Copyright
©The Author(s) 2024.
World J Psychiatry. Mar 19, 2024; 14(3): 380-387
Published online Mar 19, 2024. doi: 10.5498/wjp.v14.i3.380
Published online Mar 19, 2024. doi: 10.5498/wjp.v14.i3.380
Figure 1 Study flowchart.
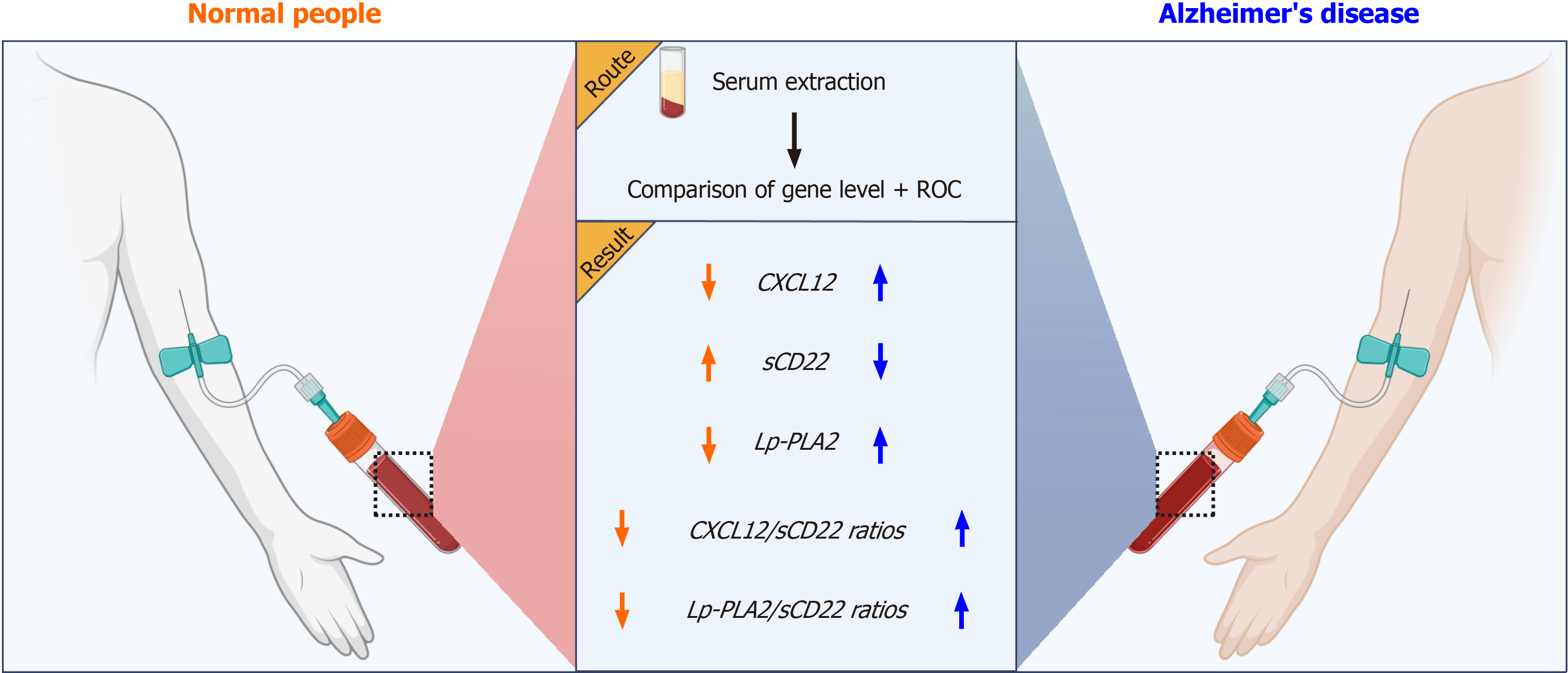
This study utilized a prospective case-control design, encompassing two distinct groups: patients with Alzheimer's disease (AD) and healthy controls (normal people). We meticulously collected serum samples from these participants and quantitatively analyzed the levels of three key biomarkers: CXCL12, sCD22, and Lp-PLA2. Our findings revealed a notable increase in the levels of CXCL12 and Lp-PLA2 in the AD cohort, contrasted by a significant decrease in sCD22 levels. Additionally, we observed marked elevations in the ratios of CXCL12/sCD22 and Lp-PLA2/sCD22. These biomarkers, along with their calculated ratios, emerged as potential diagnostic indicators for AD. This discovery holds substantial clinical value, offering crucial insights for early detection and informing targeted therapeutic strategies for AD patients.
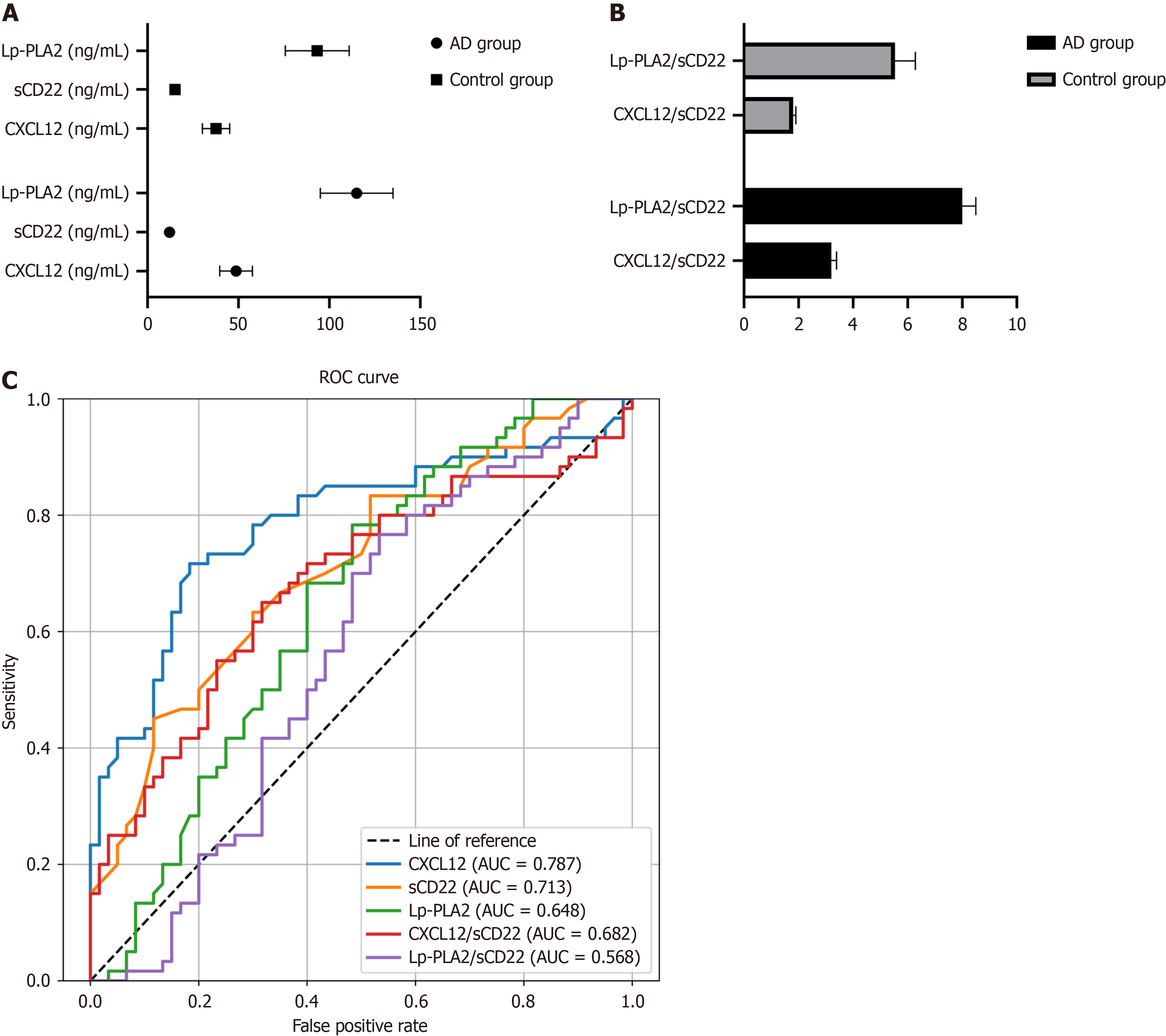
Figure 2 Peripheral blood biomarker profiles and diagnostic potential in Alzheimer's disease.
A: The concentrations of Lp-PLA2, sCD22, and CXCL12 in the peripheral blood of healthy individuals and patients with Alzheimer's disease (AD); B: Comparison of Lp-PLA2/sCD22 and CXCL12/sCD22 ratios in healthy individuals and patients with AD; C: receiver operating characteristic curves for the diagnosis of AD using Lp-PLA2, sCD22, CXCL12, Lp-PLA2/sCD22, and CXCL12/sCD22. AUC: Area under the curve; ROC: Receiver operating characteristic.
- Citation: Liu ZL, Hua FF, Qu L, Yan N, Zhang HF. Evaluating serum CXCL12, sCD22, Lp-PLA2 levels and ratios as biomarkers for diagnosis of Alzheimer's disease. World J Psychiatry 2024; 14(3): 380-387
- URL: https://www.wjgnet.com/2220-3206/full/v14/i3/380.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i3.380