Published online Oct 16, 2023. doi: 10.5494/wjh.v11.i2.12
Peer-review started: July 12, 2023
First decision: September 4, 2023
Revised: September 6, 2023
Accepted: September 26, 2023
Article in press: September 26, 2023
Published online: October 16, 2023
Processing time: 95 Days and 13.2 Hours
High blood pressure (BP), known as hypertension, is a major contributing factor to the development of cardiovascular disease. The development and pathogenesis of hypertension involve a wide array of factors including genetics, environment, hormones, hemodynamics, and inflammation. There is a significantly positive association between higher levels of colonization by Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola (etiologic bacterial burden) below the gum line, and the presence of hypertension. The use of antibiotics during pregnancy, which is likely indicative of bacterial infections severe enough to require antibiotic treatment, is associated with a slight increase in average arterial BP. Cytomegalovirus infection is a risk factor for heightened arterial BP and acts as a co-factor in the development of aortic atherosclerosis. The relationship between hypertension and coronavirus disease 2019 involves endothelial dysfunction and dysregulation of the renin-angiotensin system. The effects of gut microbiota on BP, whether beneficial or harmful, are influenced by multiple factors including genetics, epigenetics, lifestyle choices, and antibiotic usage. These variables collectively contribute to overall BP levels and the control of hypertension. Several reports have examined the BP levels of patients infected with the Zika virus. In regions with a high incidence of nasopharyngeal carcinoma, hypertension has been linked to a higher risk of Epstein-Barr virus reactivation. Also, a potential causal link has been found between malaria and elevated BP. Also, the elevated prevalence of hypertension among dengue patients during their initial visit suggests that relying solely on BP measurements to predict severe infection may not be clinically reliable.
Core Tip: Hypertension is a chronic medical condition characterized by elevated blood pressure in the arteries. It poses a significant threat to global public health and contributes to the overall burden of disease and mortality worldwide. The relationship between microbes and the pathogenesis of hypertension is an area of ongoing research, and while there is no definitive conclusion, some studies have suggested a potential connection between certain microbes and the development of hypertension. However, this article summarizes the current knowledge of microbes in the pathogenesis of hypertension which includes bacterial infection, viral infection and protozoa infection.
- Citation: Khurshid H, Rafaqat S, Rafaqat S. Overview of microbes in hypertension. World J Hypertens 2023; 11(2): 12-19
- URL: https://www.wjgnet.com/2220-3168/full/v11/i2/12.htm
- DOI: https://dx.doi.org/10.5494/wjh.v11.i2.12
High blood pressure (BP), usually referred to as hypertension, is a chronic medical disorder marked by persistently excessive BP in the arteries. It poses a significant threat to global public health and contributes substantially to the overall burden of disease and mortality worldwide. The incidence of hypertension is increasing globally, with a projected rise from 26.4% in 2000 to 29.2% by 2025[1]. Infection occurs when viruses, bacteria, or other microorganisms enter your body and start replicating. Disease, which typically affects a small portion of infected individuals, happens when the infection damages the cells in your body, leading to the emergence of signs and symptoms. When confronted with an infection, your immune system activates and takes action. White blood cells, antibodies, and various defense mechanisms work together to eliminate the foreign invader from your body. Pathogenic microorganisms present a variety of challenges to the immune system. Viruses can cause illness by either killing cells or interfering with their normal functions. In response, our bodies often trigger a fever (as higher temperatures can inactivate many viruses), release a chemical called interferon (which inhibits viral replication), or mobilize antibodies and other immune cells to target the invading virus. Similarly, many bacteria make us sick by similar means, but they also possess other strategies. Sometimes bacteria reproduce rapidly, out-competing host tissues and disrupting normal functioning. They may also directly kill cells and tissues or produce toxins that can paralyze cells, disrupt their metabolism, or trigger an overwhelming immune response that can be harmful[2].
