions patients with psychosis, and with follow-up visits at discharge or maximally 6-9 wk, 3, 6, 12 and 24 mo. Electrocardiograms were recorded on all visits. To mimic clinical shared decision-making, the patients were randomized not to a single drug, but to a sequence of the SGAs under investigation. The first drug in the sequence defined the randomization group, but the patient and/or clinician could choose an SGA later in the sequence if prior negative experiences with the first one(s) in the sequence had occurred. The study focuses on the time of, and actual use of the SGAs under investigation, that is until treatment discontinuation or change, in order to capture the direct medication effects on the QTc interval. Secondary intention-to-treat (ITT) analyses were also performed.
A total of 173 patients, with even distribution among the treatment groups, underwent ECG assessments. About 70% were males and 43% had never used antipsychotic drugs before the study. The mean antipsychotic doses in milligrams per day with standard deviations (SD) were 3.4 (1.2) for risperidone, 13.9 (4.6) for olanzapine, 325.9 (185.8) for quetiapine, and 97.2 (42.8) for ziprasidone treated groups. The time until discontinuation of the antipsychotic drug used did not differ in a statistically significant way among the groups (Log-Rank test: P = 0.171). The maximum QTc interval recorded during follow-up was 462 ms. Based on linear mixed effects analyses, the QTc interval change per day with standard error was -0.0030 (0.0280) for risperidone; -0.0099 (0.0108) for olanzapine; -0.0027 (0.0170) for quetiapine, and -0.0081 (0.0229) for ziprasidone. There were no statistically significant differences among the groups in this regard. LME analyses based on ITT groups (the randomization groups), revealed almost identical slopes with -0.0063 (0.0160) for risperidone, -0.0130 (0.0126) for olanzapine, -0.0034 (0.0168) for quetiapine, and -0.0045 (0.0225) for ziprasidone.
None of the SGAs under investigation led to statistically significant QTc prolongation. No statistically significant differences among the SGAs were found.
Core tip: Antipsychotic drugs have a bad reputation of prolonging the QTc interval, and thereby possibly leading to fatal incidents of Torsade de pointes arrhythmias and sudden cardiac death. Differential propensities for QTc prolongation among second generation antipsychotics (SGAs) have been claimed, but lack substantial support from pragmatic studies. None of the SGAs was statistically significantly prolonging the QTc interval in the present pragmatic study, and no statistically significant differences among the drug groups were found for this outcome. Even in a situation with a substantial proportion with QTc prolongation at admittance any of the SGAs under investigation seemed to be safe choices in the present study.
- Citation: Olsen RE, Kroken RA, Bjørhovde S, Aanesen K, Jørgensen HA, Løberg EM, Johnsen E. Influence of different second generation antipsychotics on the QTc interval: A pragmatic study. World J Psychiatr 2016; 6(4): 442-448
- URL: https://www.wjgnet.com/2220-3206/full/v6/i4/442.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i4.442
Sudden cardiac death (SCD) is a rare, but dramatic event in patients with schizophrenia, with a standardized mortality rate of 4.5 compared to in the general population[1]. Antipsychotic drugs have been implicated as conveying a risk for SCD because of their potential for prolonging the heart rate corrected QT (QTc) interval of the electrocardiogram (ECG) which may lead to polymorphic ventricular tachycardia [Torsade de pointes (TdP)], ventricular fibrillation and heart arrest[2,3]. Some agents have even been temporarily or permanently withdrawn from the market[4,5]. Indeed different propensities for inducing QT interval prolongation have been reported for various antipsychotic drugs, with ziprasidone among the worst offenders[2,6]. Several methodological issues have been raised however, with regards to how well differences, derived mainly from phase III randomized controlled trials (RCTs), might translate into usual clinical practice[2,7]. Potential limitations include the numerous exclusion criteria, with a risk of selection bias, and the short durations of most RCTs of antipsychotic efficacy. To combat some of the limitations, the pragmatic trial of effectiveness design has been launched in an attempt to deliver more relevant data for clinical decision makers. Effectiveness studies are characterized by heterogeneous samples and study settings more representative of usual clinical practice[8].
Pragmatic studies investigating the different propensities of QTc prolongation of second generation antipsychotics (SGAs) in real-life settings are rare, but we have previously reported QTc interval findings from a pragmatic RCT of SGAs in acutely admitted patients with psychosis and followed for 24 mo[9]. Only the intention-to-treat (ITT)/overall change of the QTc intervals during the full 24-mo follow-up were analysed in this study, regardless of the many drug changes that occurred during the study. Furthermore, we have recently published cross-sectional data on the proportion with prolonged QTc intervals at admittance and at the end of the acute treatment phase approximately 4 wk later[10]. About a quarter of the sample had borderline prolonged or prolonged QTc intervals at admittance, with a reduction of this proportion at follow-up. The substantial proportion with prolonged QTc intervals could theoretically be at particular risk if the “wrong” antipsychotic drug were initiated, and we want to compare risperidone, olanzapine, quetiapine, and ziprasidone head-to-head in a consecutive sample in the period from initiation in the acute phase of psychosis, up until discontinuation, in order to determine which drug, if any, could be considered the safest in this regard.
Accordingly, the primary aim was to investigate whether differential influence on the QTc interval existed among the SGAs under investigation. We hypothesized that ziprasidone would increase the QTc interval during follow-up, and that the QTc interval change in those receiving ziprasidone would be different from that in the other drug groups.
The Bergen Psychosis Project (BPP) compared the effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone with a 2-year follow-up[9]. The study was approved by the Regional Committee for Medical Research Ethics (RCMRE) and the Norwegian Social Science Data Services, and was sponsored independently of the pharmaceutical industry. The study investigates the first allocated antipsychotic drugs until treatment discontinuation or change to another antipsychotic drug, to isolate the effect of the drugs on the QTc interval.
The RCMRE allowed eligible patients to be included before informed consent was provided. This enabled a clinically representative sample. Adults acutely admitted for psychosis were consecutively recruited when antipsychotic drugs in the oral formulation were indicated. A symptom threshold for inclusion was set at ≥ 4 on at least one of the following items of the Positive and Negative Syndrome Scale (PANSS)[11]: Delusions, Hallucinatory behavior, Grandiosity, Suspiciousness/persecution, or Unusual thought content. Diagnostic valuations were conducted by the clinical staff (psychiatrists or specialists in clinical psychology) according to the ICD-10 (http://apps.who.int/classifications/icd10/browse/2010/en). Exclusion criteria were: Antipsychotic drugs in the oral formulation not indicated, manic psychosis because of concerns of reduced cooperativeness with assessments, other behavioral or mental reasons causing inability to cooperate, language barrier towards spoken Norwegian, electroconvulsive therapy indicated, or established clozapine treatment. Drug-induced psychosis was not an exclusion criterium when antipsychotic drug therapy was deemed indicated by the attending clinician.
Eligible patients were offered the first SGA in a random order of the investigational agents. The result of the randomization was known both to the clinical staff and the patient. The SGA that was first on the list defined the randomization group. The treating physician and/or the patient could select the next drug in the sequence if the first could not be used. Reasons for unselecting the first drug included contraindications, or negative experiences with previous use of the drug. In theory, contraindications or previous negative experiences could also include QTc-interval pathologies but this was not the case for any of the patients screened for eligibility. The same procedure was repeated if the next drug could not be used. Doses, concomitant use of other medicines, or antipsychotic drug changes were determined by the attending physician or psychiatrist. Combinations of antipsychotic drugs were not allowed except in some sporadic instances.
Importantly, the present study does not focus on the randomization groups but on the actual chosen SGA from the sequence. We have previously reported that there were no statistically significant differences among the randomization groups regarding the percentage choosing a different SGA from the first one on the list[9].
Assessments were conducted at baseline, at discharge or at 6-9 wk from baseline if still admitted, and at 3, 6, 12, and 24 mo from baseline. Other than the PANSS interview, the assessments included the Calgary Depression Scale for Schizophrenia[12], the Clinical Drug and Alcohol Use Scales[13], the Clinical Global Impression-Severity of Illness scale[14], and the Global Assessment of Functioning-Split Version, Functions scale[15]. Blood was collected between 8 am and 10 am for analysis of serum levels of the antipsychotic drugs.
Until discharge, or at 6-9 wk at the latest the study procedures were part of the hospital’s routine quality project for patients with psychosis, and the procedures were part of the patients’ medical record. At this point, the patients were asked for informed consent to be contacted and included in the follow-up project.
At follow-up visits 3, 6, 12, and 24 mo after baseline, measures of psychopathology, blood sampling, and ECG recordings were repeated, and all medications were recorded.
The QTc interval estimation was done automatically by a Philips Pagewriter Trim II cardiograph at admission and discharge/at 6-9 wk when the patient was still in hospital. At later visits after discharge, a Schiller AT-101 cardiograph was used. Bazett’s formula was used for correction. The ECG recording at baseline was done before the first administration of the study SGAs.
The baseline data of were analyzed using IBM SPSS software, version 23.0, and by means of exact χ2 tests (categorical data) and one-way ANOVAs (continuous data). These tests were also applied for baseline comparisons between those lost to follow-up before retesting and those with repeated tests.
Power analyses were run in R (http://www.r-project.org) by means of linear mixed effects (LME) models. The baseline QTc interval and standard deviations were based on the results of the model used in the present study, and slope differences between the groups deemed to be of clinical significance were used in the model. The drop-out rate was set to 3% per month, and 10000 simulations were run. Based on the power analysis the study should have 96% power to detect 2.5% QTc interval differences between the drug groups with 30 subjects in each group.
Changes in the QTc intervals were analysed in R by means of LME models (http://www.r-project.org)[16]. Fixed effects, i.e., systematic differences between the drugs, gave different linear slopes in the four treatment groups, technically a group-by-time interaction with a potential for baseline group differences. The sensitivity analyses based on the ITT groups had no baseline group differences as this was based on the randomization groups. The model calculated overall QTc interval change per day during follow-up that could be visually represented by the slope of a linear curve with time plotted against the QTc interval. The model utilized all available data and handled different numbers of visits by individual patients, as well as differences in time between visits. The LME has demonstrated superior statistical power in studies where missing data cannot be ignored[17], as is the case in the present study. Benjamini-Hochberg adjustments were applied for multiple comparisons. An α-level = 0.05, two-sided, was used as a threshold for statistical significance. The statistical review of the study was performed by a biomedical statistician.
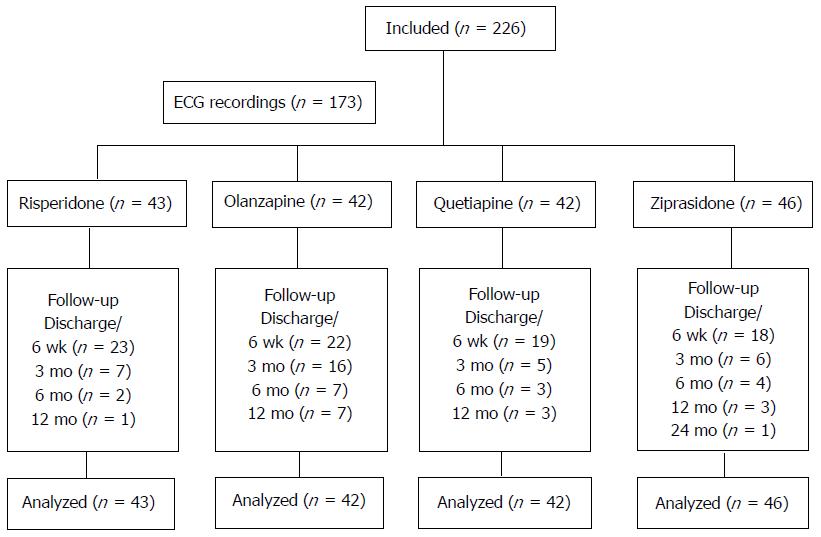
A total of 226 patients were included in the study, and 173 patients underwent ECG assessments. The study enrolment and follow-up is presented in Figure 1.
Information about baseline demographics and clinical descriptives are given in Table 1. There were no statistically significant differences between those with and without ECG assessments, respectively, for any baseline characteristic except for the distribution of alcohol use (exact χ2 test: P = 0.002), with a larger proportion with alcohol dependency among those without ECG recordings compared to those with ECG recordings (21.2% vs 7.0%). There were no statistically significant differences among the SGAs for any of the descriptives except for a higher PANSS positive subscale score in the olanzapine group compared to the risperidone group (one-way ANOVA: P = 0.025; mean difference 2.7 points; 95%CI: 0.2-5.1), and compared to the ziprasidone group (one-way ANOVA: P = 0.045; mean difference 2.5 points; 95%CI: 0.4-4.9). There were no statistically significant differences for any baseline characteristic between those with only a baseline test and those with repeated tests.
| Characteristics | Risperidone (n = 43) | Olanzapine (n = 42) | Quetiapine (n = 42) | Ziprasidone (n = 46) | All patients (n =173) |
| Gender | |||||
| Male | 34 (79.1) | 28 (66.7) | 29 (69.0) | 29 (63.0) | 120 (69.4) |
| Antipsychotic naïve | 17 (40.5) | 15 (35.7) | 21 (50.0) | 20 (44.4) | 73 (42.7) |
| Alcohol last 6 mo | |||||
| None/no misuse | 10 (23.3) | 7 (16.7) | 7 (16.7) | 10 (21.7) | 34 19.7) |
| Dependency | 2 (4.7) | 4 (9.5) | 6 (14.3) | 0 (0.0) | 12 (6.9) |
| Drugs last 6 mo | |||||
| None | 26 (60.5) | 31 (73.8) | 30 (71.4) | 32 (69.6) | 119 (68.8) |
| Misuse | 10 (23.3) | 6 (14.3) | 7 (16.7) | 7 (15.2) | 30 (17.3) |
| Diagnosis1 | n (39) | n (42) | n (41) | n (44) | n (166) |
| Schz and rel. | 22 (56.4) | 25 (59.5) | 25 (61.0) | 22 (50.0) | 94 (56.6) |
| Acute | 4 (10.3) | 4 (9.5) | 2 (4.9) | 4 (9.1) | 14 (8.4) |
| Drug-induced | 6 (15.4) | 6 (14.3) | 5 (12.2) | 5 (11.4) | 22 (13.3) |
| Affective | 4 10.3) | 4 (9.5) | 6 (14.6) | 5 (11.4) | 19 (11.4) |
| Rest | 3 (7.7) | 3 (7.1) | 3 (7.3) | 8 (18.2) | 17 (10.2) |
| Age mean (SD) | 34.5 (15.6) | 32.3 (11.2) | 37.2 (14.8) | 32.4 (13.5) | 34.1 (13.9) |
| QTc admittance mean (SD) | 422.1 (39.7) | 420.9 (33.5) | 421.1 (25.1) | 420.4 (22.0) | 421.1 (30.4) |
| PANSS total mean (SD) | 73.4 (14.0) | 76.0 (14.3) | 73.6 (14.0) | 70.8 (12.4) | 73.4 (13.7) |
| PANSS positive mean (SD) | 18.6 (4.9) | 21.3 (4.6) | 20.0 (3.6) | 18.8 (3.9) | 19.7 (7.5) |
| PANSS negative mean (SD) | 20.8 (8.1) | 18.3 (7.3) | 19.2 (7.1) | 18.4 (7.4) | 19.2 (7.5) |
| PANSS general mean (SD) | 34.0 (6.5) | 36.4 (6.6) | 34.4 (7.6) | 33.6 (6.3) | 34.6 (6.8) |
| CDSS mean (SD) | 6.8 (4.9) | 6.3 (4.9) | 6.4 (4.9) | 7.8 (6.4) | 6.9 (5.3) |
| GAF-F mean (SD) | 30.8 (5.9) | 30.1 (6.0) | 30.6 (7.2) | 32.2 (5.0) | 30.9 (6.0) |
| CGI mean (SD) | 5.2 (0.6) | 5.3 (0.7) | 5.1 (0.7) | 5.0 (0.6) | 5.2 (0.6) |
The mean antipsychotic doses in milligrams per day with standard deviations (SD) were 3.4 (1.2) for risperidone, 13.9 (4.6) for olanzapine, 325.9 (185.8) for quetiapine, and 97.2 (42.8) for ziprasidone treated groups. The mean serum levels in nanomoles per liter with SD and dose reference ranges were 73.3 (54.6) (30.0-120.0) for risperidone, 108.2 (70.9) (30.0-200.0) for olanzapine, 414.2 (548.2) (100.0-800.0) for quetiapine, and 131.0 (101.0) (30.0-200.0) for ziprasidone. The time until discontinuation of the SGAs did not differ in a statistically significant way among the groups (Log-Rank test: P = 0.171).
There were no statistically significant differences among the groups for the concomitant use of another antipsychotic drug, antidepressant, mood stabilizer, benzodiazepine, or anticholinergic drug at any visit except for a higher proportion of concomitant benzodiazepine use in the quetiapine group at the 12-mo visit (exact χ2 test: P = 0.026)
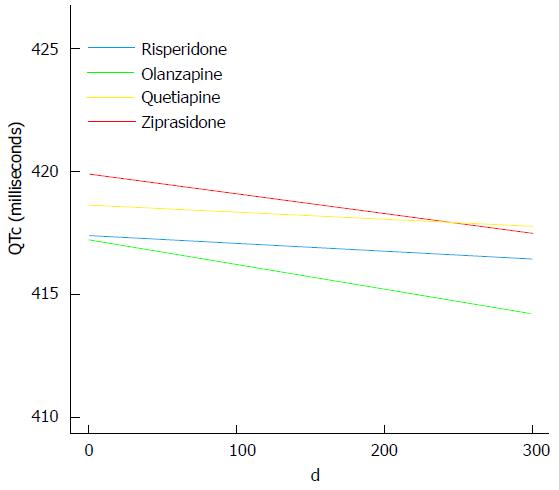
The maximum QTc interval recorded at any follow-up visit was 462 milliseconds (ms). None of the drug groups had statistically significant changes of the QTc interval (LME: P≥ 0.36 for all). The QTc interval change per day with standard error was -0.0030 (0.0280) for risperidone; -0.0099 (0.0108) for olanzapine; -0.0027 (0.0170) for quetiapine, and -0.0081 (0.0229) for ziprasidone (Figure 2).
There were no statistically significant differences among the groups for change of the QTc interval (LME: P≥ 0.72 for all). As a sensitivity analysis, we also performed LME analyses based on the ITT groups (the randomization groups), revealing almost identical slopes with -0.0063 (0.0160) for risperidone, -0.0130 (0.0126) for olanzapine, -0.0034 (0.0168) for quetiapine, and -0.0045 (0.0225) for ziprasidone.
Serum potassium, sodium, and calcium were measured at baseline and at first follow-up. There were no statistically significant differences among the drug groups for any of these electrolytes. Serum prolactin was measured at all points of follow-up. The prolactin level was higher in the risperidone group compared to the quetiapine group at baseline (one way ANOVA: P = 0.015; mean difference 350.3; 95%CI of the mean 46.7-654.0), and significantly higher in the risperidone group compared to all the other groups at first follow-up (one way ANOVA: P = 0.004). These differences did not persist at later visits.
SGAs are among the groups of drugs that have a bad reputation for prolonging the QTc interval, and thereby possibly leading to fatal incidents of TdP and SCD. This issue has received a lot of attention in previous studies, and differential propensities for QTc interval prolongation have been found among antipsychotic drugs[2]. The implications for usual clinical practice are unresolved, however, as the experimental designs of the majority of the studies may limit the generalizability of their findings[8]. The present study was conducted as close to clinical practice as possible by virtue of its pragmatic design. It aimed to investigate whether or not four first-line SGAs used in the acute treatment of psychosis increased the QTc interval - and if so - is there a basis for a ranking between these drugs, regarding the risk of such a prolongation?
The main results of the study were that none of the SGAs prolonged the QTc intervals in a statistically significant way, and that no statistically significant QTC interval differences among the SGAs were found. These findings do not support some of the previous literature, including the comprehensive meta-analysis of 15 different antipsychotics by Leucht and collaborators[6], where ziprasidone was among the top three antipsychotics with regards to QTc prolongation. A recent review also finds that ziprasidone prolongs the QTc interval, but with heterogeneous results in different studies[18]. Theoretically, short term treatment with antipsychotics may not give sufficient plasma levels to influence the heart cells but we find this unlikely as both mean doses and serum levels for the SGAs under investigation were in the therapeutic range. However, pharmacokinetic estimations are beyond the scope of the present study. Indeed, we have previously demonstrated that the distinct side-effect profiles derived from phase III RCTs are dampened in pragmatic studies[19].
Moving beyond the mean scores one might suspect the existence of a subgroup of patients with pathologically prolonged QTc intervals, but not numerous enough to alter the mean scores substantially. All the more there is reason to believe this group could be clinically very important. During follow-up, however, none of the participants had critically prolonged QTc intervals as the maximum QTc interval recorded was 462 ms. In a previous study we reported that approximately 25% of the patients had prolonged QTc intervals, or borderline prolongations, at the time of admittance to hospital[10]. Most likely, this prolongation was due to other causes than the use of SGAs including agitation, because many of the patients had never used antipsychotic drugs before, or those who had used SGAs had, in many instances, discontinued their antipsychotics some time before hospital admission. Taken together, the present study seems to indicate that, even in a situation with a substantial proportion of patients with QTc prolongation at admittance, any of the SGAs under investigation are safe choices.
Some limitations to the study need consideration. For one, attrition was substantial during follow-up. Two hundred and twenty-six patients participated in the study, but only 173 patients underwent ECG assessments because of feasibility issues in the acute phase. There were no statistically significant differences in baseline characteristics between those with and without ECG assessments except a small difference in alcohol consumption patterns. Therefore, selection bias seems unlikely. Furthermore, no baseline characteristics showed statistically significant differences between those with only baseline tests and those with repeated tests. Finally we chose LME statistics for the longitudinal data analyses to handle drop-outs and missing data.
Even though the BPP was a randomized study, the randomization was not to a single SGA but to a sequence of all of the four SGAs under investigation to mimic the clinical process of choosing a drug for a patient as closely as possible. The first drug in the sequence defined the randomization group, but about 20% chose a different SGA than the one first on the list in the sequence, although this proportion did not differ in a statistically significant way among the groups, as accounted for in a previous publication[9]. In the present study, we focused not on the randomization groups, but on the drugs actually used as we wanted to investigate the direct drug effects on the ECG. However, this also violates the effects of randomization and could introduce bias. However, the 173 patients divided themselves by chance into fairly even large groups for the four SGAs, and the only statistically significant baseline difference among the drug groups was a higher PANSS positive subscale score in the olanzapine group. Also no statistically significant differences in times to discontinuation were found. Finally, the sensitivity analyses based on ITT groups gave almost identical results. Significant bias therefore seems unlikely.
ECG assessments were carried out using two automatic measuring devices which both used Bazett’s formula for heart rate correction of the QT intervals. As this formula has a tendency to overcorrect QT intervals at higher heart rates, Frederica’s formula is now the preferred one. Both the stress associated with acute admissions as well as psychotropic medications themselves could increase the heart rate in patients with mental illness. Nielsen and collaborators[2] described incident differences in measurements by 12 ms when the heartrate was > 70/min (Bazett’s 475 ms vs Frederica’s 463 ms), and increases to 24 ms (508 vs 484) when the heartrate was > 80/min. Accordingly, the use of Bazett’s formula in our study may have led to higher QTc measures compared to if Frederica’s formula had been used. Ideally, all the recordings should have been carried out with the same equipment, but for practical reasons this was not possible. We have no reason to suspect that this should introduce bias, and believe the use of the same correction formula is the most important factor for comparable recordings.
The use of concomitant medication was equally distributed among the groups. However, it cannot be ruled out with certainty that the effects of other drugs may have influenced the slopes of the QTc recordings. The direction of any such influence would be hard to predict. There were more men than women in the study, which may have led to somewhat lower mean QTc total intervals as women have longer QTc intervals than men. This should not have introduced bias to the group comparisons as gender was evenly distributed among the drug groups. The risperidone group had higher serum prolactin levels at baseline and the first follow-up, but not thereafter. Any influence on the QTc interval from the prolactin level could only be speculative.
Despite the above mentioned limitations we conclude that our findings do not support that any of the SGAs under investigation leads to QTc prolongation. No statistically significant differences among the SGAs were found, and all the drugs on which the study is based may be considered to be safe alternatives in this regard.
We wish to thank the Division of Psychiatry, Haukeland University Hospital for financial support, and the Clinical Departments for their enthusiasm and cooperation.
The ability of antipsychotic drugs to prolong the QT interval of the electrocardiogram (ECG) has been associated with increased risk of sudden cardiac death in patients using these drugs.
Although differential propensities among various antipsychotics for QT prolongation have been found, the generalizability to, and hence the clinical relevance of these findings in, the population have been questioned.
The majority of clinical antipsychotic drug trials typically include highly selected patient samples where concomitant illicit drug use, other mental or physical comorbidities, or the need for other psychotropics are exclusion criteria. Furthermore, the follow-up is often of short duration. The main advantages of the present study include the consecutive recruitment of acutely admitted patients with psychosis; the diagnostically and symptomatically heterogeneous sample, and the long follow-up. These factors should make the results more applicable for pharmacological decision making in clinical departments.
Based on the present study, all the second-generation antipsychotics under investigation seem to be safe choices in acutely admitted patients with psychosis.
The QT interval: The ECG measure of the duration of ventricular de- and repolarization of the heart.
In this paper, the authors investigated investigate whether differential influence on the QTc interval exists among four second generation antipsychotics (SGAs) in 173 patients. They concluded that none of the SGAs under investigation led to QTc prolongation and no differences among the SGAs were found. This study is straightforward and generally well described.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tsai SJ, Wu SN S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Hou PY, Hung GC, Jhong JR, Tsai SY, Chen CC, Kuo CJ. Risk factors for sudden cardiac death among patients with schizophrenia. Schizophr Res. 2015;168:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Nielsen J, Graff C, Kanters JK, Toft E, Taylor D, Meyer JM. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25:473-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Lindström E, Farde L, Eberhard J, Haverkamp W. QTc interval prolongation and antipsychotic drug treatments: focus on sertindole. Int J Neuropsychopharmacol. 2005;8:615-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. WHO pharmaceutical newsletter, 2005. Available from: http://www.who.int/medicines/publications/newsletter/en/. [Cited in This Article: ] |
| 6. | Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1686] [Cited by in F6Publishing: 1631] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 7. | Leucht S, Heres S, Hamann J, Kane JM. Methodological issues in current antipsychotic drug trials. Schizophr Bull. 2008;34:275-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | March JS, Silva SG, Compton S, Shapiro M, Califf R, Krishnan R. The case for practical clinical trials in psychiatry. Am J Psychiatry. 2005;162:836-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Johnsen E, Kroken RA, Wentzel-Larsen T, Jørgensen HA. Effectiveness of second-generation antipsychotics: a naturalistic, randomized comparison of olanzapine, quetiapine, risperidone, and ziprasidone. BMC Psychiatry. 2010;10:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Johnsen E, Aanesen K, Sriskandarajah S, Kroken RA, Løberg EM, Jørgensen HA. QTc Prolongation in Patients Acutely Admitted to Hospital for Psychosis and Treated with Second Generation Antipsychotics. Schizophr Res Treatment. 2013;2013:375020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261-276. [PubMed] [Cited in This Article: ] |
| 12. | Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247-251. [PubMed] [Cited in This Article: ] |
| 13. | Drake RE, Rosenberg SD, Mueser KT. Assessing substance use disorder in persons with severe mental illness. New Dir Ment Health Serv. 1996;3-17. [PubMed] [Cited in This Article: ] |
| 14. | Guy W. Assessment Manual for Psychopharmacology - Revisited. Rockville: US Department of Health and Human Services 1976; . [Cited in This Article: ] |
| 15. | Karterud S, Pedersen G, Loevdahl H, Friis S. Global Assessment of Functioning - Split Version (S-GAF): Background and Scoring Manual. Oslo, Norway: Ullevaal University Hospital, Department of Psychiatry 1998; . [Cited in This Article: ] |
| 16. | Pinheiro C, Bates DM. Mixed effects models in S and S-plus. New York: Springer 2000; . [Cited in This Article: ] |
| 17. | Hedden SL, Woolson RF, Carter RE, Palesch Y, Upadhyaya HP, Malcolm RJ. The impact of loss to follow-up on hypothesis tests of the treatment effect for several statistical methods in substance abuse clinical trials. J Subst Abuse Treat. 2009;37:54-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Hasnain M, Vieweg WV. QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs. 2014;28:887-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Johnsen E, Jørgensen HA. Effectiveness of second generation antipsychotics: a systematic review of randomized trials. BMC Psychiatry. 2008;8:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |