Published online Feb 19, 2024. doi: 10.5498/wjp.v14.i2.234
Peer-review started: September 30, 2023
First decision: December 6, 2023
Revised: December 12, 2023
Accepted: December 29, 2023
Article in press: December 29, 2023
Published online: February 19, 2024
Panic disorder (PD) involves emotion dysregulation, but its underlying mechanisms remain poorly understood. Previous research suggests that implicit emotion regulation may play a central role in PD-related emotion dysregulation and symptom maintenance. However, there is a lack of studies exploring the neural mechanisms of implicit emotion regulation in PD using neurophysiological indicators.
To study the neural mechanisms of implicit emotion regulation in PD with event-related potentials (ERP).
A total of 25 PD patients and 20 healthy controls (HC) underwent clinical eva-luations. The study utilized a case-control design with random sampling, selecting participants for the case group from March to December 2018. Participants performed an affect labeling task, using affect labeling as the experimental condition and gender labeling as the control condition. ERP and behavioral data were recorded to compare the late positive potential (LPP) within and between the groups.
Both PD and HC groups showed longer reaction times and decreased accuracy under the affect labeling. In the HC group, late LPP amplitudes exhibited a dynamic pattern of initial increase followed by decrease. Importantly, a significant group × condition interaction effect was observed. Simple effect analysis revealed a reduction in the differences of late LPP amplitudes between the affect labeling and gender labeling conditions in the PD group compared to the HC group. Furthermore, among PD patients under the affect labeling, the late LPP was negatively correlated with disease severity, symptom frequency, and intensity.
PD patients demonstrate abnormalities in implicit emotion regulation, hampering their ability to mobilize cognitive resources for downregulating negative emotions. The late LPP amplitude in response to affect labeling may serve as a potentially valuable clinical indicator of PD severity.
Core Tip: This study investigates neural mechanisms of implicit emotion regulation in panic disorder (PD) using event-related potentials. PD patients exhibit anomalies during an affect labeling task, including prolonged reaction times and reduced accuracy. Neurophysiological data indicate diminished differences in late positive potential (LPP) amplitude between affect labeling and gender labeling in PD, negatively correlating with disease severity, symptom frequency, and intensity. Resultantly, PD patients demonstrate impaired implicit emotion regulation, hindering cognitive resource mobilization for negative emotion downregulation. The late LPP amplitude in response to affect labeling may serve as a valuable clinical indicator of PD severity.
- Citation: Wang HY, Li LZ, Chang Y, Pang XM, Zhang BW. Impaired implicit emotion regulation in patients with panic disorder: An event-related potential study on affect labeling. World J Psychiatry 2024; 14(2): 234-244
- URL: https://www.wjgnet.com/2220-3206/full/v14/i2/234.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i2.234
Panic disorder (PD) is a severe anxiety disorder characterized by spontaneous and recurring panic attacks and a constant fear of experiencing panic symptoms[1]. Epidemiological studies have reported a lifetime prevalence of PD at 4.7%[2]. Despite advancements in understanding PD, the neural mechanisms underlying its onset remain incompletely under-stood. Clinical observations and experimental evidence suggest that deficiencies in emotion regulation play a crucial role in the development of PD[3] because they contribute to heightened fear responses.
Emotion regulation involves individuals modifying their emotional responses in response to environmental demands, exerting control over the duration, intensity, and underlying characteristics of emotions[4]. Previous studies on emotion regulation have primarily focused on explicit strategies. In the context of PD, research on the neural mechanisms of emotion regulation has predominantly examined conscious strategies, revealing abnormal activation in the dorsolateral prefrontal cortex (DLPFC) and the ventromedial prefrontal cortex in individuals with PD during emotional regulation processes[3]. However, meta-analyses on repetitive transcranial magnetic stimulation (rTMS) targeting brain regions associated with conscious emotion regulation, such as the DLPFC, have not provided conclusive evidence regarding the efficacy of rTMS in treating PD[5,6].
According to Gross's model[7], emotion regulation strategies can be explicit (conscious) or implicit (unconscious). In clinical settings, it is observed that panic attacks in PD patients often occur suddenly and cease abruptly, with some patients even experiencing attacks during sleep[1,8]. There is no conscious emotion regulation process before or during panic attacks in patients. The catastrophic cognitive theory suggests that in PD, patients automatically evaluate both internal and external stimuli that are insufficient to elicit intense reactions as threat signals, leading to the initiation and maintenance of their anxiety[8]. Additionally, our previous findings indicate that PD patients exhibit anomalous mis-match negativity responses to acoustic and visual stimuli (both emotional and non-emotional), indicating abnormal automatic information processing in these individuals[9]. Therefore, we speculate that the core mechanism underlying emotion regulation dysfunction in PD patients may not originate from explicit abnormalities in emotion regulation but instead from implicit abnormalities in emotion regulation.
While the reported evidence suggests a significant role of implicit emotion dysregulation in the pathogenesis of PD[10], the specific mechanisms underlying these regulatory effects remain unclear. Affect labeling is an effective way to reduce unwanted emotions and the distress associated with negative events through putting feelings into words[11]. As the intent to reduce distress is not explicit, affect labeling has been conceptualized as a form of implicit emotion regulation that typically involves verbally labeling the emotional content of a facial stimulus, such as labeling an angry facial expression as "angry" or a fearful facial expression as "fearful"[11,12]. Although the regulatory effects of affect labeling on emotions have been established[11,13], the precise mechanism by which it reduces emotions, whether through direct weakening or a dynamic process of initial enhancement followed by attenuation, remains unclear, especially in PD patients. Therefore, employing high-temporal-resolution event-related potentials (ERP) can help elucidate these questions.