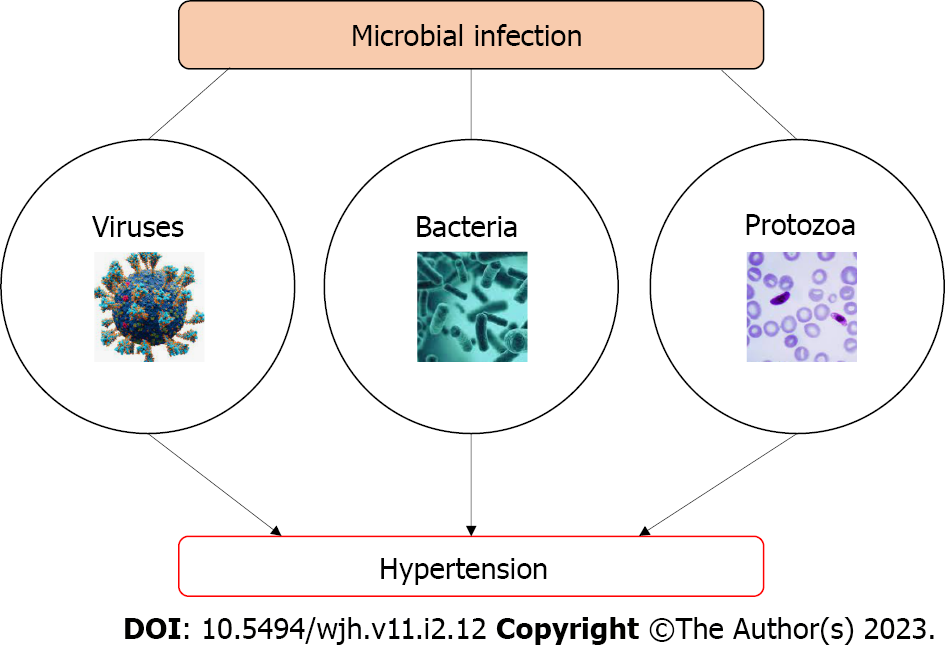
This review article summarizes the current knowledge of microbes in the pathogenesis of hypertension which includes bacterial infection, viral infection and protozoa infection as explained in Figure 1.
Science Direct, PubMed, and Google Scholar were only a few of the databases used to perform the literature review. Specific terms including "Hypertension," "Microbes," "Virus," and "Bacteria" were included in the search for articles published until May 25, 2023. Clinical research was only done on papers that were published in English. No particular timeline was set, despite the focus being on current studies. By going over the reference lists of the chosen papers, more pertinent publications were found.
One of the main risk factors for the emergence of cardiovascular disease is high BP, often known as hypertension. Recent findings from the INVEST project add important knowledge to the growing corpus of research on the relationship between periodontal diseases and hypertension. By directly analyzing the presence of periodontal bacteria rather than relying on signs of prior infection such tooth loss, attachment loss, and pocket depth, these findings offer the first clear evidence of a connection between periodontal infections and hypertension. Desvarieux et al[3] study revealed a significant positive association between higher levels of colonization by Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola (etiologic bacterial burden) below the gum line and the presence of hypertension. After adjusting for various factors, individuals in the highest tertile of etiologic bacterial burden had over a three-fold increased likelihood of having hypertension compared to those in the lowest tertile. Additionally, the highest tertile of etiologic burden was associated with a 9 mm/Hg increase in systolic BP and a 5 mm/Hg increase in diastolic BP compared to the lowest tertile. These associations persisted in both male and female subgroups, with a stronger effect observed among men. A less strict clinical definition of periodontal disease based on the percentage of mouth locations with a pocket depth of less than 3 mm was also used, and the results were still reliable[3].
The relationship between bacterial infections during pregnancy and maternal BP was investigated. In summary, Petry et al[4] presented novel evidence indicating that the use of antibiotics during pregnancy, which was likely indicative of bacterial infections severe enough to require antibiotic treatment, was associated with a slight increase in average arterial BP. This finding was partially consistent with a previous study conducted in the 1960s, which involved over 6000 women of all ages (not limited to pregnant women). It was observed that women with significant bacterial infections had systolic BP approximately 3 mmHg higher than those without bacterial infections, although statistical significance was not achieved on that occasion. Considering these factors, findings were unlikely to be random occurrences. Although the impact of bacterial infections on BP was relatively small, results support existing published evidence linking infections during pregnancy to the development of pre-eclampsia.
