Copyright
©2012 Baishideng.
World J Psychiatr. Dec 22, 2012; 2(6): 102-113
Published online Dec 22, 2012. doi: 10.5498/wjp.v2.i6.102
Published online Dec 22, 2012. doi: 10.5498/wjp.v2.i6.102
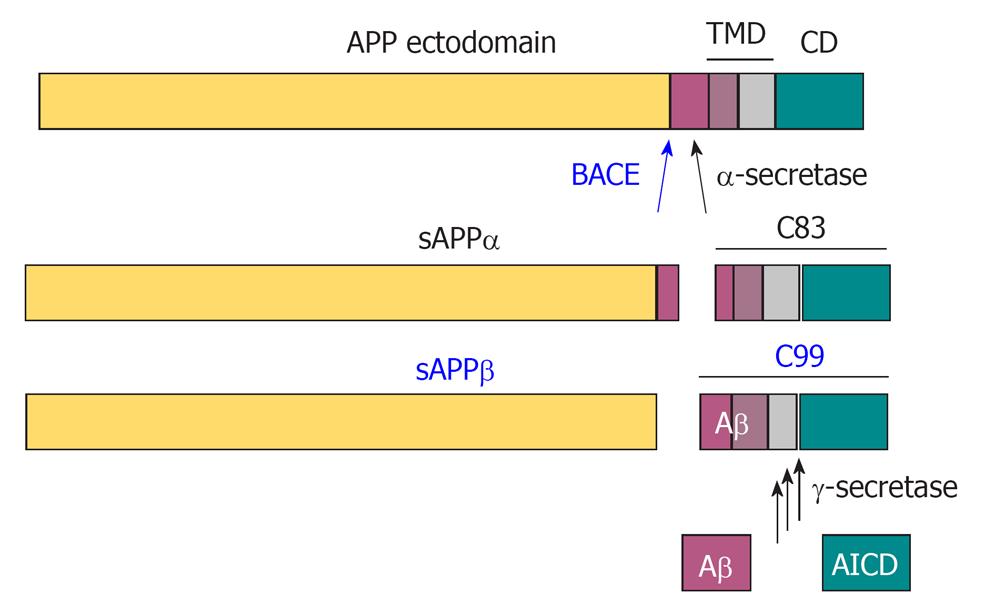
Figure 2 Proteolytic processing of amyloid precursor protein by the secretases.
In a non-amyloidogenic pathway, α-secretase cleaves amyloid precursor protein (APP) within the Aβ domain to release the α-cleaved soluble N-terminal fragment, sAPPα. β-Amyloid precursor cleaving enzyme (BACE) cleaves APP at the N-terminus of Aβ to release the β-cleaved soluble N-terminal fragment sAPPβ, and thereby initiates the amyloidogenic pathway. The remaining 99 amino acid-long C-terminal fragment (C99) is further processed at multiple sites by γ-secretase to release the APP intracellular domain (AICD) in the cytosol and Aβ peptides in the extracellular space.
- Citation: Evin G, Li QX. Platelets and Alzheimer’s disease: Potential of APP as a biomarker. World J Psychiatr 2012; 2(6): 102-113
- URL: https://www.wjgnet.com/2220-3206/full/v2/i6/102.htm
- DOI: https://dx.doi.org/10.5498/wjp.v2.i6.102