Copyright
©The Author(s) 2015.
World J Exp Med. Aug 20, 2015; 5(3): 164-181
Published online Aug 20, 2015. doi: 10.5493/wjem.v5.i3.164
Published online Aug 20, 2015. doi: 10.5493/wjem.v5.i3.164
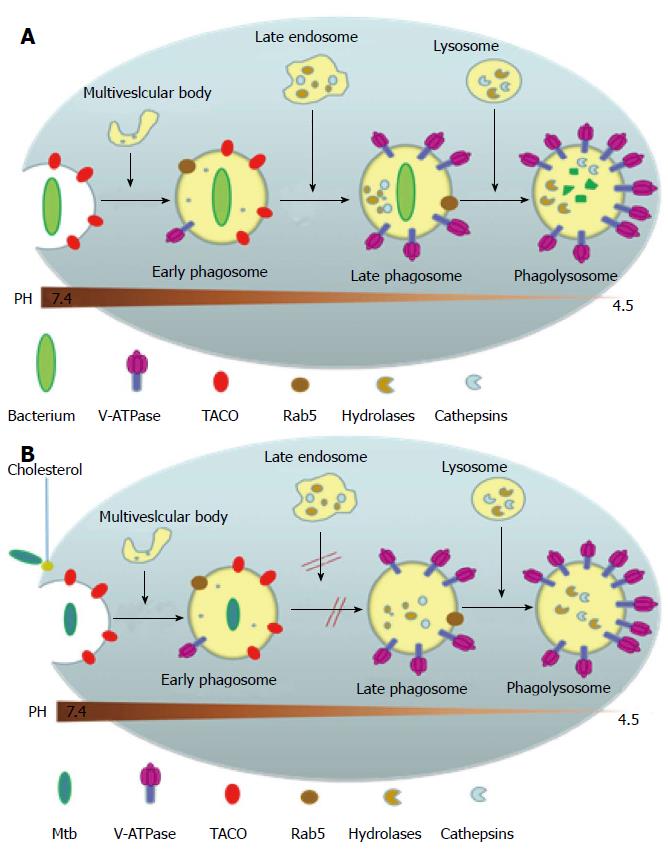
Figure 4 Phagosomal maturation or arrest following pathogen uptake (A) or Mycobacterium tuberculosis uptake (B), respectively.
(A) shows that upon entering into the body, most non-pathogenic microbes are phagocytized by the macrophage into a phagosome which then goes on to mature by fusing with the vesicles of the endocytic pathway and to finally fuse with lysosomes. These phagosomes undergo acidification due to the presence of proton-ATPase molecules from vacuolar membranes and the lysosomes, and this increased level of acidification activates the lysosomally derived acid hydrolases, cathepsins and other enzymes, along with reactive oxygen and nitrogen intermediates, to destroy the pathogen. Phagocytosis also initially triggers the recruitment of TACO around the particle to be ingested, as a result of the latter’s initial association with cell cortex microtubules, but is released prior to the lysosomal delivery of the bacteria; (B) Shows the effect of M. tuberculosis on phagosome maturation. Cholesterol serves as a docking site for the mycobacteria and its cell surface receptor there by facilitating its phagocytosis at cholesterol-rich regions. Cholesterol plays a crucial role in not only the entry of mycobacteria into macrophages but also mediates the phagososomal association of TACO (Coronin 1), a coat protein associated with cholesterol-rich regions which is actively retained on the phagosomal membrane housing the mycobacteria through a yet unknown mechanism, which prevents the degradation of the mycobacteria in the lysosomes. TACO: Tryptophan-aspartate containing coat protein.
- Citation: George R, Cavalcante R, Jr CC, Marques E, Waugh JB, Unlap MT. Use of siRNA molecular beacons to detect and attenuate mycobacterial infection in macrophages. World J Exp Med 2015; 5(3): 164-181
- URL: https://www.wjgnet.com/2220-315X/full/v5/i3/164.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i3.164