Typically, during pregnancy, the macrolide antibiotic spiramycin is administered over an extended period to treat Toxoplasma infection and prevent fetal malformations. Interestingly, another study noticed an empirical trend where treated patients rarely developed pregnancy-induced hypertension (PIH), a common and serious pregnancy disorder with disputed causes and mechanisms. Some clinical and experimental data suggest that infection may contribute to the development of PIH. The findings indicate that prolonged spiramycin treatment during pregnancy reduces the risk of developing PIH, potentially by preventing the occurrence of infections that could complicate the pregnancy. These results introduce new possibilities for PIH prevention. Nevertheless, larger prospective studies are necessary to corroborate the hypothesis that infection plays a role in the pathogenesis of certain hypertensive disorders of pregnancy. Ultimately, a randomized trial was needed to justify the use of antibiotic treatment as a preventive strategy in clinical practice[5].
The understanding of the gut microbiome expands, and a growing body of evidence suggests that these microorganisms can have significant impacts, both positive and negative, on BP and related conditions. The metabolites produced by bacteria in the gut can exert broad effects on various tissues and organs throughout the body. It was evident that the extensive metabolic functions disrupted by an imbalanced gut microbiome cannot be fully restored by a single metabolite or strain of bacteria alone. Instead, combinations of bacteria and complementary therapies are likely to offer a more comprehensive approach to effectively managing hypertension[6].
The immune response triggered by viral infections is another significant factor that can exacerbate high BP also is discussed. The ongoing interactions between humans and viruses can have various consequences that potentially contribute to the development of hypertension and negatively impact cardiovascular and kidney health. Human-virus interactions have been ongoing since the emergence of species. Apart from the direct effects caused by viruses, their ability to modulate the immune response and induce chronic inflammation can have implications for the function of various organs. The impact of viral infections on cardiovascular and kidney health is extensive and continues to be explored for many different viruses. Numerous mechanisms leading to hypertension have been identified, including the involvement of the immune system through factors like interferon gamma, interleukins, cytokines, pathogen-associated molecular patterns, damage-associated molecular patterns, and the generation of reactive oxygen species. Immune system activation can also contribute to collapsing glomerulopathy in individuals with high-risk apolipoprotein L1 alleles. Certain viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), cytomegalovirus (CMV), and coxsackievirus, have a direct affinity for cardiovascular organs (such as the heart, vasculature, and kidneys), resulting in organ dysfunction. The renin-angiotensin system (RAS) appears to be disrupted in several viral diseases. The past 2 years have focused extensively on unravelling the association between hypertension, cardiovascular and kidney disease, and the RAS in the context of the SARS-CoV-2 virus. The key link to the cardiovascular effects of SARS-CoV-2 is utilization of the angiotensin-converting enzyme 2 (ACE2) protein for cell entry. ACE2 is expressed in various organs crucial for cardiovascular health. Imbalances between the two arms of the RAS, characterized by an increased ACE to ACE2 ratio, have previously been associated with the development of hypertension and kidney disease in animal models before the coronavirus disease 2019 (COVID-19) era. Indeed, downregulation of ACE2 has been observed in other viral respiratory infections like respiratory syncytial virus and influenza[7].
In the same way, the connections among emerging viral infections, hypertension, and cardiovascular disease, compelling evidence was found indicating that preexisting cardiovascular disease (CVD) and hypertension contribute to increased severity and mortality of COVID-19 infections in Sub-Saharan Africa (SSA). Weaker evidence suggests that COVID-19 may also raise the incidence of CVD in SSA. While there was considerable variation in findings across studies, the variability among high-quality studies with larger sample sizes focusing on COVID-19 was relatively limited. Considering this evidence, along with data from other regions worldwide, a bidirectional relationship between emerging viral infections, hypertension, and CVD in SSA. It is important to acknowledge that unique environmental, social, genetic, and healthcare system factors may be involved in this context[8].
Moreover, CMV infection is a prevalent condition in adults, with a high global seropositivity rate ranging from 60% to 99%. It is associated with cardiovascular diseases, in line with established risk factors such as hypertension and atherosclerosis. Other viral infections, including human herpes virus 8 and human immunodeficiency virus-1 (HIV-1), have also been linked to hypertension. However, the specific mechanisms by which viral infections contribute to increased BP or hypertension are not yet clearly defined. Cheng et al[9] investigated the role of CMV infection in causing elevated BP and the formation of aortic atherosclerotic plaques. Through viral replication kinetics and plaque formation assays, the study revealed that an active and persistent CMV infection in endothelial cells (EC) and the expression of viral genes may play a role in the underlying molecular mechanism. The findings indicate that CMV infection is a risk factor for heightened arterial BP and acts as a co-factor in the development of aortic atherosclerosis. The persistence of the viral infection in ECs may be a key aspect of this mechanism. Controlling CMV infection could be a potential strategy to limit the occurrence of hypertension and atherosclerosis in the cardiovascular system. These findings highlight the importance of developing interventions to manage CMV infection to mitigate the risks associated with elevated BP and atherosclerotic plaque formation.
In COVID-19, hypertension and cardiovascular diseases are significant factors contributing to the progression of severe illness. However, the precise causes and effects of the main anti-hypertensive therapies, such as ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), in the context of COVID-19 remain unclear. By combining clinical data from 144 patients and single-cell sequencing data of airway samples from 48 individuals, along with in vitro experiments, Trump et al[10] observed a distinct inflammatory predisposition of immune cells in hypertensive patients that correlated with the critical progression of COVID-19. ACEI treatment was associated with a reduction in COVID-19-related hyperinflammation and an increase in cell-intrinsic antiviral responses. On the other hand, ARB treatment was linked to enhanced interactions between epithelial cells and immune cells. Macrophages and neutrophils from hypertensive patients, particularly those under ARB treatment, exhibited higher expression levels of pro-inflammatory cytokines C-C motif chemokine ligand 3 (CCL3) and CCL4, as well as the chemokine receptor C-C chemokine receptor type 1. While the size of the cohort was limited, which prevented definitive clinical efficacy from being established, data suggest that further investigation is warranted to explore the potential clinical benefits of ACEI treatment in hypertensive patients with COVID-19.
Individuals with hypertension are at a higher risk of experiencing severe complications from COVID-19. The relationship between hypertension and COVID-19 involves endothelial dysfunction and dysregulation of the RAS. The entry of the SARS-CoV-2 virus into host cells is facilitated by ACE2, an enzyme that plays a crucial role in maintaining BP balance. As a result, alterations in the RAS may impact the development and progression of COVID-19. Initially, there were hypotheses suggesting that certain antihypertensive medications like ACEI and ARBs could worsen the disease. However, no clinical evidence has confirmed these hypotheses. Presently, no clinical data indicate that ACEIs or ARBs improve or worsen COVID-19 cases or act as a risk factor for COVID-19 infection. Similarly, there is no substantial evidence supporting the discontinuation of ACEI or ARBs or the use of alternative pharmacotherapy to manage hypertension in COVID-19 patients. Large-scale studies that account for potential biases and confounding factors were necessary to establish the relationship between preexisting hypertension and the severity of COVID-19 and to develop improved pharmacological management strategies for COVID-19 patients with hypertension[11].
The global spread of SARS-CoV-2 has emerged as a significant public health concern, posing a considerable threat to human well-being. Reports have indicated that individuals with hypertension who contract COVID-19 experience higher rates of illness severity and mortality. To contribute to the international community’s knowledge and comprehension of the relationship between COVID-19 and hypertension were aimed to provide a more comprehensive understanding of this important issue. In comparison to those in the normotensive group, the median ages of those with hypertension who had mild or severe COVID-19 instances were both noticeably higher. The distribution of gender between the normo
The presence of Firmicutes and Bacteroidetes, two types of gut microbes, has been linked to elevated BP in various hypertension models, such as spontaneously hypertensive rats and Dahl salt-sensitive rats. Modifying the gut microbiota through the use of antibiotics has shown contradictory effects on BP, which can be influenced by genetic factors. Probiotics' beneficial effects may also arise from epigenetic modifications, potentially involving microRNAs. The fermentation products produced by gut microbiota from ingested nutrients can impact BP regulation by influencing energy expenditure, the metabolism of catecholamines in the intestines, and ion transport in the gastrointestinal and renal systems, particularly related to salt sensitivity. The effects of gut microbiota on BP, whether beneficial or harmful, are influenced by multiple factors, including genetics, epigenetics, lifestyle choices, and antibiotic usage. These variables collectively contribute to the overall BP levels and the control of hypertension[13].
Indeed, Zika virus infection can affect individuals irrespective of their BP levels, including those with hypertension, hypotension, or normal BP. There was no scientific evidence suggesting that patients with pre-existing hypertension experience any particular additional issues when infected with the Zika virus. However, when looking at a similar infection, such as dengue fever, it has been observed that individuals with underlying hypertension have an increased risk of developing a more severe form of the illness, known as dengue hemorrhagic fever (DHF)[14]. Several reports have examined the BP levels of patients infected with the Zika virus. According to Wiwanitkit's[15] findings, there were no notable fluctuations in BP among individuals affected by the Zika virus.
Hypertension may elevate the susceptibility to various viral infections. However, the connection between hypertension and the reactivation of the Epstein-Barr virus (EBV) remains largely unsupported by substantial evidence. In regions with a high incidence of nasopharyngeal carcinoma (NPC), hypertension has been linked to a higher risk of EBV reactivation. Interestingly, the utilization of β-blockers and ACEIs has been shown to mitigate this risk, suggesting their potential use for preventing NPC in areas where it is endemic[16].
Hypertension poses a risk for organ damage and mortality, and its prevalence is higher among individuals with HIV compared to the general population. Researchers have explored various mechanisms involved in the development of hypertension. Existing evidence indicates that the epithelial sodium channel (ENaC) plays a crucial role in BP regulation by facilitating the movement of sodium and water across kidney tubule membranes, leading to sodium and water retention and an imbalance in fluid levels. However, limited information is available regarding the specific role of ENaC in HIV and its potential contribution to the increased risk or progression of hypertension[17].
Exposure to malaria during childhood may play a role in the development of high BP in adulthood. Etyang et al[18] utilized sickle cell trait (SCT) and α-thalassemia, genetic variations that provide partial protection against malaria, as tools. It found that SCT was associated with lower BP and a reduced prevalence of hypertension in Kilifi, Kenya, but not in Nairobi, Kenya, or Jackson, Mississippi. This observation suggests a potential causal link between malaria and elevated BP. The findings have important implications. First, they suggest that eliminating malaria could lead to broader health benefits beyond what is currently recognized. Second, understanding the mechanisms by which malaria contributes to increase BP could pave the way for new preventive strategies targeting hypertension and subsequent cardiovascular diseases.
The development and pathogenesis of hypertension involve a wide array of factors including genetics, environment, hormones, hemodynamics, and inflammation. Increasing evidence suggests that the gut microbiome plays a significant role in hypertension. The gastrointestinal tract, which contains a sizable number of immune cells, acts as a point of contact between the host and the outside world. The microbiome influences lifestyle variables and is changed by those same factors, changing the likelihood of developing hypertension. A good illustration of this is the ingestion of dietary fibers, which produces short-chain fatty acids and encourages the expansion of immune cells that fight inflammation, providing defense against the development of hypertension. Fasting is one dietary strategy that affects hypertension via the microbiota. Comparatively to conventional research paradigms, examining the involvement of the microbiome in hypertension presents special difficulties. Preclinical studies must take into account the microbiome, and cutting-edge methods, like the wildling mouse model, can open up new possibilities for translational research. Future research must take a number of technical factors into account because the microbiome's role in hypertension is complicated and under constant investigation. The interaction between the host and the microbiome, summarizing the evidence highlighting its importance in BP regulation. Furthermore, recommendations for ongoing and future research were aimed at integrating significant findings from the broader field of microbiome research into the context of hypertension[19].
Maruyama et al[20] identified specific gut bacteria associated with individuals who respond positively to barley consumption in terms of hypertension. A hypertensive non-responder was defined as someone who consumes barley but either has BP levels above a defined threshold or requires hypertension treatment. The ability to categorize individuals based on their BP response to barley intake provides novel information for addressing individual variations in the effectiveness of functional studies involving different foods. Notably, the study developed a machine-learning model to classify responders, enabling the assessment of compatibility between barley and an individual based on their gut bacteria. This research will offer valuable insights for designing personalized dietary interventions to prevent cardiovascular diseases in the future.
The monitoring of BP is an integral part of managing dengue illness. However, there is a lack of comprehensive studies examining the temporal trends of BP in adults with dengue. Yeung et al[21] investigate the differences in the time trends of systolic BP (SBP) and diastolic BP (DBP) among patients with severe dengue (SD), DHF, pre-existing hypertension, and between elderly and non-elderly patients. In cases of SD or DHF, SBP showed a decline, reaching its lowest point around the defervescence day (DD), and subsequently recovering to levels surpassing those observed during the febrile phase by day 2 or 3 post-defervescence. Elderly patients and those with pre-existing hypertension maintained higher levels of both SBP and DBP throughout the dengue infection. Also, the elevated prevalence of hypertension among dengue patients during their initial visit suggests that relying solely on BP measurements to predict severe infection may not be clinically reliable[22].
