Published online Jan 19, 2024. doi: 10.5498/wjp.v14.i1.148
Peer-review started: October 17, 2023
First decision: November 30, 2023
Revised: December 9, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: January 19, 2024
The detection rate of depression among university students has been increasing in recent years, becoming one of the main psychological diseases that endangers their physical and mental health. According to statistics, self-harm and suicide, for which there is no effective intervention, are the second leading causes of death.
To explore the relationship between different elements and levels of physical activity and college students’ depression-symptom-specific working memory indicators.
Of 143 college students were analyzed using the Beck Depression Self-Rating Scale, the Physical Activity Rating Scale, and the Working Memory Task.
There was a significant difference between college students with depressive symptoms and healthy college students in completing verbal and spatial working memory (SWM) tasks correctly (all P < 0.01). Physical Activity Scale-3 scores were significantly and positively correlated with the correct rate of the verbal working memory task (r = 0.166) and the correct rate of the SWM task (r = 0.210) (all P < 0.05). There were significant differences in the correct rates of verbal and SWM tasks according to different exercise intensities (all P < 0.05) and different exercise durations (all P < 0.05), and no significant differences in the correct rates of verbal and SWM tasks by exercise frequency (all P > 0.05).
An increase in physical exercise among college students, particularly medium- and high-intensity exercise and exercise of 30 min or more, can improve the correct rate of completing working memory tasks.
Core Tip: This study discusses physical exercise in university students with depression and the specificity of their working memory. In addition, this study analyzes the relationships between the three variables through cross-sectional research, the relationship between different factors, performance of physical exercise, and working memory of university students with depression.
- Citation: Zhao Q, Wang X, Li SF, Wang P, Wang X, Xin X, Yin SW, Yin ZS, Mao LJ. Relationship between physical activity and specific working memory indicators of depressive symptoms in university students. World J Psychiatry 2024; 14(1): 148-158
- URL: https://www.wjgnet.com/2220-3206/full/v14/i1/148.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i1.148
The World Health Organization predicts that depression will become the leading contributor to the global burden of disease by 2030[1]. Early adulthood is a critical period for the physiological and psychological development of individuals, and is also a high-risk period for developing depression[2]. College students are generally in the early adulthood stage from 18 to 25 years of age, and because of their immature physical and mental development, they are prone to internal conflicts when facing multiple pressures, such as complicated study tasks and life events, which can induce depression and other undesirable emotions, and in severe cases, can lead to suicide and other crisis events[3,4]. Some studies have shown that the detection rate of depression among Chinese college students ranges from 21.6% to 37.6%, with an increasing trend year by year[5-7]. Therefore, the prevention and intervention of depressive symptoms in college students is of great significance.
Working memory impairment occurs in patients with depressive symptoms. Working memory, a capacity-limited cognitive system that temporarily stores relevant information[8,9], is one of the core elements of the human cognitive system and plays an important role in learning, reasoning, and completing complex tasks. Typical symptoms of depression (e.g., pleasure deficit) are highly correlated with impairment of working memory[8,10-12]. Related brain imaging studies have found that depressed individuals show greater activation in the cingulate cortex and prefrontal lobe when completing N-back tasks compared to healthy individuals[13-17], suggesting that to achieve the same level of performance on working memory tasks as healthy individuals, depressed individuals need to mobilize more cognitive resources and exert greater cognitive effort. Comparisons were made between depressed and non-depressed college students on a working memory task, and it was found that the correct rate of depressed subjects was lower than that of healthy subjects, and the reaction time was higher than that of healthy subjects, both of which were statistically significant[18]. The working memory model proposed by Baddeley includes the Central Executive, Visuo- spatial Sketchpad, and Phonological Loop[19]. According to the manner in which information is processed and handled, working memory can be divided into visuospatial and verbal working memory (VWM)[20]. People with depressive symptoms may experience impairments in different dimensions of working memory.
Physical exercise is closely associated with depressive symptoms and working memory. Physical exercise is an effective means of alleviating negative mood in depressed patients, with the advantages of high adherence, low adverse effects, and stable effects[21,22]. Previous cross-sectional studies have found that physical activity is significantly negatively correlated with depressive symptoms[23,24], and the higher the level of participation in sports, the lower the risk of depression detection[25]; Physical activity can also improve depressive symptoms by improving working memory. Weuve et al[26] found that those with higher weekly physical activity had better performance in reverse-order memory breadth, and the decline in homework performance was lower than the decline in physical activity performance in the second test two years later. The decline in homework performance was lower in this group than that of those with low physical activity; and the findings of Szabo et al[27] suggest that cardiorespiratory fitness levels in older adults can directly influence hippocampal gyrus volume, which in turn promotes overall correctness and speed of response in spatial working memory (SWM). Depressive disorder severity significantly affects working memory and may be related to altered frontal executive control circuit functioning in patients[28], who show consistent abnormalities in limbic-subcortical calcium cycle functioning during working memory processing[29]; disruption of working memory updating is mainly characterized by altered activity in the connections between visual association areas and the prefrontal cortex[30], and physical activity can significantly affect prefrontal activation, which in turn affects performance on working memory tasks, thereby ameliorating depressive symptoms.
