Published online Nov 19, 2021. doi: 10.5498/wjp.v11.i11.1065
Peer-review started: May 30, 2021
First decision: July 14, 2021
Revised: July 21, 2021
Accepted: September 22, 2021
Article in press: September 22, 2021
Published online: November 19, 2021
Recent epidemiological and genetic studies have revealed an interconnection between schizophrenia and breast cancer. The mutual underlying pathophy
Core Tip: The frequent occurrence of breast carcinoma in female patients with schizophrenia could indicate a specific immunological perturbation in both diseases. A common denominator could be interleukin-33 (IL-33). In this review article, we aim to describe the IL-33/suppressor of tumorigenicity 2 axis as a crossroad in schizophrenia-breast cancer comorbidity. Considering that raloxifene could be tissue-specific and improve cognition and that tamoxifen resistance in breast carcinoma could be improved by strategies targeting IL-33, these selective estrogen receptor modulators could be useful in complementary treatment. These observations could guide further somatic, as well as psychiatric therapeutical protocols by incorporating what is known about immunity in schizophrenia.
- Citation: Borovcanin MM, Vesic K. Breast cancer in schizophrenia could be interleukin-33-mediated. World J Psychiatr 2021; 11(11): 1065-1074
- URL: https://www.wjgnet.com/2220-3206/full/v11/i11/1065.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i11.1065
The initial question is whether patients with schizophrenia are resistant or more susceptible to developing breast cancer. Recently, it has been reported that the prevalence rate of schizophrenia of 0.3%-0.7% in the world population remains stable[1]. There is a possibility that some mechanisms underlying the pathogenesis of schizophrenia also have beneficial or even protective properties for the onset of some somatic disorders, while others lead to somatic comorbidity and mortality.
Schizophrenia is a neurodegenerative disease with a complex pathogenesis and pathophysiology. This type of disorder is characterized by a chronic course, exacerbations, and progression leading to neurodegeneration over time. Genetic predisposition, individual and environmental factors, and specific immune responses have a significant impact on disease onset and clinical presentation. Moreover, genome-wide association studies in schizophrenia have revealed similar genetic backgrounds with some immunological properties and genetic overlap with breast carcinoma[2,3].
Scientific advances in the last decade have led to the recognition that interleukin-33 (IL-33) and neuroinflammation play a role in some aspects of neurodegenerative diseases. It is possible that IL-33 has various biological activities at different stages of the disease. It is necessary to consider disease progression with exacerbations and neurodegeneration, and to discuss the impact of IL-33 on somatic disturbances.
The alarmin molecule IL-33 as a marker of innate immunity was first studied in different phases of schizophrenia, but also in breast carcinoma patients[4]. In summary, we will try to further elucidate the possibility of the involvement of IL-33 in the onset of breast cancer in schizophrenia patients.
Disturbingly, approximately 2.1 million new cases of breast cancer were diagnosed worldwide in 2018, accounting for nearly one in four cancers in women and resulting in approximately 630000 deaths[5]. Previous epidemiological studies suggest that breast cancer is more common in patients with severe mental disorders than in the general population[6,7]. Among mental illnesses, a higher incidence of breast cancer has been observed in schizophrenia than in mood disorders[8]. Patients with schizophrenia have an increased risk of breast cancer, but not cancer overall[9,10]. The mortality cancer risk in schizophrenia is estimated to be 40% higher than in the general population and 51% higher than in individuals without schizophrenia[11].
The aspects of genetics, as well as epigenetic disturbances due to unhealthy lifestyle and harmful habits should be considered in this comorbidity. Recently, the genetic overlap of schizophrenia and breast cancer has been extensively reported[12]. A study by Lu et al[3] reported the phenotypic and genetic positive association of schizophrenia and breast cancer, estimated the percentage of genetic overlap to be 0.14 [95% cumulative incidence (CI): 0.09-0.19], and identified a shared locus at 19p13 (GATA
In recent years, epidemiological evidence of a controversial association between cancer and neurodegenerative diseases has been increasing. Common etiological factors may play opposing roles in the pathogenesis of neurodegeneration and breast cancer[14,15]. Characteristic brain pathology leads to neuronal cell death and neurodegeneration over time, whereas cancer is dominated by the process of unlimited cell proliferation[16,17]. Evidence suggests that common biological mechanisms such as oxidative stress, metabolic dysregulation and inflammation underlie both diseases, all of which could promote apoptosis and cell proliferation[18-20].
Some immune-metabolic specifiers observed in patients with schizophrenia, such as diabetes, hyperinsulinemia, insulin resistance, and hyperlipidemia, may also be involved in the development of breast cancer[21-23]. Inflammatory changes that occur in patients with schizophrenia could also be marked as a cancer risk factor[24] (Figure 1). IL-33 is significantly higher in serum, indicating tumor recurrence, which also indicates poor prognosis in patients with breast cancer[25-27]. Similarly, higher serum levels of IL-33 are measured in schizophrenia during psychotic episodes[4].
So far, many contributing risk factors have been considered for the onset of breast carcinoma, and they seem to overlap with those considered lifestyle-related qualifiers in patients with schizophrenia. Behavioral habits such as smoking, alcohol consum
It is particularly important to acknowledge gender specificities in schizophrenia. Gender differences in breast carcinoma risk have been observed in people without mental illnesses, but also in patients with schizophrenia. Taiwanese women with schizophrenia were observed to have a 1.94-fold higher risk of breast cancer than non-schizophrenic controls[32]. In addition, hormonal disturbances have been implicated as a risk factor for schizophrenia and breast cancer. According to previous findings, estrogen levels in women decrease during certain periods of life, such as during menstruation, after childbirth, and menopause, which can often lead to an exacerbation of schizophrenia symptoms and resistance to antipsychotic treatment[33,34]. At the same time, estrogen intermediates carcinoma development[35].
IL-33 is a multifunctional cytokine, and an alarmin, a damage-associated molecular pattern (DAMP), an endogenous molecule released into the extracellular space upon cellular stress and damage. DAMPs can correct altered physiological states and regulate homeostasis at low concentrations, propagate inflammatory reactions at high concentrations, or even lead to trauma and activation of surrounding cells and recruitment of distant cells in excessive release[36]. The role of DAMPs in neurodegenerative diseases has already been recognized in sterile inflammation. As recently summarized by Pandolfo et al[37], acute or chronic stress may trigger sterile inflammation associated with DAMPs and may be an etiopathogenetic mechanism for affective disorders.
In recent years, there is increasing evidence for an important immunomodulatory role of IL-33 in neurodegenerative diseases. IL-33 is a multifunctional cytokine that acts intracellularly as a nuclear factor and extracellularly as a cytokine[38]. By binding to the trans-membranes full length receptor (sST2), IL-33 exerts its biological activity through the IL-33/suppressor of tumorigenicity 2 (ST2) signaling pathway[39]. In contrast, the soluble form functions as a decoy receptor and limits the biological activity of IL-33[40]. IL-33 is highly expressed in the brain and released from astrocytes and oligodendrocytes, while the ST2 receptor is expressed in glial cells[41]. It has a dual function and may exert pro-inflammatory or anti-inflammatory effects in the central nervous system (CNS)[42,43]. Up-regulated expression of IL-33 in peripheral cells contributes to blood-brain barrier disruption[44]. By binding to the ST2 receptor, IL-33 promotes microglial activation and proliferation and enhances production of different cytokines, leading to an acute inflammatory response[45]. IL-33 signaling is associated with regulatory T and B cell responses[46,47]. By inducing microglial and macrophage polarization to an anti-inflammatory type 2 phenotype and phagocytosis, IL-33 exerts a neuroprotective and reparative role in the CNS[45,48].
In Alzheimer’s disease (AD), soluble decoys trap IL-33 already produced at lower levels, and its concentrations in serum and cerebrospinal fluid are significantly reduced[49]. IL-33 could exert a neuroprotective effect by inducing innate immunity to reduce soluble amyloid β levels and amyloid plaque deposition[50]. Carlock et al[51] in 2017 pointed out that deficiency of IL-33 caused tau abnormality and late-onset neurodegeneration in the cerebral cortex and hippocampus, accompanied by memory impairment. A recent study suggested that IL-33 gene mutations affect susceptibility to late-onset AD, which in turn confirms that IL-33 may exacerbate neuroinflammation and cognitive decline[52,53].
As a neuroinflammatory and neurodegenerative disease, multiple sclerosis (MS) is associated with increased expression of IL-33 in the periphery, white matter, and plaque areas of the MS brain[54-57]. However, the exact role of IL-33 has not been fully elucidated, and data in the current literature are inconsistent. Previous studies have supported the involvement of IL-33 as a proinflammatory cytokine in disease pathology[56]. However, several recent observations suggest a dominant neuroreparative role of IL-33 in MS. Studies on experimental autoimmune encephalomyelitis (EAE) reported a detrimental effect of IL-33 treatment on EAE severity. The identification of ST2 expression by oligodendrocytes indicates an important role in the myelination process during CNS development and the repair phase of MS[57,58].
Recently, the protective effect of IL-33 in some acute neurological states has been reported. For example, Miao et al[59] in 2021 highlighted that increased IL-33 levels might reduce brain damage in patients with intracerebral hemorrhage. The reviewed results support the evidence of elevated IL-33 serum levels in the acutisation of schizophrenia and depression, suggesting the involvement of this axis in the processes of relapse and recurrence of mental disorder[37].
IL-33 exerts its function by binding to the ST2 receptor, expressed on T helper (Th) 2 cells, but skewing toward Th1 cytokines has been found in MS[60,61]. IL-33 has been categorized as a promoter of Th2 immunity by inducing the production of IL-4, IL-5 and IL-13, M2 polarization of macrophages, and eosinophil recruitment[62]. The Th2-related cytokine can act as a pro-tumorigenic factor by limiting anti-tumor immunity and promoting extracellular matrix remodeling, but the localization of IL-33 is crucial for accurately distinguishing its effect in tumor biology[63].
IL-33 stimulates innate type 2 lymphoid cells (ILC2s), leading to the release of proinflammatory cytokines and type-2 inflammation[64]. An association with allergic disease has been observed in patients with autism spectrum disorder, and an immune pattern initiated by IL-33, ILC2 and mast cells has been confirmed[65]. To date, there are no data on the role of ILC2s in schizophrenia, and these findings in autism spectrum disorder as a neurodegenerative disease may serve as a basis for further investigation in psychosis. ILC2s are the largest subset in the lung and skin associated with pro-allergic and antiparasitic immunity. Dysregulation of their signaling circuitry may accelerate fibrotic responses and have predominantly carcinogenic activities[66]. In the breast cancer model, increased endogenous IL-33 was observed during cancer progression, further facilitating the intratumoral accumulation of immunosuppressive IL-13-producing innate lymphoid cells and promoting the growth of breast cancer and metastases in the lungs[67]. Escalation of systemic IL-33 secretion could precipitate the carcinoma development in neurodegenerative diseases and schizophrenia as a representative.
The first line of treatment for schizophrenia is antipsychotics, but nowadays it is surprising that these drugs could also have anticancer effects[68]. Previous studies have shown that some antipsychotics may cause higher prolactin levels, called “prolactin-raising”, and thus an increased risk of breast cancer by promoting carcinogenesis and transition to invasive carcinoma[69]. Breast cancer risk was higher in patients receiving first-generation antipsychotics and second-generation antipsychotics alone, as well as a combination of both, regardless of the mean exposure dose[32]. However, it must be emphasized that according to the World Federation of Societies of Biological Psychiatry, breast cancer is not listed as an antipsychotic-induced hyperprolactinemia adverse effect[70]. De Hert et al[71] reported that studies in patients with idiopathic hyperprolactinemia, prolactinomas, and Parkinson’s disease (PD) found no carcinogenic effects of prolactin. Hoehn et al[72] first described the possible inverse association between PD and neoplasms. This may suggest that excessive dopamine or prolactin release is not the exclusive mechanism leading to breast cancer development, but rather a milieu for more complex interactions. Our study exploring the impact of risperidone and paliperidone as prolactin-raising long-acting injections suggested a decrease in IL-33 serum levels in patients with schizophrenia in remission with possibly balancing and antitumorigenic properties[4].
The clinical presentation of schizophrenia differs in men and women and could be partly attributed to the neuroprotective properties of estrogens[73]. Modulation of dopamine 2 receptor occupancy of antipsychotics can also be associated with estrogens[74]. Estrogens protect women from infections and prevent mortality associated with inflammation by downregulating levels of pro-inflammatory cytokines IL-1β, IL-10, and tumor necrosis factor alpha[75].
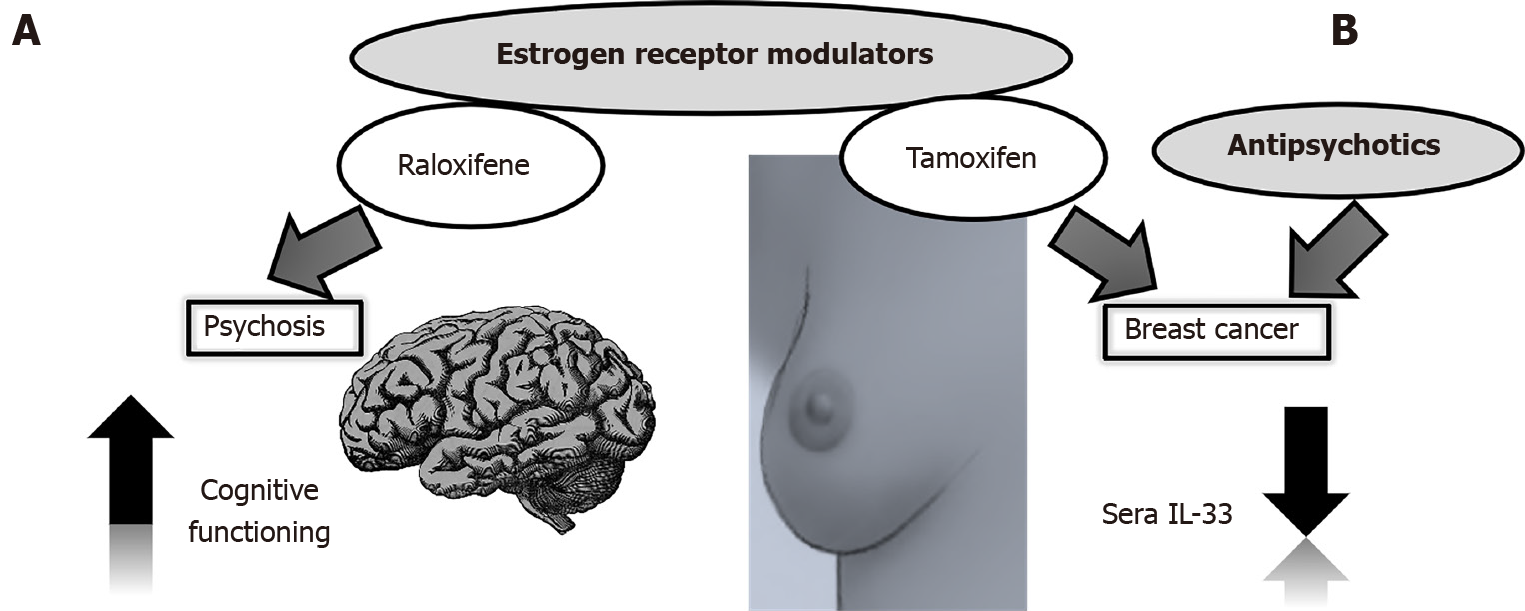
Selective estrogen receptor modulators such as raloxifene have an antiestrogenic effect in the breast and uterus, but not in the brain and bone tissue[76]. Of particular interest to us was that raloxifene was not only effective in improving schizophrenia, but even more effective in improving cognitive symptoms in postmenopausal women with low estrogen levels[77,78] (Figure 2A). This tissue-specific dual action of the drug could be simultaneously used for targeted breast cancer therapy and mental state in schizophrenia patients and specifically explored in the context of IL-33 secretion.
Guidelines recommend tamoxifen as a standard of care for premenopausal women for 5-10 years[79]. In vitro studies could guide further investigations on the beneficial properties of the prolactin-elevating antipsychotics thioridazine and chlorpromazine in enhancing the effect of tamoxifen in tamoxifen-resistant human breast cancer cells[80]. IL-33 overexpression in breast cancer cells results in resistance to tamoxifen-induced tumor growth inhibition, while IL-33 knockdown corrects this problem[81]. This knockdown could be achieved through the action of antipsychotics (Figure 2B).
Individualized treatment is of great importance in modern medicine, as patients with schizophrenia must be treated equally. Diagnosis and treatment of somatic states in schizophrenia patients could influence behavioral changes and improve outcome. Prevention strategies for breast carcinoma onset in schizophrenia patients could be developed by understanding and recognizing the genetic background, lifestyle, and individual factors, altogether resulting in phase-specific immune dysregulation. IL-33 as a marker of innate immunity and an alarmin has been discussed in other neurodegenerative diseases. Further exploration of IL-33 as an alarmin in mental disorders should take into account gender, age at onset, duration of illness, frequency of disease acutisation, antipsychotic treatment, and a variety of comorbid somatic states including breast cancer. There appears to be an impact on the occurrence of positive symptoms and exacerbation of schizophrenia, but also on the progression of breast cancer, making IL-33 a candidate for centered therapy. We have shown that antipsychotics with their anticarcinogenic properties could be beneficial, possibly through prolactin elevation, tissue-specific estrogen-sparing drugs, and additional IL-33 downregulation.
This review was enriched by the valuable collaboration with the Center for Molecular Medicine and Stem Cell Research, at the Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia. We would like to thank Bojana Mircetic for language editing.
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Psychiatry
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Valencia GA S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Guo X
| 1. | McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1256] [Cited by in F6Publishing: 1314] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 2. | Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5668] [Cited by in F6Publishing: 5327] [Article Influence: 532.7] [Reference Citation Analysis (0)] |
| 3. | Lu D, Song J, Lu Y, Fall K, Chen X, Fang F, Landén M, Hultman CM, Czene K, Sullivan P, Tamimi RM, Valdimarsdóttir UA. A shared genetic contribution to breast cancer and schizophrenia. Nat Commun. 2020;11:4637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Borovcanin MM, Janicijevic SM, Jovanovic IP, Gajovic N, Arsenijevic NN, Lukic ML. IL-33/ST2 Pathway and Galectin-3 as a New Analytes in Pathogenesis and Cardiometabolic Risk Evaluation in Psychosis. Front Psychiatry. 2018;9:271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 51120] [Article Influence: 8520.0] [Reference Citation Analysis (122)] |
| 6. | Girardi P, Schievano E, Fedeli U, Braggion M, Nuti M, Amaddeo F. Causes of mortality in a large population-based cohort of psychiatric patients in Southern Europe. J Psychiatr Res. 2021;136:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Solmi M, Firth J, Miola A, Fornaro M, Frison E, Fusar-Poli P, Dragioti E, Shin JI, Carvalho AF, Stubbs B, Koyanagi A, Kisely S, Correll CU. Disparities in cancer screening in people with mental illness across the world versus the general population: prevalence and comparative meta-analysis including 4 717 839 people. Lancet Psychiatry. 2020;7:52-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Nordentoft M, Plana-Ripoll O, Laursen TM. Cancer and schizophrenia. Curr Opin Psychiatry. 2021;34:260-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Zhuo C, Triplett PT. Association of Schizophrenia With the Risk of Breast Cancer Incidence: A Meta-analysis. JAMA Psychiatry. 2018;75:363-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Zhuo C, Tao R, Jiang R, Lin X, Shao M. Cancer mortality in patients with schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2017;211:7-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Byrne EM, Ferreira MAR, Xue A, Lindström S, Jiang X, Yang J, Easton DF, Wray NR, Chenevix-Trench G. Is Schizophrenia a Risk Factor for Breast Cancer? Schizophr Bull. 2019;45:1251-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Shi J, Wu L, Zheng W, Wen W, Wang S, Shu X, Long J, Shen CY, Wu PE, Saloustros E, Chang-Claude J, Brenner H, Shu XO, Cai Q. Genetic Evidence for the Association between Schizophrenia and Breast Cancer. J Psychiatr Brain Sci. 2018;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ganoci L, Trkulja V, Živković M, Božina T, Šagud M, Lovrić M, Božina N. ABCB1, ABCG2 and CYP2D6 polymorphism effects on disposition and response to long-acting risperidone. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Rojas NG, Cesarini M, Etcheverry JL, Prat GAD, Arciuch VA, Gatto EM. Neurodegenerative diseases and cancer: sharing common mechanisms in complex interactions. J Integr Neurosci. 2020;19:187-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kong W, Mou X, Deng J, Di B, Zhong R, Wang S, Yang Y, Zeng W. Differences of immune disorders between Alzheimer's disease and breast cancer based on transcriptional regulation. PLoS One. 2017;12:e0180337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25:59-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1163] [Cited by in F6Publishing: 1393] [Article Influence: 232.2] [Reference Citation Analysis (0)] |
| 17. | Wood CE, Hukkanen RR, Sura R, Jacobson-Kram D, Nolte T, Odin M, Cohen SM. Scientific and Regulatory Policy Committee (SRPC) Review: Interpretation and Use of Cell Proliferation Data in Cancer Risk Assessment. Toxicol Pathol. 2015;43:760-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP. Transcriptional evidence for the "Reverse Warburg Effect" in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer's disease, and "Neuron-Glia Metabolic Coupling". Aging (Albany NY). 2010;2:185-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Hedskog L, Zhang S, Ankarcrona M. Strategic role for mitochondria in Alzheimer's disease and cancer. Antioxid Redox Signal. 2012;16:1476-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Harris RA, Tindale L, Cumming RC. Age-dependent metabolic dysregulation in cancer and Alzheimer's disease. Biogerontology. 2014;15:559-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Cazzaniga M, Bonanni B. Relationship Between Metabolic Disorders and Breast Cancer Incidence and Outcomes. Is There a Preventive and Therapeutic Role for Berberine? Anticancer Res. 2018;38:4393-4402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired Glucose Homeostasis in First-Episode Schizophrenia: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017;74:261-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 23. | Misiak B, Stańczykiewicz B, Łaczmański Ł, Frydecka D. Lipid profile disturbances in antipsychotic-naive patients with first-episode non-affective psychosis: A systematic review and meta-analysis. Schizophr Res. 2017;190:18-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Hodgson R, Wildgust HJ, Bushe CJ. Cancer and schizophrenia: is there a paradox? J Psychopharmacol. 2010;24:51-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Liu J, Shen JX, Hu JL, Huang WH, Zhang GJ. Significance of interleukin-33 and its related cytokines in patients with breast cancers. Front Immunol. 2014;5:141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Jafarzadeh A, Minaee K, Farsinejad AR, Nemati M, Khosravimashizi A, Daneshvar H, Mohammadi MM, Sheikhi A, Ghaderi A. Evaluation of the circulating levels of IL-12 and IL-33 in patients with breast cancer: influences of the tumor stages and cytokine gene polymorphisms. Iran J Basic Med Sci. 2015;18:1189-1198. [PubMed] [Cited in This Article: ] |
| 27. | Yang ZP, Ling DY, Xie YH, Wu WX, Li JR, Jiang J, Zheng JL, Fan YH, Zhang Y. The Association of Serum IL-33 and sST2 with Breast Cancer. Dis Markers. 2015;2015:516895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105:515-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 29. | Jung S, Wang M, Anderson K, Baglietto L, Bergkvist L, Bernstein L, van den Brandt PA, Brinton L, Buring JE, Eliassen AH, Falk R, Gapstur SM, Giles GG, Goodman G, Hoffman-Bolton J, Horn-Ross PL, Inoue M, Kolonel LN, Krogh V, Lof M, Maas P, Miller AB, Neuhouser ML, Park Y, Robien K, Rohan TE, Scarmo S, Schouten LJ, Sieri S, Stevens VL, Tsugane S, Visvanathan K, Wilkens LR, Wolk A, Weiderpass E, Willett WC, Zeleniuch-Jacquotte A, Zhang SM, Zhang X, Ziegler RG, Smith-Warner SA. Alcohol consumption and breast cancer risk by estrogen receptor status: in a pooled analysis of 20 studies. Int J Epidemiol. 2016;45:916-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Elme A, Utriainen M, Kellokumpu-Lehtinen P, Palva T, Luoto R, Nikander R, Huovinen R, Kautiainen H, Järvenpää S, Penttinen HM, Vehmanen L, Jääskeläinen AS, Ruohola J, Blomqvist C, Saarto T. Obesity and physical inactivity are related to impaired physical health of breast cancer survivors. Anticancer Res. 2013;33:1595-1602. [PubMed] [Cited in This Article: ] |
| 31. | Schoemaker MJ, Jones ME, Wright LB, Griffin J, McFadden E, Ashworth A, Swerdlow AJ. Psychological stress, adverse life events and breast cancer incidence: a cohort investigation in 106,000 women in the United Kingdom. Breast Cancer Res. 2016;18:72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Wu Chou AI, Wang YC, Lin CL, Kao CH. Female schizophrenia patients and risk of breast cancer: A population-based cohort study. Schizophr Res. 2017;188:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Seeman MV, González-Rodríguez A. Use of psychotropic medication in women with psychotic disorders at menopause and beyond. Curr Opin Psychiatry. 2018;31:183-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Searles S, Makarewicz JA, Dumas JA. The role of estradiol in schizophrenia diagnosis and symptoms in postmenopausal women. Schizophr Res. 2018;196:35-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang N, Conaway M, Wang H, Korach KS, Bocchinfuso W, Santen R. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127:1748-1757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Klegeris A. Regulation of neuroimmune processes by damage- and resolution-associated molecular patterns. Neural Regen Res. 2021;16:423-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Pandolfo G, Genovese G, Casciaro M, Muscatello MRA, Bruno A, Pioggia G, Gangemi S. IL-33 in Mental Disorders. Medicina (Kaunas). 2021;57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Küchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 39. | Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551-2555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 381] [Cited by in F6Publishing: 400] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 40. | Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 707] [Cited by in F6Publishing: 759] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 41. | Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 42. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2564] [Cited by in F6Publishing: 2731] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 43. | Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond). 2011;8:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 44. | Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, Kim J, Kim YM, Kwon YG. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117-3126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 45. | Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Production and functions of IL-33 in the central nervous system. Brain Res. 2011;1385:8-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 46. | Pastille E, Wasmer MH, Adamczyk A, Vu VP, Mager LF, Phuong NNT, Palmieri V, Simillion C, Hansen W, Kasper S, Schuler M, Muggli B, McCoy KD, Buer J, Zlobec I, Westendorf AM, Krebs P. The IL-33/ST2 pathway shapes the regulatory T cell phenotype to promote intestinal cancer. Mucosal Immunol. 2019;12:990-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 47. | Siede J, Fröhlich A, Datsi A, Hegazy AN, Varga DV, Holecska V, Saito H, Nakae S, Löhning M. IL-33 Receptor-Expressing Regulatory T Cells Are Highly Activated, Th2 Biased and Suppress CD4 T Cell Proliferation through IL-10 and TGFβ Release. PLoS One. 2016;11:e0161507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 48. | Marsh SE, Abud EM, Lakatos A, Karimzadeh A, Yeung ST, Davtyan H, Fote GM, Lau L, Weinger JG, Lane TE, Inlay MA, Poon WW, Blurton-Jones M. The adaptive immune system restrains Alzheimer's disease pathogenesis by modulating microglial function. Proc Natl Acad Sci U S A. 2016;113:E1316-E1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 49. | Saresella M, Marventano I, Piancone F, La Rosa F, Galimberti D, Fenoglio C, Scarpini E, Clerici M. IL-33 and its decoy sST2 in patients with Alzheimer's disease and mild cognitive impairment. J Neuroinflammation. 2020;17:174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Fu AK, Hung KW, Yuen MY, Zhou X, Mak DS, Chan IC, Cheung TH, Zhang B, Fu WY, Liew FY, Ip NY. IL-33 ameliorates Alzheimer's disease-like pathology and cognitive decline. Proc Natl Acad Sci U S A. 2016;113:E2705-E2713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 51. | Carlock C, Wu J, Shim J, Moreno-Gonzalez I, Pitcher MR, Hicks J, Suzuki A, Iwata J, Quevado J, Lou Y. Interleukin33 deficiency causes tau abnormality and neurodegeneration with Alzheimer-like symptoms in aged mice. Transl Psychiatry. 2017;7:e1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Zhong X, Liu MY, He M, Du K, Wei MJ. Association of interleukin-33 gene polymorphisms with susceptibility to late onset Alzheimer's disease: a meta-analysis. Neuropsychiatr Dis Treat. 2017;13:2275-2284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Reverchon F, de Concini V, Larrigaldie V, Benmerzoug S, Briault S, Togbé D, Ryffel B, Quesniaux VFJ, Menuet A. Hippocampal interleukin-33 mediates neuroinflammation-induced cognitive impairments. J Neuroinflammation. 2020;17:268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 54. | Alsahebfosoul F, Rahimmanesh I, Shajarian M, Etemadifar M, Sedaghat N, Hejazi Z, Naderi S. Interleukin-33 plasma levels in patients with relapsing-remitting multiple sclerosis. Biomol Concepts. 2017;8:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Allan D, Fairlie-Clarke KJ, Elliott C, Schuh C, Barnett SC, Lassmann H, Linnington C, Jiang HR. Role of IL-33 and ST2 signalling pathway in multiple sclerosis: expression by oligodendrocytes and inhibition of myelination in central nervous system. Acta Neuropathol Commun. 2016;4:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Christophi GP, Gruber RC, Panos M, Christophi RL, Jubelt B, Massa PT. Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin Immunol. 2012;142:308-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 57. | Fairlie-Clarke K, Barbour M, Wilson C, Hridi SU, Allan D, Jiang HR. Expression and Function of IL-33/ST2 Axis in the Central Nervous System Under Normal and Diseased Conditions. Front Immunol. 2018;9:2596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 58. | Ljunggren-Rose Å, Natarajan C, Matta P, Pandey A, Upender I, Sriram S. Anacardic acid induces IL-33 and promotes remyelination in CNS. Proc Natl Acad Sci U S A. 2020;117:21527-21535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Miao Y, Zhang ZX, Feng X, Sun WM. IL-33 as a Novel Serum Prognostic Marker of Intracerebral Hemorrhage. Oxid Med Cell Longev. 2021;2021:5597790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Kamekura R, Kojima T, Takano K, Go M, Sawada N, Himi T. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis. Clin Exp Allergy. 2012;42:218-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 834] [Cited by in F6Publishing: 871] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 62. | Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1241] [Cited by in F6Publishing: 1320] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 63. | Mora J, Weigert A. IL-1 family cytokines in cancer immunity - a matter of life and death. Biol Chem. 2016;397:1125-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Toki S, Goleniewska K, Zhang J, Zhou W, Newcomb DC, Zhou B, Kita H, Boyd KL, Peebles RS Jr. TSLP and IL-33 reciprocally promote each other's lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. 2020;75:1606-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 65. | Di Salvo E, Casciaro M, Quartuccio S, Genovese L, Gangemi S. Do Alarmins Have a Potential Role in Autism Spectrum Disorders Pathogenesis and Progression? Biomolecules. 2018;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Wang S, Wu P, Chen Y, Chai Y. Ambiguous roles and potential therapeutic strategies of innate lymphoid cells in different types of tumor. Oncol Lett. 2020;20:1513-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 68. | Zhuo C, Xun Z, Hou W, Ji F, Lin X, Tian H, Zheng W, Chen M, Liu C, Wang W, Chen C. Surprising Anticancer Activities of Psychiatric Medications: Old Drugs Offer New Hope for Patients With Brain Cancer. Front Pharmacol. 2019;10:1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Harvey PW, Everett DJ, Springall CJ. Adverse effects of prolactin in rodents and humans: breast and prostate cancer. J Psychopharmacol. 2008;22:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Greenland S, Fairweather WR. Re: "Comparing Proportion Exposed in Case-Control Studies Using Several Control Groups". Am J Epidemiol. 1990;131:944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 71. | De Hert M, Peuskens J, Sabbe T, Mitchell AJ, Stubbs B, Neven P, Wildiers H, Detraux J. Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand. 2016;133:5-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 72. | Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8028] [Cited by in F6Publishing: 8072] [Article Influence: 141.6] [Reference Citation Analysis (1)] |
| 73. | Brand BA, de Boer JN, Sommer IEC. Estrogens in schizophrenia: progress, current challenges and opportunities. Curr Opin Psychiatry. 2021;34:228-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 74. | Eugene AR, Masiak J. A pharmacodynamic modelling and simulation study identifying gender differences of daily olanzapine dose and dopamine D2-receptor occupancy. Nord J Psychiatry. 2017;71:417-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Hidalgo-Lanussa O, Baez-Jurado E, Echeverria V, Ashraf GM, Sahebkar A, Garcia-Segura LM, Melcangi RC, Barreto GE. Lipotoxicity, neuroinflammation, glial cells and oestrogenic compounds. J Neuroendocrinol. 2020;32:e12776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf. 2015;14:921-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | de Boer J, Prikken M, Lei WU, Begemann M, Sommer I. The effect of raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a systematic review and meta-analysis. NPJ Schizophr. 2018;4:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 78. | Gurvich C, Hudaib A, Gavrilidis E, Worsley R, Thomas N, Kulkarni J. Raloxifene as a treatment for cognition in women with schizophrenia: the influence of menopause status. Psychoneuroendocrinology. 2019;100:113-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1194-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 1060] [Article Influence: 265.0] [Reference Citation Analysis (0)] |
| 80. | Yde CW, Clausen MP, Bennetzen MV, Lykkesfeldt AE, Mouritsen OG, Guerra B. The antipsychotic drug chlorpromazine enhances the cytotoxic effect of tamoxifen in tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells. Anticancer Drugs. 2009;20:723-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Hu H, Sun J, Wang C, Bu X, Liu X, Mao Y, Wang H. IL-33 facilitates endocrine resistance of breast cancer by inducing cancer stem cell properties. Biochem Biophys Res Commun. 2017;485:643-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |