Copyright
©The Author(s) 2015.
World J Exp Med. May 20, 2015; 5(2): 84-102
Published online May 20, 2015. doi: 10.5493/wjem.v5.i2.84
Published online May 20, 2015. doi: 10.5493/wjem.v5.i2.84
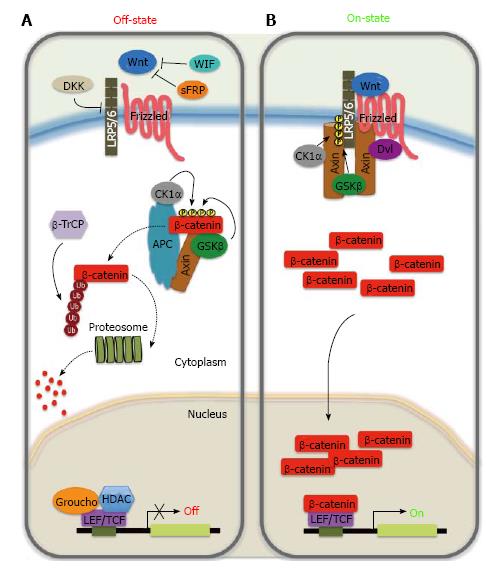
Figure 1 Wnt/β-catenin signalling pathway.
A: In the absence of Wnt ligands, cytoplasmic β-catenin binds to the ‘‘destruction’’ complex composed by Axin, adenomatous polyposis coli (APC), the glycogen synthase kinase 3β (GSK3β) and casein kinase 1α (CK1α). β-catenin is phosphorylated by the kinases of the complex on a set of conserved Ser and Thr residues in its amino terminus. The phosphorylated form of β-catenin is recognized by an E3 ubiquitin ligase (β-TrCP) and then targeted to proteosomal degradation, resulting in low cytosolic levels. In the absence of nuclear β-catenin, T cell-specific factors (TCF)/lymphoid enhancer-binding factor (LEF) proteins repress target genes expression through a direct association with transcriptional inhibitors of the Groucho family and histone deacetylases (HDACs); B: In the presence of Wnt ligand, secreted Wnt binds to their receptor complex, consisting of Frizzled and one member of the low-density lipoprotein receptor family (LRP5/6). This binding disrupts the ‘‘destruction complex’’ by recruiting Dishevelled (Dvl) to the cytoplasmic domain of Frizzled, which induces the delocalization of cytoplasmic Axin to the cytoplasmic tail of LRP and phosphorylation by CK1α and GSK3β of the latter. The disruption of the ‘‘destruction’’ complex prevents phosphorylation/degradation of β-catenin, leading to accumulation of β-catenin in the cytoplasm. Active ß-catenin translocates to the nucleus, where it acts as a transcriptional co-activator with TCFLEF to activate Wnt-responsive target genes. Extracellular Wnt signaling can be inhibited by binding of members of the secreted frizzled related protein (sFRP) and Wnt inhibitory factor (WIF) families to Wnt ligands, or by the interaction of soluble Dickkopf (DKK) with LRP.
- Citation: Chiurillo MA. Role of the Wnt/β-catenin pathway in gastric cancer: An in-depth literature review. World J Exp Med 2015; 5(2): 84-102
- URL: https://www.wjgnet.com/2220-315X/full/v5/i2/84.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i2.84