Previous studies have shown that physical exercise, working memory, and depressive symptoms are closely related to each other, and that impairment of working memory is a prominent manifestation of cognitive impairment in patients with depression. There is a basic consensus that physical exercise improves working memory, and that improvement of working memory alleviates depressive symptoms. We found that previous studies did not clarify whether there was a difference in working memory task performance between healthy college students and college students with depressive symptoms, nor did they mention the relationship between the elements of physical exercise and working memory. The present study thus adopted a cross-sectional design paradigm to explore the correlation between various elements of physical activity and working memory-specific indicators, as well as the relationship between different levels of physical activity and working memory under each element, to clarify the indicators of depression-symptom-specific working memory in college students with a view to provide evidence for future clinical practice.
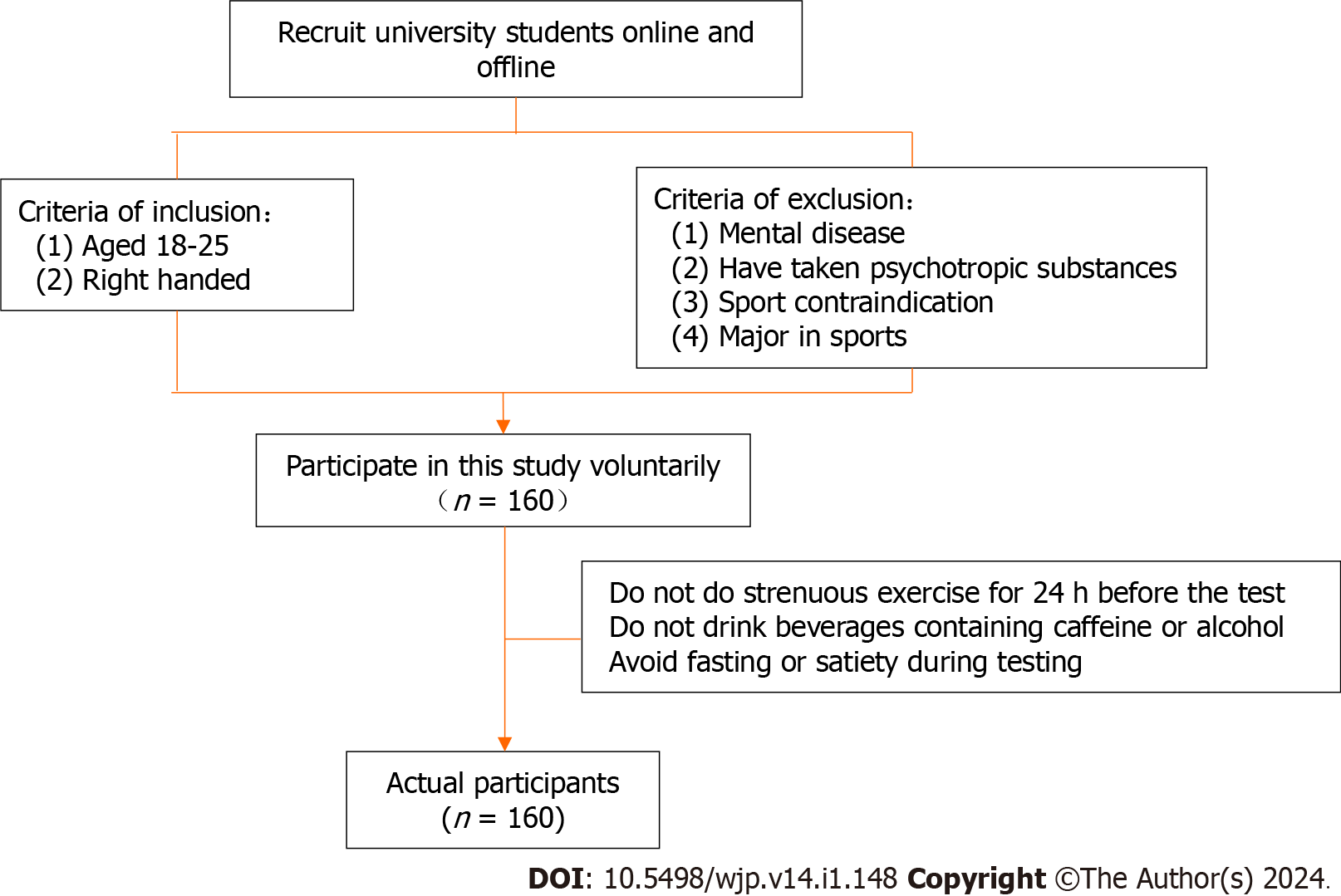
Based on the principle of voluntariness, university students were randomly recruited from Songjiang University in Shanghai. All subjects were required to be 18-26 years old; have no mental illness; have never taken barbiturates, benzodiazepines, or chloral hydrate; be non-sports majors or high-level athletes; and have no sports contraindications. All participants signed informed consent forms, and the study was approved by the Ethics Committee of Shanghai University of Sport, with an ethical code of 102772023RT075. The recruitment process for the participants is shown in Figure 1.
General information questionnaire: Participants’ basic information, such as age, sex, height, weight, and family status was obtained, as well as information on any mental illness, on the use of barbiturates, benzodiazepines, and chloral hydrate, whether they were professional athletes, and whether they had sports contraindications.
Beck Depression Inventory-II: This widely used 21-item self-assessment scale was used to assess depressive symptoms. Responses were rated on a 4-point Likert scale ranging from 0 (no symptoms) to 3 (severe symptoms). Total scores of 0-13, 14-19, 20–28, and 29–63 indicate no, mild, moderate, and severe depression, respectively. The internal consistency coefficient was 0.948[16].
The Physical Activity Scale-3: The Physical Activity Scale-3 (PARS-3), translated and revised by Liang et al[31] is currently recognized as an effective adult physical activity measurement questionnaire. This scale defines the amount of exercise = intensity × duration × frequency. The intensity and frequency are divided into five grades, with 1-5 points respectively. The five grades of duration are 0-4 points respectively, and the score range is 0-100 points. The evaluation criteria are: ≤ 19 means a small amount of exercise, 20-42 a moderate amount of exercise, and ≥ 43 a large amount of exercise. In this study, Cronbach α coefficient of the scale is 0.740.
Working memory task: A verbal n-back spatial n-back task paradigm was used to measure the refreshing ability of the participants’ working memory, where n was 2. VWM and SWM were tested according to different processing levels of working memory. The experimental procedure was completed using the subjects’ keystrokes on a computer, and the verbal and spatial n-back tasks used the same experimental materials and procedures but differentiated between the two experimental procedures using different experimental instructions. Eight distinct uppercase letters-B, D, H, K, M, P, S, and Y-were chosen for the experimental material to avoid confusion in the subjects. The screen background was black and eight letters were randomly presented at eight positions on the screen.
The verbal n-back task required participants to memorize the letters themselves, ignoring their spatial location, and consisted of 55 trials, including 5 practice trials and 50 formal trials. After completing the 5 practice trials, the participant was asked whether he/she was familiar with the task and the procedure, and if he/she did not receive an affirmative answer, he/she was given the option of pressing the “Q” key to practice again; if he/she received an affirmative answer, he/she was given the option of pressing the “Enter” key to enter the formal experiment. Each experiment consisted of 50 trials. There were 50 trials in the formal experiment, and the specific experimental procedure was as follows: first, a gaze point “+” was presented for 3000 ms, and then a picture with a letter was presented sequentially for 2000 ms, with a stimulus interval of 1000 ms. Participants were asked to memorize the letter itself, ignore the spatial location of the letter, and remember the letter if it was different from the second letter. If the currently presented letter was the same as the second letter in the previous interval, the “J” key was pressed, and if it was different, the “F” key was pressed. The statistics show the response times and correct rates for the verbal n-back task (Figure 2).
The spatial n-back task was the same as the verbal n-back task in terms of the number of trials and the complete experimental procedure, with the difference that participants were required to memorize the spatial position of the letter, ignoring the letter itself. Participants were asked to press the “J” key to respond if the position of the currently presented letter was the same as that of the previous penultimate letter (in this case, there would be a situation in which the letters are different, but the spatial position of the letter is the same), and press the "F" key if they are different. The statistics represent the response times, press the “J” key to respond, and if they are different, press the “F” key. The statistics represent the response times and correct rates for the spatial N-back task (Figure 3).
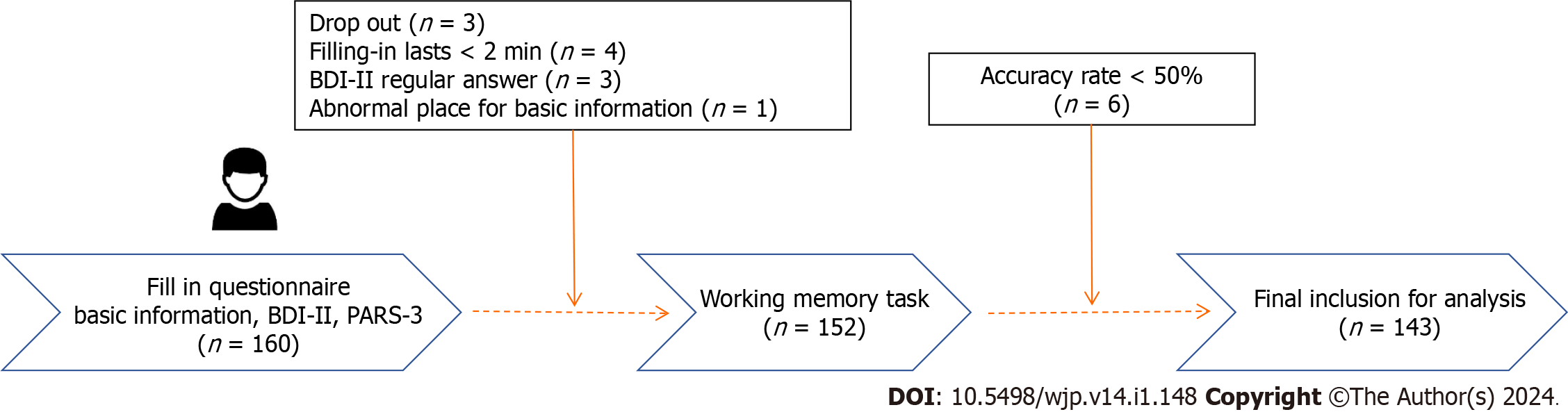
Questionnaires (basic information form, Beck Depression Inventory-II, and PARS-3) were distributed to the participants. Before filling in the questionnaires, the chief examiner read out the guidelines and explained the entries, making it clear that the data obtained were only used for scientific research, and emphasizing that the answers were true, independent and voluntary. In the process of filling in the questionnaires, the subjects were prompted to answer the questionnaires according to the requirements. After filling in the questionnaires, the chief examiner checked the questionnaires that had omitted any item or whose responses were against common sense, and ensured the completeness of the information through filling and re-filling the questionnaires (Figure 4).
IBM SPSS Statistics 26.0 software was used to statistically analyze the data. For the questionnaire, there was a non-random lack of data to avoid the use of a simple deletion method to make the estimated coefficients biased; then the same kind of mean interpolation processing was performed. The common method bias test was conducted by using the Harman one-way test; the data distribution was observed through frequency histograms, and the independent sample t-test was used for comparison between groups of the measured data that conformed to normal distribution or nearly normal distribution; the Mann-Whitney U nonparametric test was used for comparison of the measured data that were obviously skewed; the count data were described by n (%), and the χ2 test was used for comparison between groups. One-way analysis of variance (ANOVA) and least significant difference post-hoc multiple tests were used to compare the effects of different exercise intensities, times, and frequencies on the correct rate of verbal and SWM tasks, and Pearson correlation analysis and linear regression analysis were used to explore the relationship between physical exercise, depressive symptoms, and working memory. One-way ANOVA was used to investigate the relationship between physical exercise (intensity and duration) and working memory.
Overall, 143 participants were included, with age 19.53 ± 1.149 year-old, and a 21.68% detection rate of depression. Comparing the demographic features between depressed and non-depressed university students, as shown in Table 1, we discovered significant differences in the groups in terms of registered households, only children, study pressure, interpersonal relationships, and social activities. Further analysis found that compared to non-depressed university students, depressed university students had significantly higher study pressure, a lower proportion of only children, more tense interpersonal relationships, and fewer social activities. This result shows that being in a senior grade, not being an only child, high study pressure, more tense interpersonal relationships, and fewer social activities are indicators of depressive symptoms in the high-risk groups.
| Demographic indicators | Overall | Without depression | With depression | Comparison among groups | |
| (n = 143) | (n = 112) | (n = 31) | χ²/t | P value | |
| Gender (male) | 47.55 | 48.21 | 45.16 | 0.091 | 0.763 |
| Age (yr) | 19.53 ± 1.149 | 19.52 ± 1.139 | 19.58 ± 1.205 | 0.959 | 0.789 |
| BMI (kg/m²) | 21.483 ± 3.939 | 21.729 ± 4.039 | 20.592 ± 3.468 | 0.245 | 0.156 |
| Registered household (city) | 52.45 | 58.04 | 32.26 | 6.469 | 0.011a |
| Only child (yes) | 46.15 | 50.89 | 29.03 | 4.669 | 0.031a |
| Single-parent family (yes) | 9.09 | 9.82 | 6.45 | 0.334 | 0.564 |
| Average education year of parents (yr) | 11.119 ± 3.598 | 11.371 ± 3.370 | 10.210 ± 4.260 | 0.117 | 0.112 |
| Drinking habit (yes) | 20.28 | 18.75 | 25.81 | 0.748 | 0.387 |
| Smoking habit (yes) | 2.10 | 2.68 | 0 | 0.848 | 0.357 |
| Family relations | |||||
| Amiable | 50.35 | 53.57 | 38.71 | 3.466 | 0.177 |
| General | 47.55 | 43.75 | 61.29 | ||
| Many conflicts | 2.10 | 2.68 | 0 | ||
| Study pressure | |||||
| Easy | 22.38 | 25.89 | 9.68 | 7.107 | 0.029a |
| General | 54.55 | 55.36 | 51.61 | ||
| Difficult | 23.08 | 18.75 | 38.71 | ||
| Interpersonal relationship | |||||
| Good | 74.13 | 83.04 | 41.94 | 22.009 | < 0.001b |
| General | 23.78 | 16.07 | 51.61 | ||
| Bad | 2.10 | 0.89 | 6.45 | ||
| Social activities | |||||
| > Three times/wk | 2.80 | 1.79 | 6.45 | 13.625 | 0.001b |
| ≤ Three times/wk | 83.22 | 89.29 | 61.29 | ||
| Hardly | 13.99 | 8.93 | 32.26 | ||
As shown in Table 2 and Figure 5, there was a significant difference in the rate of correct completion of both verbal and SWM tasks between college students with depressive symptoms and healthy college students (all P < 0.01).
| Variable | Without depression, (n = 134) | With depression, (n = 31) | F value | P value |
| VWM accuracy | 0.868 ± 0.080 | 0.762 ± 0.170 | 50.963 | 0.002 |
| VWM reaction time | 995.18 ± 146.27 | 913.45 ± 290.846 | 42.502 | 0.14 |
| SWM accuracy | 0.875 ± 0.084 | 0.773 ± 0.187 | 73.121 | 0.006 |
| SWM reaction time | 955.41 ± 168.20 | 880.81 ± 249.87 | 10.484 | 0.125 |
To facilitate the analysis of different physical exercise elements and levels, the participants were divided into different exercise intensities, durations, and frequencies according to the PARS-3 questionnaire. Exercise intensity was classified as light exercise (level 1), medium-low intensity (levels 2 and 3), and high intensity (levels 4 and 5), exercise duration was classified as < 30 min (levels 1-3), 30-59 min (level 4), and ≥ 60 min (level 5), and exercise frequency was classified as ≤ 1 time/mo (level 1), 2 times/mon-2 times/wk (levels 2 and 3), and ≥ 3 times/wk (levels 4 and 5).
In order to analyze the characteristics of working memory in students with different exercise intensity, duration, and frequency according to the PARS-3 questionnaire, exercise intensity was divided into light exercise (level 1), medium and low intensity (levels 2 and 3), and high intensity (levels 4 and 5), and exercise duration was divided into < 30 min (levels 1-3), 30–59 min (levels 4) and ≥ 60 min (level 5), frequency of exercise was divided into ≤ once/month (level 1, twice/month-twice/week (level 2 and 3) and ≥ three times/week (level 4 and 5).
As shown in Table 3, there were significant differences in VWM and SWM accuracies among the different exercise intensities (all P < 0.05). After multiple comparisons, significant differences were found between high-intensity and medium-intensity physical exercise and low-intensity physical exercise (all P < 0.05), suggesting that medium intensity had already taken effect, and there was little difference between medium and high intensity.
| Variable | Overall | Different intensity | F value | P value | Multiple comparisons | ||||
| (n = 143) | Low (n = 27) | Medium (n = 94) | High (n = 22) | Low vs medium | Low vs high | Medium vs high | |||
| Accuracy rate of VWM | 0.845 ± 0.114 | 0.795 ± 0.102 | 0.849 ± 0.104 | 0.889±0.048 | 4.45 | 0.013 | 0.028 | 0.004 | 0.137 |
| Accuracy rate of SWM | 0.853 ± 0.121 | 0.786 ± 0.171 | 0.862 ± 0.106 | 0.894±0.069 | 6.008 | 0.003 | 0.004 | 0.002 | 0.241 |
As shown in Table 4, there were significant differences in the VWM and SWM accuracy rates for different exercise durations (all P < 0.05). After multiple comparisons, significant differences were found in the VWM accuracy rate between physical exercise of middle- and low duration (P < 0.05). In terms of the accuracy rate of SWM, there were significant differences between the group of low-duration and the groups of high-duration and medium-duration (all P < 0.05), suggesting that the exercise duration takes effect when it exceeds 30 min, and there is little difference between medium-duration and high-duration physical exercise.
| Variable | Overall | Duration | F value | P value | Multiple comparisons | ||||
| (n = 143) | < 30 min (n = 33) | 30-59 min (n = 63) | ≥ 60 min (n = 47) | Low vs medium | Low vs high | Medium vs high | |||
| Accuracy rate of VWM | 0.845 ± 0.114 | 0.808 ± 0.165 | 0.869 ± 0.084 | 0.838 ± 0.114 | 3.344 | 0.038 | 0.012 | 0.243 | 0.151 |
| Accuracy rate of SWM | 0.853 ± 0.121 | 0.798 ± 0.172 | 0.874 ± 0.084 | 0.862 ± 0.111 | 4.675 | 0.011 | 0.003 | 0.018 | 0.608 |
As shown in Table 5, there was no significant difference in the VWM and SWM accuracy rates for different exercise frequencies (all P > 0.05), suggesting that different exercise frequencies pose no effect on the completion of working memory tasks.
| Variable | Overall | Frequency | F value | P value | ||
| (n = 143) | ≤ Once/mo (n = 1) | Twice/month-twice/week (n = 103) | ≥ Three times/week (n = 39) | |||
| Accuracy rate of VWM | 0.845 ± 0.114 | 0.766 ± 0.000 | 0.840 ± 0.117 | 0.861 ± 0.114 | 0.709 | 0.494 |
| Accuracy rate of SWM | 0.853 ± 0.121 | 0.797 ± 0.000 | 0.847 ± 0.114 | 0.870 ± 0.139 | 0.623 | 0.538 |
The results of this study showed that the correct rate when completing a working memory task is a depression-symptom-specific working memory indicator for college students and that physical activity participation has a positive correlation with the correct rate when completing a working memory task, which is consistent with the results of previous studies. The results of previous studies found[32,33] that depressed individuals had lower rates of correctness and slower responses when completing tasks compared to healthy individuals, and the severity of working memory impairment was positively correlated with the severity of symptoms in depressed patients[34]. In terms of research on physical exercise to improve working memory in depressed groups, previous researchers have found through cross-sectional studies[35] that there is a positive correlation between physical activity and cognition and a significant positive correlation between physical activity and its rate of correctness in completing working memory[36-38]. Overseas studies have found that depressed patients all have abnormal activation of the frontal and parietal lobes when performing working memory tasks and also have failure of inhibition of the limbic system[39,40].
This study found that the higher the exercise intensity and longer the duration of physical exercise for college students, the better their performance in working memory tasks, whereas the positive facilitation effect of exercise frequency on working memory task performance was not significant. Evidence shows[41] that physical exercise can provide sufficient nutrition and energy to the brain by increasing neurotransmitter content, promoting glial cell regeneration, improving synaptic plasticity, effectively regulating neurotrophic factor concentration, glucocorticoid hormone levels, morphology and structure of specific parts of the central nervous system, as well as the release of pro-inflammatory cytokines, and at the same time increasing brain plasticity and improving working memory. Furthermore, physical exercise increases the area of grey and white matter in the prefrontal, parietal and temporal lobes[42], induces structural changes in the hippocampal volume and the vascular system[43], and significantly increases the number of newborn neurons[44], which, in turn, improves working memory capacity. Vazou et al[45] conducted a cross-sectional cognitive test on and showed that subjects who exercised more performed better. Sibley et al[46] and Griffin et al[47] found that both moderate- and high-intensity exercise resulted in a significant increase in working memory capacity in university students.
We also found that depressive symptoms were significantly negatively correlated with the correct rate of completion of the working memory task, but not with the response time of completing the working memory task, which is inconsistent with the results of previous studies[17,48], which may be due to the difference in task paradigms, as previous studies of working memory used both the 1-back and 2-back working memory tasks, whereas the present study used only the 2-back task. This result may be due to the difference in task paradigms, as the 1-back task is simpler for college students, and a “ceiling effect” may occur[49]. Another inconsistent result is that there is no significant difference in college students’ performance on the working memory task between different exercise frequencies[50]. This may be due to the difference in the study population; previous researchers selected healthy college students, whereas the present study included college students with depressive symptoms. Cross-sectional studies on the effect of physical activity frequency on working memory have rarely been reported at home and abroad, and high-quality studies are still necessary in the future to supplemented these findings.
Limitations: This study used a cross-sectional design, which needs to be confirmed by longitudinal studies in the future. The physical activity scale, as a subjective report, may have some bias, and some objective indicators such as accelerometers and heart rate bands can be used to measure physical activity data in future studies. Larger samples are required for future investigations.
The more physical exercise college students engage in, the higher is the correct rate of completing working memory tasks. Among the elements of physical exercise, exercise intensity of medium intensity or more and exercise duration of more than 30 minutes can improve the correct rate of working memory tasks. Therefore, college students with depressive symptoms should be encouraged to increase their physical activity to improve their working memory and pay attention to changes in working memory, which may reduce scores on depressive symptoms.
Depression is an important factor contributing to the global burden of disease, and the detection rate of depressed mood among Chinese university students ranges from 21.6 per cent to 37.6 percent, with a tendency to increase year by year.
Reduce the prevalence of depressive symptoms in college students.
This paper aims to discuss the relationship between different factors, the performance of sports exercises, and the working memory of university students with depression.
One-way analysis of variance and Pearson’s correlation were used to explore the correlations and interaction pathways between variables.
There was a significant difference between depressive symptomatic and healthy college students in the completion of both verbal and spatial working memory tasks correctly. Physical Activity Scale-3 scores were significantly and positively correlated with verbal working memory (VWM) correctness and spatial working memory (SWM) correctness. High- and moderate-intensity physical exercise were significantly different from low-intensity physical exercise. In terms of VWM correctness, there was a significant difference between medium-duration compared with low-duration physical exercise; in terms of SWM correctness, there was a significant difference between high-duration and medium-duration physical exercise compared with low-duration physical exercise. There was no significant difference in the correct VWM and SWM rates between the different exercise frequencies.
Colleges and universities should encourage students with depressive symptoms to increase their physical activity and improve their working memory. This is particularly evident with increased intensity and duration of physical activity, which may reduce the incidence of depressive symptoms.
The use of objective measurement tools is recommended for future studies, and longitudinal studies are necessary to further define the course of action.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosak L, Czech Republic; Stogov MV, Russia S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
| 1. | Lépine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7:3-7. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Hayley AC, Skogen JC, Sivertsen B, Wold B, Berk M, Pasco JA, Øverland S. Symptoms of Depression and Difficulty Initiating Sleep from Early Adolescence to Early Adulthood: A Longitudinal Study. Sleep. 2015;38:1599-1606. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Zhang R, Yang CY, Zhang YD. Influencing Factors of Depression in Chinese College Students: A Meta-analysis. Zhongguo Quanke Yixue. 2020;23:4497-4502. [DOI] [Cited in This Article: ] |
| 4. | Cao C, Wang MP, JI LQ, Wei X, Cao YN, Zhang WX. The MAOA rs6323 polymorphism interacts with maternal supportive parenting in predicting adolescent depression:Testing the diathesis-stress and differential susceptibility hypotheses. Xinli Xuebao. 2016;48:22-35. [DOI] [Cited in This Article: ] |
| 5. | Tang H, Ding LL, Song XL, Huang ZW, Qi Q, He LP, Yao YS. Meta-analysis of detection rate of depressed mood among Chinese college students from 2002 to 2011. Jilin Daxue Xuebao (Yixueban). 2013;39:965-969. [Cited in This Article: ] |
| 6. | Hu YQ, Liu ZH. An Intervention Study of Psychological Health of Depressed College Students:the Different Effects of Different Types of School Support. Huan Shifan Daxue Jiaoyu Kexue Xuebao. 2019;18:120-125. [DOI] [Cited in This Article: ] |
| 7. | Wang MY, Liu J, Wu X, Li L, Hao XD, Shen Q, Huang MT, Sun RH. The prevalence of depression among students in Chinese universities over the past decade: A Me-ta-analysis. Hainan Yixueyuan Xuebao. 2020;26:686-93+99. [DOI] [Cited in This Article: ] |
| 8. | Chen NT, Clarke PJ, Watson TL, MacLeod C, Guastella AJ. Attentional bias modification facilitates attentional control mechanisms: evidence from eye tracking. Biol Psychol. 2015;104:139-146. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Eriksson J, Vogel EK, Lansner A, Bergström F, Nyberg L. Neurocognitive Architecture of Working Memory. Neuron. 2015;88:33-46. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cogn Emot. 2010;24:281-298. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | LeMoult J, Gotlib IH. Depression: A cognitive perspective. Clin Psychol Rev. 2019;69:51-66. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44:2029-2040. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehéricy S, Allilaire JF, Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26:860-869. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 2006;29:203-215. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Matsuo K, Glahn DC, Peluso MA, Hatch JP, Monkul ES, Najt P, Sanches M, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao JH, Soares JC. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry. 2007;12:158-166. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, Egan GF. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp. 2008;29:490-501. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Nikolin S, Tan YY, Martin D, Moffa A, Loo CK, Boonstra TW. Behavioural and neurophysiological differences in working memory function of depressed patients and healthy controls. J Affect Disord. 2021;295:559-568. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Wang X. A study of the effects of depressed mood on working memory in college students. M.Sc. Thesis, Dalian Maritime University. 2017.. [DOI] [Cited in This Article: ] |
| 19. | Baddeley AD, Hitch GJ, Allen RJ. From short-term store to multicomponent working memory: The role of the modal model. Mem Cognit. 2019;47:575-588. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Wang Z, Jia DM. Evolution of a theoretical model of working memory and its application. Changji Xueyuan Xuebao. 2009;98-101. [DOI] [Cited in This Article: ] |
| 21. | Wang P, Wang J, Zhao JL, Wang X, Xin X, Qiu SL, Zang YH. Relationship Between Physical Activity Level and Depressive Symptoms in College Students:A Pathway Analysis Based on EEG. Shanghai Tiyu Xueyuan Xuebao. 2023;47:51-60. [DOI] [Cited in This Article: ] |
| 22. | Hallgren M, Stubbs B, Vancampfort D, Lundin A, Jääkallio P, Forsell Y. Treatment guidelines for depression: Greater emphasis on physical activity is needed. Eur Psychiatry. 2017;40:1-3. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | da Costa BGG, Chaput JP, Lopes MVV, Malheiros LEA, Silva KS. Movement behaviors and their association with depressive symptoms in Brazilian adolescents: A cross-sectional study. J Sport Health Sci. 2022;11:252-259. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Sun WX, Wang X, Yu MX, Zhao QY, Zhou XJ. Physical activity participation levels and depressive symptoms among college students: the mediating role of social support. Zhongguo Weisheng Tongji. 2023;40:421-4+8. [DOI] [Cited in This Article: ] |
| 25. | Zhang SH, Dai YX, Zhang XH, Li YJ, Zhang JX. Impact of social sports activities on depression among junior middle school students. Zhongguo Xuexiao Weisheng. 2020;41:551-3+7. [DOI] [Cited in This Article: ] |
| 26. | Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454-1461. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Szabo AN, McAuley E, Erickson KI, Voss M, Prakash RS, Mailey EL, Wójcicki TR, White SM, Gothe N, Olson EA, Kramer AF. Cardiorespiratory fitness, hippocampal volume, and frequency of forgetting in older adults. Neuropsychology. 2011;25:545-553. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Watters AJ, Carpenter JS, Harris AWF, Korgaonkar MS, Williams LM. Characterizing neurocognitive markers of familial risk for depression using multi-modal imaging, behavioral and self-report measures. J Affect Disord. 2019;253:336-342. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Wang XL, Du MY, Chen TL, Chen ZQ, Huang XQ, Luo Y, Zhao YJ, Kumar P, Gong QY. Neural correlates during working memory processing in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:101-108. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Le TM, Borghi JA, Kujawa AJ, Klein DN, Leung HC. Alterations in visual cortical activation and connectivity with prefrontal cortex during working memory updating in major depressive disorder. Neuroimage Clin. 2017;14:43-53. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Liang DQ. Stress levels and their relationship with physical activity among university students. Zhongguo Xinli Weisheng Zazhi. 1994;8: 5-6. [Cited in This Article: ] |
| 32. | Li M, Feng L, Liu X, Zhang M, Fu B, Wang G, Lu S, Zhong N, Hu B. Emotional working memory in patients with major depressive disorder. J Int Med Res. 2018;46:1734-1746. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Nikolin S, Tan YY, Schwaab A, Moffa A, Loo CK, Martin D. An investigation of working memory deficits in depression using the n-back task: A systematic review and meta-analysis. J Affect Disord. 2021;284:1-8. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci. 2006;17:172-179. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Fedewa AL, Ahn S. The effects of physical activity and physical fitness on children's achievement and cognitive outcomes: a meta-analysis. Res Q Exerc Sport. 2011;82:521-535. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Weng TB, Pierce GL, Darling WG, Voss MW. Differential Effects of Acute Exercise on Distinct Aspects of Executive Function. Med Sci Sports Exerc. 2015;47:1460-1469. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Gothe N, Pontifex MB, Hillman C, McAuley E. The acute effects of yoga on executive function. J Phys Act Health. 2013;10:488-495. [PubMed] [DOI] [Cited in This Article: ] |
| 38. | Shi H. Time-course effects of high-intensity interval exercise on executive function in young people with high and low cardiorespiratory fitness. M.Sc. Thesis, Shandong Tiyu Xueyuan. 2022.. [DOI] [Cited in This Article: ] |
| 39. | Rodríguez-Cano E, Sarró S, Monté GC, Maristany T, Salvador R, McKenna PJ, Pomarol-Clotet E. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychol Med. 2014;44:3263-3273. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Lee TW, Liu HL, Wai YY, Ko HJ, Lee SH. Abnormal neural activity in partially remitted late-onset depression: an fMRI study of one-back working memory task. Psychiatry Res. 2013;213:133-141. [PubMed] [DOI] [Cited in This Article: ] |
| 41. | Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525-544. [PubMed] [DOI] [Cited in This Article: ] |
| 42. | Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR, Drollette ES, Moore RD, Wu CT, Kamijo K. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics. 2014;134:e1063-e1071. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H. The effects of aerobic activity on brain structure. Front Psychol. 2012;3:86. [PubMed] [DOI] [Cited in This Article: ] |
| 44. | van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427-13431. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Vazou S, Pesce C, Lakes K, Smiley-Oyen A. More than one road leads to Rome: A narrative review and meta-analysis of physical activity intervention effects on cognition in youth. Int J Sport Exerc Psychol. 2019;17:153-178. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Sibley BA, Beilock SL. Exercise and working memory: an individual differences investigation. J Sport Exerc Psychol. 2007;29:783-791. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Griffin ÉW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934-941. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Ren LJ, Han MF, Li YS, Xia J, Long X. Working Memory in Depression Patients : a fMRI Study. Hangtian Yixue Yu Yixue Gongcheng. 2013;26:402-404. [DOI] [Cited in This Article: ] |
| 49. | Wang YL, Chen CX, Ma SH, Dou N, Li D. The ceiling effects and correlation of three balance scales in stroke patients. Zhongguo Kangfu Yixue Zazhi. 2015;30:679-683. [Cited in This Article: ] |
| 50. | Liu JY. “Dosage Effect” of the Relationship Between Aerobic Exercise and College Students’ Executive Function. Beijing Tiyu Daxue Xuebao. 2017;40:58-64. [DOI] [Cited in This Article: ] |