The late positive potential (LPP) is a prominent component of ERP, typically occurring around 300 ms after stimulus onset. It is widely utilized in the study of emotion regulation[14], and its amplitude is known to increase with the subjective intensity of emotional experience, serving as an indicator of emotional regulation effectiveness. While previous LPP research suggests that affect labeling can reduce emotional experience[11,13], these findings are based on measuring the overall effect after labeling. Affect labeling tasks involve cognitive and motivation-related processing, and the LPP reflects changes in cognitive resources and motivation-related stimulus processing[15]. As a result, the amplitude of the LPP may dynamically vary instead of simply weakening.
To date, no studies have investigated the neural correlates of affect labeling in PD using ERP. In our current investigation, we recorded LPP data during affect labeling tasks performed by both PD patients and healthy individuals. We used emotional faces (negative and positive) as stimuli, building upon findings from research on implicit emotion dysregulation in anxiety disorders[16]. Building on clinical observations and previous studies, we developed two hypotheses: (1) In healthy individuals, the amplitude of the LPP is expected to dynamically fluctuate rather than simply decrease when using affect labeling to downregulate emotions; and (2) Compared to healthy controls (HC), individuals with PD are anticipated to show a deficit in the regulatory effect of affect labeling, potentially attributed to impaired cognitive processing.
Twenty-five individuals (7 males, 18 females) diagnosed with clinically predominant PD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV)[17], and 20 HC (9 males, 11 females) without a history of DSM-IV Axis I disorders were recruited from the outpatient and emergency departments of the First Affiliated Hospital of Dalian Medical University and the surrounding communities. The sample size was determined based on previously published ERP studies related to PD[9]. The PD group scored 14 or higher on the Hamilton Anxiety Rating Scale (HAMA) and 7 or lower on the Hamilton Depression Rating Scale (HAMD). Benzodiazepine medication was discontinued for at least one week, and a standardized Structured Clinical Interview for DSM-IV was conducted by a qualified physician to screen for other Axis I diagnoses. The HC group was recruited during the same period and matched the demographic characteristics of the PD group. The HC group scored 7 or lower on the HAMA and HAMD. PD patients completed the PD Severity Scale (PDSS)[18] and the Panic-Associated Symptom Scale (PASS)[19] to assess the severity of PD and associated symptoms. All participants scored ≥ 24 on the Mini-Mental State Examination and abstained from using psychoactive substances in the 24 h preceding the examination. Participants with other psychiatric disorders, severe physical illnesses, brain disorders, or substance dependence were excluded. Informed consent was obtained from all participants prior to their participation, following the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the First Hospital of Dalian Medical University.
From the Chinese Facial Affective Picture System[20], a set of 60 negative emotional face pictures (including anger, fear, and sadness) and 60 positive emotional face pictures were selected. The distribution of male and female pictures was equal in both categories. Statistical analysis revealed no significant differences in valence and arousal ratings between male and female pictures (t = -1.54, P > 0.05; t = 0.19, P > 0.05). The mean valence ratings for positive male and female pictures were 5.6 (SD = 0.6) and 6.3 (SD = 0.7), respectively, while negative male and female pictures received valence ratings of 2.8 (SD = 0.3) and 3.0 (SD = 0.5), respectively. Arousal ratings showed that positive male and female pictures had mean scores of 4.6 (SD = 1.1) and 4.8 (SD = 1.0), respectively, whereas negative male and female pictures had mean scores of 6.1 (SD = 1.2) and 5.8 (SD = 1.2), respectively.
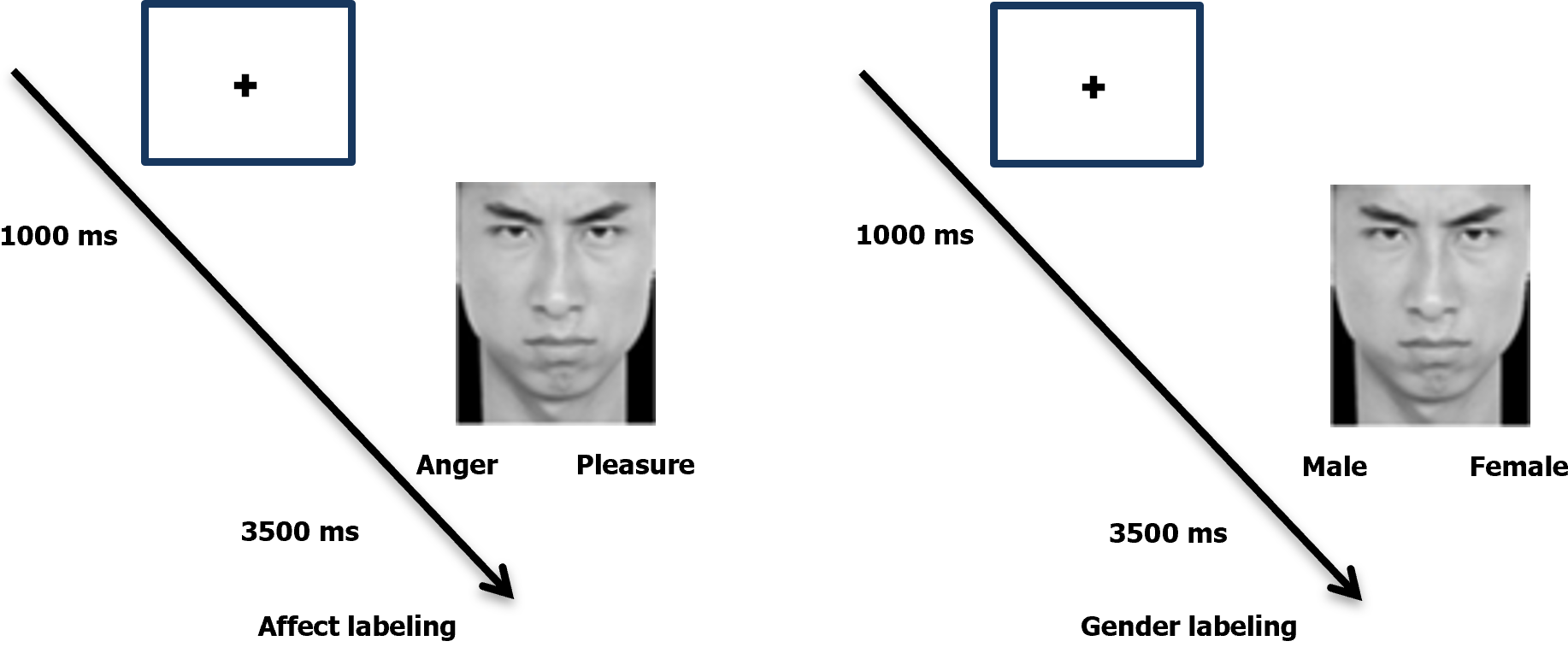
The experiment followed a classic paradigm[21] that involved two tasks: Affect labeling (experimental group) and gender labeling (control group). Participants first fixated on a "+" in the center of the screen for 1000 ms. Then, for 3500 ms, they viewed randomly presented label words and targeted emotional facial expressions. In the gender labeling task, participants determined the gender of the facial image and pressed the left or right key accordingly. The gender labels used were "male" and "female." The affect labeling task followed the same procedure, with participants selecting the label word that best described the emotion. Each trial followed the procedure depicted in Figure 1, with the next trial commencing after the image disappeared. The experiment comprised a practice phase and a formal experiment phase, with the practice images excluded from the formal phase. The practice phase continued until participants demonstrated comprehension of the experimental procedure and achieved an accuracy rate of 80% or higher. The formal experiment phase consisted of 240 trials, with 120 trials allocated to affect labeling (randomly divided into E1 and E2 groups) and 120 trials to gender labeling (randomly divided into S1 and S2 groups). Each set of trials constituted a block. To ensure balance between participant groups, the experiment was conducted in two versions: E1-S1-E2-S2 and S2-E2-S1-E1.
Stimulus presentation and behavioral response data (reaction time, accuracy) were controlled and recorded using E-prime 2.0 software. Electroencephalogram (EEG) data were collected using a Neuroscan SynAmps2 amplifier with a 64-channel Ag/AgCl electrode cap following the standardized 10-20 system. Continuous EEG signals were recorded and analyzed offline using NeuroScan 4.5 software. During EEG recording, an average reference was applied, and bipolar electrodes were used to capture horizontal eye movements and vertical eye movements. The data were sampled at a rate of 500 Hz in direct current mode and filtered with a bandpass of 0.05 to 100 Hz. Electrode impedance was maintained below 5 kΩ throughout the data collection process.
The participants' EEG waveforms were analyzed offline using NeuroScan 4.5 software. Bilateral mastoids served as the average reference electrodes. Manual removal of eye movements and artifacts was performed. The EEG data were filtered with a low-pass filter at 30 Hz and a high-pass filter at 0.5 Hz. Segmentation of the EEG data yielded two time windows: Early LPP and late LPP. The early LPP time window extended from 200 ms before stimulus onset to 1000 ms after stimulus onset, while the late LPP was defined as the time window spanning from 200 ms before response onset to 1000 ms after response onset. Artifacts were rejected, and the data were averaged to obtain the grand average waveform. The measured ERP components, based on prior literature, comprised the early LPP (300-800 ms after stimulus onset, reflecting emotional experience following stimulus presentation) and the late LPP (300-1000 ms after task response, reflecting emotional processing and experience during task performance). To investigate the dynamic changes of the late LPP, the late LPP was further divided into two time windows (350-630 ms, 630-1000 ms) based on the grand average waveform from this experiment. Five electrode sites along the midline of the frontal-parietal region were recorded: Frontal midline (Fz), frontocentral midline (FCz), central midline (Cz), centroparietal midline (CPz), and parietal midline (Pz).
The behavioral data (reaction time, accuracy) and EEG components were analyzed using SPSS 22.0. A 2 × 2 repeated measures analysis of variance (RMANOVA) was conducted on the behavioral data, with group (PD, HC) as the between-subjects factor and task type (affect labeling, gender labeling) as the within-subjects factor. The LPP amplitude data were analyzed using a 2 × 2 × 5 RMANOVA, considering group, task type, and electrode location (Fz, FCz, Cz, CPz, Pz). Separate within-group RMANOVAs (2 × 5) were performed for the HC and PD groups, with task type and electrode location as the within-subjects’ factors. Covariance analysis was applied, taking into account covariates based on the behavioral results. Effect sizes were measured with partial eta-squared (ηp²). Greenhouse-Geisser correction was applied, when necessary, to adjust degrees of freedom and P-values for main effects and interaction effects. Pearson correlation analysis was used to examine the relationships between electrophysiological data, clinical data, and behavioral data in the PD group. Two-tailed tests were conducted, and statistical significance was set at P < 0.05.
Demographic and clinical characteristics of the HC and PD groups are presented in Table 1. There were no significant differences among the three groups in terms of gender, age, body mass index, and education. However, significant differences were observed in HAMA and HAMD scores (P < 0.001), with the PD group exhibiting the highest scores for both HAMA and HAMD.
| PD (n = 25) | HC (n = 20) | t/χ2 | P value | |
| Age (yr) | 47.20 ± 10.928 | 49.65 ± 12.766 | 0.681 | 0.500 |
| Gender (male/female) | 7/18 | 9/11 | 1.401 | 0.236 |
| Education in years | 13.52 ± 10.689 | 14.05 ± 12.037 | 0.156 | 0.877 |
| BMI | 24.89 ± 3.612 | 24.77 ± 3.222 | 0.120 | 0.905 |
| HAMA | 19.40 ± 4.830 | 2.95 ± 3.000 | 13.299 | < 0.001a |
| HAMD | 12.76 ± 2.85 | 2.85 ± 1.599 | 10.581 | < 0.001a |
| PDSS | 8.56 ± 3.906 | N/A | N/A | N/A |
| PASS | 6.84 ± 2.954 | N/A | N/A | N/A |
Descriptive statistics for reaction time and accuracy of the two groups under the two conditions are presented in Table 2. Reaction time analysis showed no significant interaction between group and task type (F1, 41 = 1.209, P = 0.278, ηp2 = 0.029). Task type had a significant main effect (F1, 41 = 18.54, P < 0.001, ηp2 = 0.311), indicating longer reaction times in the affect labeling task. The main effect of group was not significant (F1, 41 = 2.339, P = 0.134, ηp² = 0.054), despite the PD group having longer reaction times in both tasks.
| PD | HC | ||||||||
| Affect labeling | Gender labeling | Affect labeling | Gender labeling | ||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Reaction time | 0.9271 | 4.62 | 0.9594 | 4.85 | 0.9145 | 13.94 | 0.9382 | 14.21 | |
| Accuracy | 1389.32 | 221.43 | 1239.97 | 275.19 | 1247.50 | 261.76 | 1158.9 | 255.87 | |
Accuracy analysis showed no significant interaction between group and task type (F1, 41 = 0.453, P = 0.505, ηp2 = 0.011). The main effect of group was not statistically significant (F1, 41 = 0.468, P = 0.498, ηp2 = 0.011), indicating similar accuracy between the two groups. However, there was a significant difference between the two groups in the affect labeling and gender labeling conditions (F1, 41 = 19.174, P < 0.001, ηp² = 0.319), with higher accuracy in the gender labeling task.
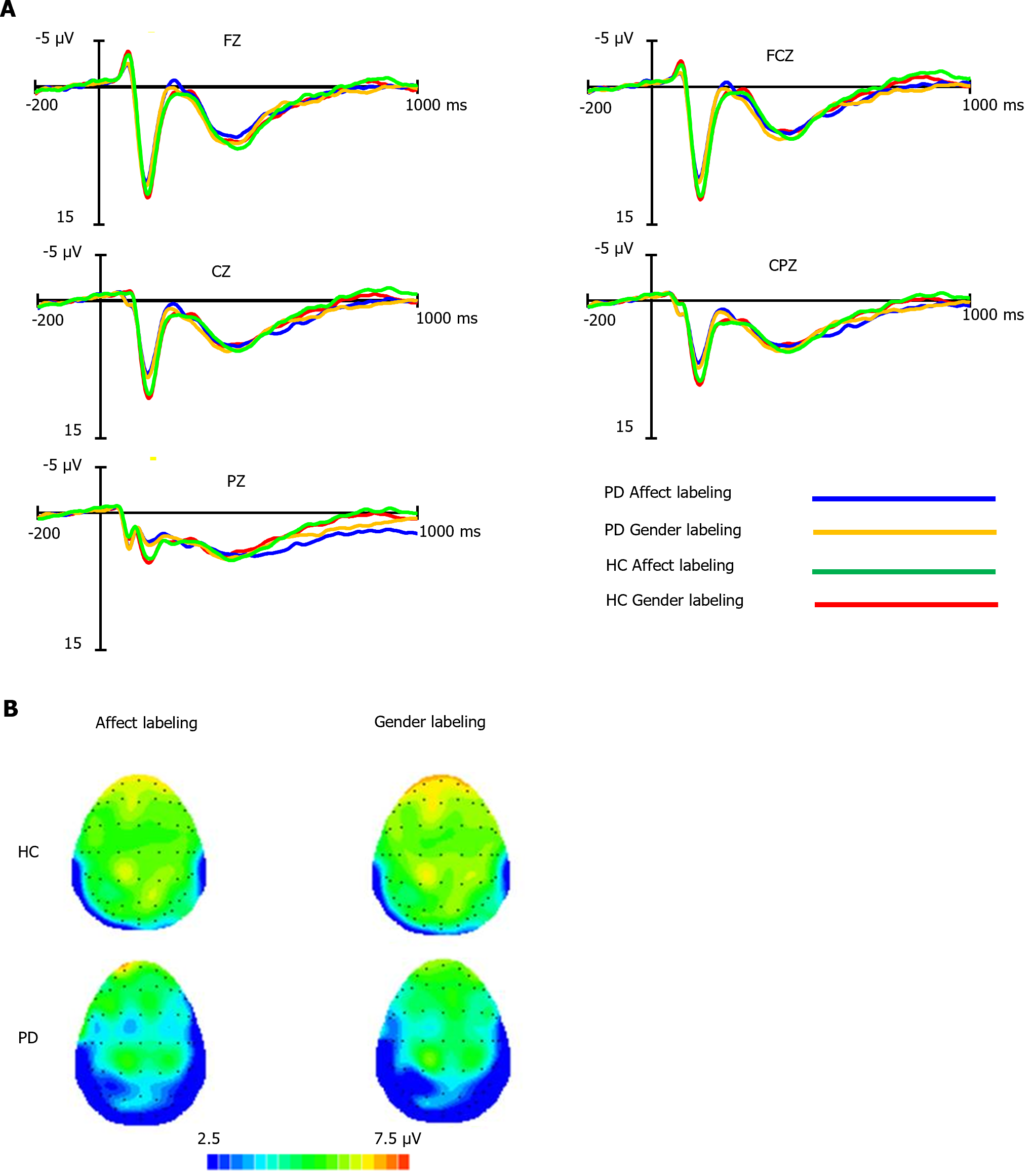
The average early LPP amplitudes for both groups under the two conditions are shown in Supplementary Table 1, with waveform plots in Figure 2A and scalp topography in Figure 2B. Covariance analysis, with accuracy and reaction time as covariates, showed no significant differences in early LPP amplitudes among the groups, paradigm types, electrode locations, and their interactions (P > 0.05 for all comparisons).
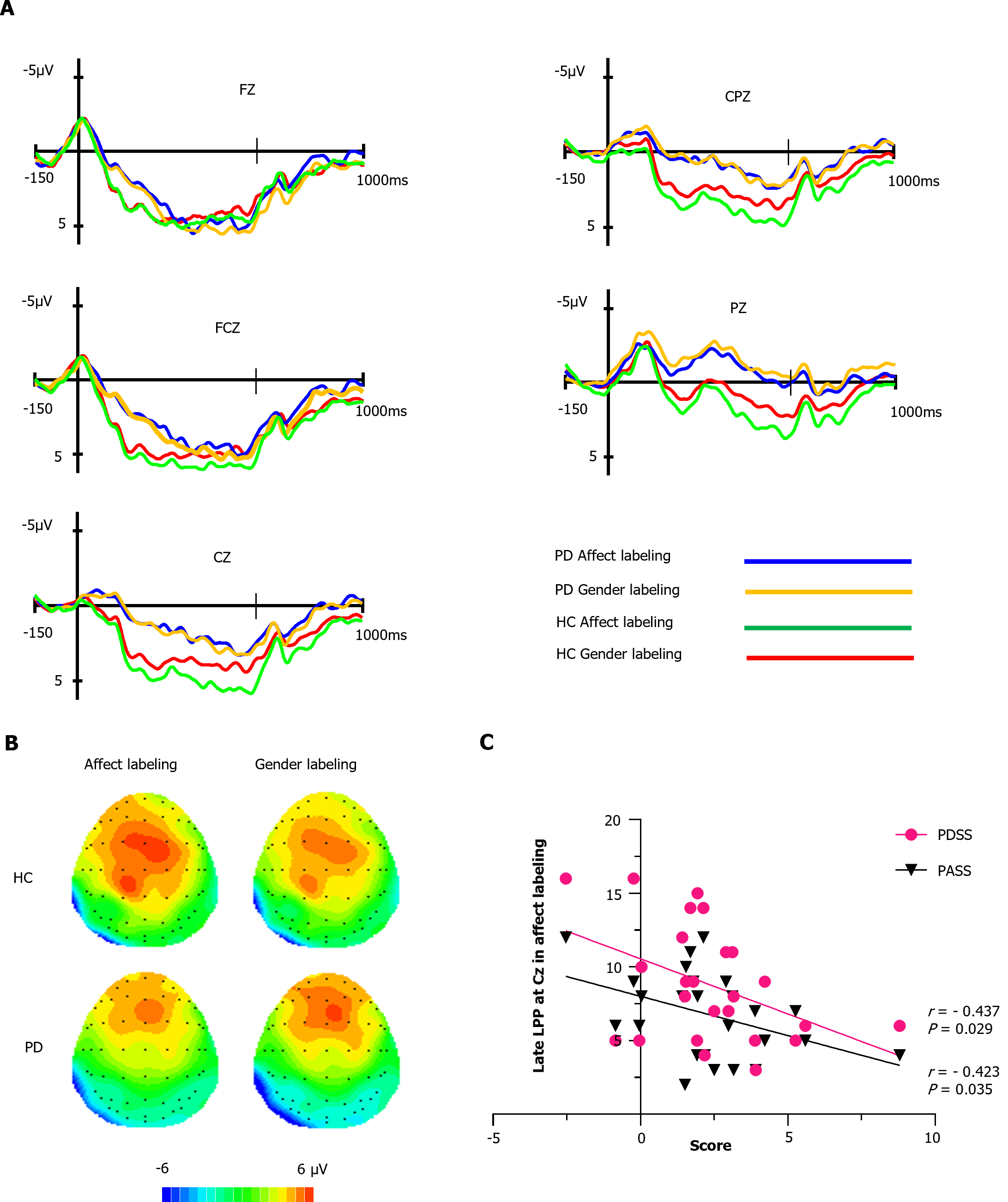
As depicted in Figure 3A, the HC group demonstrated higher amplitudes compared to the PD group at FCz, Cz, CPz, and Pz electrode sites. We conducted an analysis of the overall average late LPP amplitude (300-1000 ms). Within the HC group, the affect labeling condition exhibited larger LPP amplitudes than the gender labeling condition [(2.77 ± 2.75 μV) vs (2.15 ± 2.42 μV)]. Conversely, in the PD group, there was minimal difference in late LPP amplitudes between the affect labeling and gender labeling conditions [(1.49 ± 2.29 μV) vs (1.79 ± 2.63 μV)], which aligns with the observed scalp voltage distribution in Figure 3B. Supplementary Table 2 provides the statistical values for the overall late LPP amplitudes.
A 2 × 2 × 5 RMANOVA was conducted to analyze overall average late LPP amplitude within the 350-630 ms time window. The results revealed significant effects. Firstly, there was a significant main effect of electrode location (F1.46,63 = 33.68, P < 0.001, ηp2 = 0.4394). Specifically, the FCz electrode showed significantly higher amplitudes compared to the Fz, Cz, CPz, and Pz electrodes (P < 0.01 for all comparisons). Furthermore, the analysis indicated significant differences in late LPP amplitudes between the two groups at the Cz (P = 0.015), CPz (P = 0.01), and Pz (P = 0.004) electrode sites.
Importantly, there was a significant task type × group interaction effect (F1,41 = 6.32, P = 0.02, ηp2= 0.14) within the 350-630 ms time window. Follow-up RMANOVA on the HC group revealed that affect labeling LPP (3.88 ± 3.60 μV) was significantly higher than gender labeling LPP (2.33 ± 3.48 μV) (P = 0.044). However, in the PD group, the difference between affect labeling LPP (3.14 ± 3.65 μV) and gender labeling LPP (2.44 ± 4.04 μV) did not reach statistical significance (P > 0.05), suggesting a lack of affect labeling effect. Late LPP amplitudes within the 630-1000 ms time window exhibited no significant differences in late LPP amplitudes across the groups, paradigm types, electrode locations, and their interactions (P > 0.05 for all comparisons).
Notably, in healthy individuals, the affect labeling LPP amplitudes were significantly higher than the gender labeling amplitudes within the 350-630 ms window, which does not indicate a decline in emotional response. However, after 630 ms, there was a trend towards convergence between the affect labeling and gender labeling LPP amplitudes. Further analysis in the 630-1000 ms window revealed a smaller difference in LPP amplitudes between affect labeling and gender labeling [(1.65 ± 2.35 μV) vs (1.36 ± 2.16 μV)], compared to the 350-630 ms window [(3.88 ± 3.60 μV) vs (2.33 ± 3.48 μV)], with no statistically significant difference of the 630-1000 ms window LPP amplitudes (F1, 19 = 1.06, P = 0.316, ηp2 = 0.05), indicating a decline in emotional response. This suggests that the unique nature of affect labeling led to an initial increase followed by a decrease in LPP amplitudes.
We performed Pearson correlation analyses to investigate the associations between late LPP amplitudes (350-630 ms) at 5 electrode sites during affect labeling and demographic data, behavioral measures, and clinical scale scores in the PD group. As shown in Figure 3C, we found significant negative correlations between late LPP amplitudes and PDSS scores at the Cz electrode site (r = -0.437, P = 0.029). Additionally, there were significant negative correlations between late LPP amplitudes and PASS scores at the same Cz electrode site (r = -0.423, P = 0.035).
This study investigated the implicit emotion regulation capacity in individuals with PD using ERP and affect labeling paradigms, while also examining the dynamic changes in affect labeling effects observed in the HC. Our findings revealed that both the PD and HC groups demonstrated prolonged reaction times and reduced accuracy in the affect labeling task compared to the gender labeling task, potentially indicating a greater demand for cognitive resources during affect labeling[22]. Additionally, the LPP amplitudes in the affect labeling condition displayed an initial increase followed by a subsequent decrease, suggesting that the process of emotion regulation through labeling is not immediate. Importantly, we observed an interaction effect between group and task condition on the late LPP. However, in the PD group, affect labeling did not result in significant neural modulation, indicating the ineffectiveness of this strategy in reducing negative emotions among PD patients and highlighting compromised implicit emotion regulation abilities in PD. Lastly, the LPP amplitudes in PD exhibited a negative correlation with PDSS and PASS scores, implying that the inability to effectively attenuate negative emotions during implicit emotion regulation may be associated with disease severity, episode frequency, and symptom intensity.
The behavioral data showed that, in the HC group, reaction times were longer for affect labeling compared to gender labeling, while accuracy was higher for gender labeling, which is in line with previous findings[23]. This performance difference can be attributed to task difficulty[24]. Moreover, the RMANOVA analysis demonstrated a significant main effect of condition but did not reveal a main effect of group, indicating that individuals with PD do not exhibit differences in reaction times and accuracy compared to HC.
The dynamic changes in LPP amplitudes during affect labeling, characterized by an initial increase followed by a decrease, reflect a distinct psychological process worthy of discussion. Torrisi et al[22] (2013) discovered that individuals utilize the prefrontal-amygdala circuit to suppress emotions during affect labeling, leading to the downregulation of negative emotional experiences[22]. This process involves the consumption of cognitive resources. Our study involved participants translating perceived facial emotions into verbal expressions through the affect labeling task, which required semantic processing of emotional features and the utilization of cognitive resources[23]. While both affect labeling and gender labeling tasks involve transforming features into language, affect labeling specifically deals with emotionally arousing information, representing a more complex cognitive processing[11] that demands higher cognitive resource consumption. Consequently, this is likely to lead to a more pronounced increase in LPP amplitudes during affect labeling. Furthermore, the decline in LPP amplitudes, reaching a level comparable to or even lower than gender labeling, indicates the regulatory influence of affect labeling on emotions[11,12]. However, the absence of a significant decrease in LPP amplitudes after affect labeling in this study can be attributed to the relatively lower level of arousal elicited by the negative images employed[23,25].
In this study, we observed an interaction effect between group and task type, indicating that the relationship between these factors influenced the outcomes. Specifically, when analyzing the late LPP amplitudes in the PD group, we found no significant differences between affect labeling and gender labeling tasks. These findings suggest a lack of effect of affect labeling on emotion regulation in individuals with PD. Previous research has consistently reported cognitive biases and interpretive tendencies in individuals with PD[8,26], where they exhibit a tendency to prioritize attention towards threatening stimuli and engage in catastrophic interpretations of emotional cues. This cognitive bias hinders effective downregulation of negative emotions, as individuals with PD tend to perseverate on negative aspects and struggle to mobilize sufficient cognitive resources for successful regulation of negative emotions. Furthermore, prior functional magnetic resonance imaging (fMRI) studies have consistently demonstrated compromised top-down unconscious cognitive control from the prefrontal cortex to the amygdala in individuals with PD[3,27]. Our study aligns with these findings as we did not observe a significant enhancement in the late LPP amplitude (350-630 ms) in the electrophysiological signals, providing further support for the notion of impaired implicit emotion processing in individuals with PD.
Interestingly, we observed a significant negative correlation between disease severity and late LPP amplitudes in the PD group. Specifically, greater severity of PD symptoms in the past month or higher frequency and intensity of symptoms in the past week were associated with impaired implicit emotion regulation abilities. These findings support the potential use of LPP as an electrophysiological marker for monitoring disease progression and assessing treatment effectiveness.
This study has a few limitations. Firstly, the level of arousal elicited by the emotional stimuli was not strong enough, potentially impacting the full capture of the decline in late LPP[23,24]. However, data and waveform analysis support the existence of the regulatory process involving enhancement followed by attenuation through affect labeling. Secondly, the study exclusively utilized "negative" labels. Future research can explore more specific labels (e.g., neutral and positive) to further investigate related aspects. Lastly, the study design lacked longitudinal treatment evaluations. Future studies should consider incorporating follow-up assessments of the patients to track changes over time. This would not only enhance our understanding of the causal relationship between affect labeling and neural activity changes in PD but also allow for an evaluation of whether successful treatment leads to the disappearance of the observed dysregulation.
This study reveals that the regulation process of affect labeling is not immediate, as evidenced by an initial increase followed by a decrease in LPP amplitude. Importantly, it identifies impaired implicit emotion regulation in PD patients and provides the first electrophysiological evidence of this impairment. Specifically, the diminished LPP modulation supports the abnormal affect labeling in PD patients. These findings serve as a valuable marker for future research investigating therapeutic interventions for PD that rely on implicit emotion regulation, such as cognitive behavioral therapy[28,29], and neuromodulatory interventions like transcranial magnetic stimulation[6].
The background of this study is rooted in clinical observations and research findings that identify emotion regulation dysfunction as a significant factor in the occurrence of panic disorder (PD). However, the neurophysiological mechanisms underlying implicit emotion regulation abnormalities in patients with PD remain unclear.
We aim to analyze neurophysiological changes in PD patients during implicit emotion regulation, identifying a concise and effective electrophysiological marker to assess potential anomalies in implicit emotion regulation.
The study aims to determine if there are anomalies in implicit emotion regulation in PD. Past research suggests abnormal Late Positive Potential (LPP) during emotion regulation in PD patients, indicating that LPP in event-related potentials (ERP) could be an effective tool for assessing implicit emotion regulation deficits.
We assessed PD patients using clinical and psychological scales, conducting an emotion labeling task and recording behavioral and ERP data.
In the control group, late LPP initially increased, then decreased. A significant group × condition interaction effect was observed. Simple effect analysis showed reduced differences in late LPP amplitudes between affect labeling and gender labeling conditions in PD compared to controls. Additionally, under affect labeling, late LPP in PD negatively correlated with disease severity.
PD patients have implicit emotion regulation impairments, and the late LPP amplitude in response to affect labeling may be a valuable clinical indicator of PD severity.
Future considerations should include longitudinal treatment studies with follow-ups, assessing whether PD patients regain regulatory function post-successful treatment.
We would like to express our sincere gratitude to the patients and their families for their invaluable support in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garcia-Campayo J, Spain Kar SK, India S-Editor: Li L L-Editor: A P-Editor: Chen YX
| 1. | Roy-Byrne PP, Craske MG, Stein MB. Panic disorder. Lancet. 2006;368:1023-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11472] [Cited by in F6Publishing: 11056] [Article Influence: 581.9] [Reference Citation Analysis (1)] |
| 3. | Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage. 2017;151:105-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 4. | Gross JJ, editor. Handbook of emotion regulation. 2nd ed. New York (NY): The Guilford Press, 2014. [Cited in This Article: ] |
| 5. | Cox J, Thakur B, Alvarado L, Shokar N, Thompson PM, Dwivedi AK. Repetitive transcranial magnetic stimulation for generalized anxiety and panic disorders: A systematic review and meta-analysis. Ann Clin Psychiatry. 2022;34:e2-e24. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 6. | Li H, Wang J, Li C, Xiao Z. Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults. Cochrane Database Syst Rev. 2014;2014:CD009083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 595] [Cited by in F6Publishing: 618] [Article Influence: 68.7] [Reference Citation Analysis (1)] |
| 8. | Clark DM. A cognitive approach to panic. Behav Res Ther. 1986;24:461-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1618] [Cited by in F6Publishing: 1652] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 9. | Chang Y, Xu J, Pang X, Sun Y, Zheng Y, Liu Y. Mismatch negativity indices of enhanced preattentive automatic processing in panic disorder as measured by a multi-feature paradigm. Biol Psychol. 2015;105:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Li XL, Wang HY. Unconscious cognitive dysfunction in emotion dysregulation and psychopathology of panic disorder: evidence from the late positive potential. Neuroreport. 2018;29:6-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 569] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 12. | Memarian N, Torre JB, Haltom KE, Stanton AL, Lieberman MD. Neural activity during affect labeling predicts expressive writing effects on well-being: GLM and SVM approaches. Soc Cogn Affect Neurosci. 2017;12:1437-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Tabibnia G, Lieberman MD, Craske MG. The lasting effect of words on feelings: words may facilitate exposure effects to threatening images. Emotion. 2008;8:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol. 2010;35:129-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 718] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 15. | Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 838] [Cited by in F6Publishing: 852] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 16. | Burklund LJ, Craske MG, Taylor SE, Lieberman MD. Altered emotion regulation capacity in social phobia as a function of comorbidity. Soc Cogn Affect Neurosci. 2015;10:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York: New York State Psychiatric Institute; 2002. [Cited in This Article: ] |
| 18. | Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Gorman JM, Papp LA. Multicenter collaborative panic disorder severity scale. Am J Psychiatry. 1997;154:1571-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 605] [Cited by in F6Publishing: 621] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 19. | Argyle N, Deltito J, Allerup P, Maier W, Albus M, Nutzinger D, Rasmussen S, Ayuso JL, Bech P. The Panic-Associated Symptom Scale: measuring the severity of panic disorder. Acta Psychiatr Scand. 1991;83:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Gong X, Huang YX, Wang Y, Luo YJ. [Revision of the Chinese Facial Affective Picture System]. Zhongguo Xinli Weisheng Zazhi. 2011;25:40-46. [DOI] [Cited in This Article: ] |
| 21. | Ferri J, Eisendrath SJ, Fryer SL, Gillung E, Roach BJ, Mathalon DH. Blunted amygdala activity is associated with depression severity in treatment-resistant depression. Cogn Affect Behav Neurosci. 2017;17:1221-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Torrisi SJ, Lieberman MD, Bookheimer SY, Altshuler LL. Advancing understanding of affect labeling with dynamic causal modeling. Neuroimage. 2013;82:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Deng H, Jiang Q. [When Does Labeling Happen: An ERP Study of Affect Labeling]. Zhongguo Linchuang Xinlixue Zazhi. 2017;25:231-236. [DOI] [Cited in This Article: ] |
| 24. | Liang J, Lin H. Current and lasting effects of affect labeling on late positive potential (LPP) amplitudes elicited by negative events. Brain Behav. 2023;13:e3065. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 25. | McRae K, Taitano EK and Lane RD. The effects of verbal labelling on psychophysiology: Objective but not subjective emotion labelling reduces skin-conductance responses to briefly presented pictures. Cogn Emot. 2010;24:829-839. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Lang AJ, Sarmiento J. Relationship of attentional bias to anxiety sensitivity and panic. Depress Anxiety. 2004;20:190-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Korgaonkar MS, Tran J, Felmingham KL, Williams LM, Bryant RA. Neural correlates of emotional processing in panic disorder. Neuroimage Clin. 2021;32:102902. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 28. | Strauss AY, Kivity Y, Huppert JD. Emotion Regulation Strategies in Cognitive Behavioral Therapy for Panic Disorder. Behav Ther. 2019;50:659-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Koole SL, Webb TL and Sheeran PL. Implicit emotion regulation: feeling better without knowing why. Curr Opin Psychol. 2015;3:6-10. [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |