Copyright
©The Author(s) 2015.
World J Exp Med. Aug 20, 2015; 5(3): 164-181
Published online Aug 20, 2015. doi: 10.5493/wjem.v5.i3.164
Published online Aug 20, 2015. doi: 10.5493/wjem.v5.i3.164
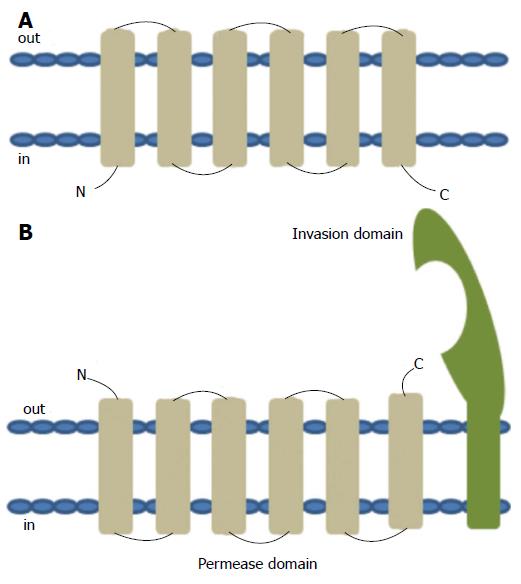
Figure 6 Topology of typical ATP binding cassette transmembrane permease (A) and the predicted topology of YRBE permease and MCE invasion domain (B).
ATP binding cassette (ABC) transport systems in both prokaryotes and eukaryotes consists of four domains, two cytoplasmic ABC domains and two hydrophobic membrane spanning permease domains (A). Each of the membrane spanning domains typically contains 6 hydrophobic transmembrane segments anchored in the cell membrane with the C- and N-termini located towards the cytoplasmic side. ABC transporters in prokaryotes that function as importers also typically require additional extracytoplasmic helper proteins called ‘substrate binding proteins’ for function. Each of the mce operons contains two YRBE domains that have structural homology to the permease domain of the ABC transporters (B). Each YRBE permease contains five or six transmembrane segments with the C-terminal end located on the extracytoplasmic side and the N-terminal end being either cytoplasmic or extracellular. The MCE protein, MCE4A, consists of a patch of 400 amino acid residues, with a hydrophobic stretch anchored in the cell membrane and a 22-amino acid invasion domain exposed outside to the membrane, which is predicted to be structurally similar to the substrate binding domain of ABC importers.
- Citation: George R, Cavalcante R, Jr CC, Marques E, Waugh JB, Unlap MT. Use of siRNA molecular beacons to detect and attenuate mycobacterial infection in macrophages. World J Exp Med 2015; 5(3): 164-181
- URL: https://www.wjgnet.com/2220-315X/full/v5/i3/164.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i3.164