Dengue infection is a highly prevalent viral infection transmitted by mosquitoes worldwide. Coinciding with this, there has been an increase in the occurrence of non-communicable comorbidities. The link between these comorbidities and the development of SD was investigated. A noteworthy association between hypertension and the development of SD in adult patients were indicated. From a clinical perspective, this suggests that dengue patients with pre-existing hypertension should receive closer monitoring to detect any deterioration. A significant correlation between SD and various abnormal laboratory parameters was observed, which aligns with previous research. Although further validation through larger prospective studies is desirable, this association can potentially contribute to the dengue triaging process, providing valuable insights for healthcare practitioners[23].
The occurrence of dengue in elderly individuals is becoming more prevalent. Monitoring BP and pulse rate (PR) is crucial, yet there is a lack of large-scale studies examining the daily trends in dengue patients. Among 6070 confirmed dengue inpatients, 296 (4.87%) were classified as elderly, with 64.7% being male and 71.3% being of Chinese ethnicity. Among the elderly cohort, 8% had hypertension, 26.3% had DHF, and 0.31% required intensive care or resulted in death. The median duration of illness upon hospital admission was 5 d (ranging from the 5th to 95th quantile: 3 to 7). On admission, the mean SBP, DBP, pulse pressure (PP), and PR readings in the elderly cohort were higher compared to the controls, with values of 117 vs 106, 65 vs 62, 47 vs 40, and 86 vs 92, respectively. Throughout the illness, the elderly cohort consistently exhibited higher mean BP readings (SBP, DBP, and PP) compared to the controls. Both cohorts showed similar declining trends in BP readings during the febrile phase. However, during the critical phase, the controls exhibited a higher rate of decrease in BP readings. During the early recovery phase, both cohorts displayed similar increasing trends in BP readings until normalization. The lowest BP readings were observed on the DD, with comparable downward and upward trends occurring 3 d before and after DD. Regarding PP, although the elderly cohort demonstrated a relatively stable course, the controls exhibited a steep downward trend, resulting in a lower PP at normalization compared to the elderly cohort. The crossover of PP trends between the elderly and controls occurred 1 d after DD[24].
A growing body of research shows that the gut microbiota has a substantial impact on hypertension and its side effects, such as chronic kidney disease, stroke, heart failure, and myocardial infarction. This association makes sense given that the gut microbiota may be affected by a number of major risk factors for hypertension including age, sex, medication, and nutrition. For instance, the intake of sodium and fermentable fiber has been extensively studied concerning both hypertension and gut microbiota. The identification of certain metabolites, such as short-chain fatty acids and trimethylamine N-oxide, and the bacteria that produce them have been made possible by recent developments in sequencing technology, especially third-generation sequencing, in conjunction with metabolomics techniques. These metabolites have been found to have profound effects on host physiology and the cardiovascular system. Furthermore, it has been shown that receptors that bind these metabolites, including well-known short-chain fatty acid receptors such as G protein-coupled receptor 41 (GPR41), GPR43, GPR109a, and olfactory receptor 78 in mice, play crucial roles in controlling BP and heart function. Prebiotics, probiotics, and postbiotics, such as acetate, propionate, and butyrate, have been shown to reduce BP in animal models, making them promising therapy possibilities. However, the underlying mechanisms of these effects were not yet fully understood, and their translation to hypertensive patients is still in progress. The available data on the connection between hypertension, the gut microbiota, and issues related to the cardiorenal system were evaluated. In this dynamic and quickly expanding discipline, suggest potential future study areas as well[25].
Hypertension has been associated with significant disruptions in the gut microbiota, characterized by decreased diversity within individual samples and shifts in microbial composition. Through a metagenome-wide association study, Yan et al[26] 53953 microbial genes with a false discovery rate of 0.05 were discovered to have altered distributions in hypertension patients compared to healthy controls. Sixty-eight groups representing different bacterial species were possible for these genes to be grouped into. Opportunistic pathogenic taxa like Klebsiella spp., Streptococcus spp., and Parabacteroides merdae had a higher prevalence in the hypertensive gut microbiome, whereas Roseburia spp. and Faecalibacterium prausnitzii, which produce beneficial short-chain fatty acids, had higher concentrations in the healthy controls. The number of species associated with hypertension also demonstrated a stronger correlation with disease severity. Functionally, the hypertensive gut microbiome exhibited elevated activities related to membrane transport, lipopolysaccharide biosynthesis, and steroid degradation, whereas control samples showed higher metabolic functions associated with amino acid, cofactor, and vitamin metabolism. Furthermore, the potential of the gut microbiota in predicting hypertension is highlighted by the discovery that microbial indicators may distinguish between hypertensive and healthy patients, attaining a promising area under the receiver operator characteristic curve of 0.78. These results offer important new understandings of the unique modifications in the hypertensive gut microbiome's microbial diversity, genes, species, and functions. New approaches to the treatment and prevention of hypertension and the disorders it is connected with may be made possible by more study examining the causal association between gut microbiota and hypertension[26].
CMV belongs to the β-herpesviruses group and is widespread, infecting a significant percentage of the human population (ranging from 50% to 99%) depending on ethnic and socioeconomic factors. CMV establishes lifelong, dormant infections within its host. The reawakening of CMV typically occurs without noticeable symptoms, but in individuals with weakened immune systems, it can result in severe illness and even death. Additionally, herpesvirus infections, including CMV, have been linked to various cardiovascular and post-transplant ailments, such as stroke, atherosclerosis, post-transplant vasculopathy, and hypertension. Herpesviruses, CMV included, possess viral G-protein-coupled receptors (vGPCRs) that manipulate host cells by hijacking crucial signaling pathways vital for both the viral life cycle and the development of these cardiovascular disorders. The authors of the study explore the pharmacological and signaling characteristics of these vGPCRs and their role in causing hypertension. All in all, these vGPCRs show promise as potential targets in the ongoing development of innovative treatments for hypertension[27].
This review article concluded that microbes including bacteria, viruses and protozoa play a significant role in hypertension. However, there are other microorganisms which have not been reported in hypertension. Further studies are required to identify the exact mechanism of action of these microbes in hypertension. Medications used to treat microbial infections, such as certain antibiotics or antiviral drugs, might have side effects that can affect BP. It is important to consult with a healthcare professional to understand the potential impact of medications on BP management.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Webb RC, Brazil S-Editor: Lin C L-Editor: Filipodia P-Editor: Lin C
| 1. | Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2858] [Cited by in RCA: 3813] [Article Influence: 190.7] [Reference Citation Analysis (1)] |
| 2. | What You Need to Know About Infectious Disease. Washington (DC): National Academies Press (US); 2010– . [PubMed] |
| 3. | Desvarieux M, Demmer RT, Jacobs DR Jr, Rundek T, Boden-Albala B, Sacco RL, Papapanou PN. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. 2010;28:1413-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Petry CJ, Ong KK, Hughes IA, Acerini CL, Dunger DB. Associations between bacterial infections and blood pressure in pregnancy. Pregnancy Hypertens. 2017;10:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Todros T, Verdiglione P, Oggè G, Paladini D, Vergani P, Cardaropoli S. Low incidence of hypertensive disorders of pregnancy in women treated with spiramycin for toxoplasma infection. Br J Clin Pharmacol. 2006;61:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Cookson TA. Bacterial-Induced Blood Pressure Reduction: Mechanisms for the Treatment of Hypertension via the Gut. Front Cardiovasc Med. 2021;8:721393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Savedchuk S, Raslan R, Nystrom S, Sparks MA. Emerging Viral Infections and the Potential Impact on Hypertension, Cardiovascular Disease, and Kidney Disease. Circ Res. 2022;130:1618-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Yan LD, Matuja SS, Pain KJ, McNairy ML, Etyang AO, Peck RN. Emerging Viral Infections, Hypertension, and Cardiovascular Disease in Sub-Saharan Africa: A Narrative Review. Hypertension. 2022;79:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Cheng J, Ke Q, Jin Z, Wang H, Kocher O, Morgan JP, Zhang J, Crumpacker CS. Cytomegalovirus infection causes an increase of arterial blood pressure. PLoS Pathog. 2009;5:e1000427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Trump S, Lukassen S, Anker MS, Chua RL, Liebig J, Thürmann L, Corman VM, Binder M, Loske J, Klasa C, Krieger T, Hennig BP, Messingschlager M, Pott F, Kazmierski J, Twardziok S, Albrecht JP, Eils J, Hadzibegovic S, Lena A, Heidecker B, Bürgel T, Steinfeldt J, Goffinet C, Kurth F, Witzenrath M, Völker MT, Müller SD, Liebert UG, Ishaque N, Kaderali L, Sander LE, Drosten C, Laudi S, Eils R, Conrad C, Landmesser U, Lehmann I. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat Biotechnol. 2021;39:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 11. | Muhamad SA, Ugusman A, Kumar J, Skiba D, Hamid AA, Aminuddin A. COVID-19 and Hypertension: The What, the Why, and the How. Front Physiol. 2021;12:665064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Wang S, Zhang Q, Wang P, Ye H, Jing X, Zhang Z, Zhu S, Luo T, Zheng Z. Clinical features of hypertensive patients with COVID-19 compared with a normotensive group: Single-center experience in China. Open Med (Wars). 2021;16:367-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Baig Mirza AM, Fida M, Murtaza G, Niazi R, Hanif A, Irfan K, Masud F. Association of metabolic factors with dengue viral infection on admission triage which predict its clinical course during Lahore dengue epidemic. J Pak Med Assoc. 2016;66:1102-1106. [PubMed] |
| 15. | Wiwanitkit V. Heart rate and blood pressure in Zika virus infection: Findings? Indian Heart J. 2016;68:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Lin K, Zeng Z, Li X, Chen W, Lin D, Xie S, Wu Z, Li M, Cao S, Du J. Association between hypertension and Epstein-Barr virus reactivation among the population in a high-risk area for nasopharyngeal carcinoma. Virus Res. 2023;331:199117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Mutengo KH, Masenga SK, Mwesigwa N, Patel KP, Kirabo A. Hypertension and human immunodeficiency virus: A paradigm for epithelial sodium channels? Front Cardiovasc Med. 2022;9:968184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 18. | Etyang AO, Kapesa S, Odipo E, Bauni E, Kyobutungi C, Abdalla M, Muntner P, Musani SK, Macharia A, Williams TN, Cruickshank JK, Smeeth L, Scott JAG. Effect of Previous Exposure to Malaria on Blood Pressure in Kilifi, Kenya: A Mendelian Randomization Study. J Am Heart Assoc. 2019;8:e011771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, Rosshart SP, Forslund SK, Müller DN. The Gut Microbiome in Hypertension: Recent Advances and Future Perspectives. Circ Res. 2021;128:934-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 20. | Maruyama S, Matsuoka T, Hosomi K, Park J, Nishimura M, Murakami H, Konishi K, Miyachi M, Kawashima H, Mizuguchi K, Kobayashi T, Ooka T, Yamagata Z, Kunisawa J. Characteristic Gut Bacteria in High Barley Consuming Japanese Individuals without Hypertension. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Yeung W, Lye DCB, Thein TL, Chen Y, Leo YS. Blood pressure trend in hospitalized adult dengue patients. PLoS One. 2020;15:e0235166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Wiwanitkit V. LBPS 02-57 Hypertension in Dengue Patients Important Concern. J Hypertens. 2016;34:e521. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Ng WY, Atan R, Mohd Yunos N, Bin Md Kamal AH, Roslan MH, Quah KY, Teh KX, Zaid M, Kassim M, Mariapun J, Ngim CF, Dhanoa A, Yeo TW. A double whammy: The association between comorbidities and severe dengue among adult patients-A matched case-control study. PLoS One. 2022;17:e0273071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Ng EL, Thein TL, Hao Y, Lee L, Lye D, Leo YS. Vital signs in elderly dengue patients: Trends of blood pressure and pulse rate. In J Infect Dis. 21 Suppl 1:447. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Muralitharan RR, Jama HA, Xie L, Peh A, Snelson M, Marques FZ. Microbial Peer Pressure: The Role of the Gut Microbiota in Hypertension and Its Complications. Hypertension. 2020;76:1674-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, Fang Z, Zhou J, Guan X, Ding Y, Wang S, Khan M, Xin Y, Li S, Ma Y. Alterations of the Gut Microbiome in Hypertension. Front Cell Infect Microbiol. 2017;7:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 27. | Bomfim GF, Priviero F, Poole E, Tostes RC, Sinclair JH, Stamou D, Uline MJ, Wills MR, Webb RC. Cytomegalovirus and Cardiovascular Disease: A Hypothetical Role for Viral G-Protein-Coupled Receptors in Hypertension. Am J Hypertens. 2023;36:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |