Published online Nov 12, 2014. doi: 10.5318/wjo.v4.i4.92
Revised: August 26, 2014
Accepted: September 16, 2014
Published online: November 12, 2014
Processing time: 167 Days and 17.2 Hours
Retinal vein occlusion (RVO) is the second vascular retinal cause of visual loss and defined by the occlusion of a retinal vein. It is divided into branch retinal vein occlusion or central retinal vein occlusion, depending on the location of occlusion. RVO has severe medical, financial and social implications on the patients. The diagnosis of the disease is easier nowadays with the use of spectral domain optical coherence tomography and fluorescein angiography. The treatment options for RVO have changed dramatically over the past few years with the introduction of the intravitreal injections of dexamethasone (Ozurdex), bevacizumab (Avastin), ranibizumab (Lucentis) and aflibercept (EYLEA), along with the panretinal laser photocoagulation, abandoning former treatment modalities and surgical solution. This manuscript is a review of current literature about RVO with emphasize on the pathophysiology, risk factors and prevention, diagnosis and sub-group categorization and treatments including medical and surgical. Since no official guidelines are available for the treatment of RVO patients, and considering the latest developments in the treatment options, and the variety of follow-up and treatment modalities, this manuscript aims to provide tools and knowledge to guide the physician in treating RVO patients, based on the latest publications from the literature and on several of the patients characteristics.
Core tip: Retinal vein occlusion (RVO) is the second vascular retinal cause of visual loss and is defined by the occlusion of a retinal vein. The diagnosis of the disease is easier with the common use of spectral domain optical coherence tomography and fluorescein angiography. The treatment options for RVO, has changed over the past years with the introduction of the intravitreal injections of dexamethasone (Ozurdex), bevacizumab (Avastin), ranibizumab (Lucentis) and aflibercept (EYLEA). This manuscript is a review of current literature about RVO and provides tools and knowledge to guide the physician in treating patients.
- Citation: Keren S, Loewenstein A, Coscas G. Pathogenesis, prevention, diagnosis and management of retinal vein occlusion. World J Ophthalmol 2014; 4(4): 92-112
- URL: https://www.wjgnet.com/2218-6239/full/v4/i4/92.htm
- DOI: https://dx.doi.org/10.5318/wjo.v4.i4.92
Retinal vein occlusion (RVO) is a retinal vascular disorder. Its main characteristic is the (partial) occlusion of the central retinal vein (CRVO) or of a branch retinal vein (BRVO) followed by the associated veins becoming engorged and dilated, intraretinal haemorrhages and edema in the retina and mainly the macula. In some cases retinal ischemia is seen which consists of areas of non-perfusion of retinal capillary bed, more or less extensive, in the periphery or in the macular area. The ischemia is associated with deep large hemorrhages and sometimes cotton wool spots (CWS)[1-7].
RVO is considered the second vascular retinal cause of visual loss, after diabetic retinopathy, and is responsible for up to 12% of severe visual loss[8-11]. RVO occurs most commonly in middle-aged and elderly individuals of age 50 and more. The incidence of RVO is 0.7% of the population between ages of 49 and 60, and rises to 4.6% above the age of 80, with about 15%-20% of patients having CRVO and the rest BRVO[12,13]. Hemiretinal vein occlusion involves the blockage of one of the two central retinal vein trunks, an extraordinary anatomical change found in up to 20% of the population, making it a less common RVO[14].
The pathogenesis of RVO is believed to be a compression, externally, on the wall of the retinal vein in the lamina cribrosa (CRVO) or at an arterio-venous crossing (BRVO) by the adjacent artery[15]. The type of RVO and clinical picture is the result of the location of interruption. In CRVO the whole venous system, in all 4 quadrants, is involved and characterized by optic disk edema, retinal veins in all 4 quadrants become dilated and torturous, CWS, and large areas of capillary nonperfusion. Haemorrhages are a significant clinical finding in CRVO, and are found in all four quadrants. CRVO can be further divided clinically into perfused (non-ischemic) or non-perfused (ischemic). The haemorrhages in CRVO can be divided into deep retinal haemorrhages in ischemic CRVO, and superficial dot and flame-shaped haemorrhages in non-ischemic CRVO.
BRVO consists of the same clinical findings in one major retinal vein and its quadrant.
Another subtle, less frequent finding is a macular venule occlusion, which does not involve any major arcade but only a small branch draining the macula, and is frequently missed[16].
The pathogenesis of RVO is not fully understood and it appears to be multifactorial and different for BRVO and CRVO. Both types however share an arterial disease as part of the etiology as part of a systemic cardiovascular risk profile[14].
BRVO occurs at a retinal arteriovenous crossing, where both artery and vein share a common adventitia[8]. The compression of the artery on the vein results in the formation of a turbulent flow which can be demonstrated by fluorescein angiography and can lead to thrombus formation[9].
CRVO is the result of arterial compression on the vein in the lamina cribrosa where both vessels share a common fibrous sleeve. The central retinal vein usually tends to narrow in aging eyes of otherwise healthy individuals in that location, and causing a disturbance to the normal laminar flow. This disturbance increases the chance of turbulent flow and thrombus formation[17].
Several factors such as blood dyscrasia, degenerative or inflammatory disease, hypotension and obstructive sleep apnea, were suggested to take part in the pathogenesis[17-20]. In young patients under the age of 50, a complete work up is warranted to find the cause for RVO.
Macular edema (ME) is the main complication in RVO patients and is a result of an increase in retinal and macular capillary permeability and leakage leading to hypoxic environment in the retina and changes, resulting in expression of many mediators of inflammation and later to BRB break down[21,22].
Inflammation plays a major role in macular edema development with many mediators having a role including: cytokines, interleukins, chemokines, angiotensin 2, vascular endothelial growth factor (VEGF), prostaglandins, P and E-selectins, vascular cell adhesion molecule 1(VCAM-1), ICAM-1 and particularly activation of resident cells like microglia, macrophages and neutrophils[22]. Macular edema consists of accumulation of fluid with initial swelling of the Muller cells and intra retinal fluid accumulation in the outer plexiform and inner nuclear layers.
VEGF A (VEGF-A) is a very important regulator in angiogenesis and vascular permeability and has been shown to have a key part in the pathogenesis of neovascularisation (NV) and macular edema in RVO. VEGF-A is required, along with other mediators, for blood vessel growth in pathological angiogenesis[10].
Several risk factors have been implicated as having a role in RVO.
Glaucoma: Open angle glaucoma is an ocular risk factor which is most commonly connected to RVO patients and plays a role in RVO pathogenesis due to compromised venous flow and stasis induction in the face of high intraocular pressures (IOP)[23-27]. This process usually occurs in the lamina cribrosa and leads to CRVO formation and increase of severity[28]. History of glaucoma may be found in up to 4.5% of CRVO patients[29]. IOP lowering medications may improve perfusion in patients with CRVO and is considered as preventive treatment in the fellow eye, which has a 10% risk of developing RVO as reported in the Branch Vein Occlusion Study (BVOS)[14,30,31].
Hypertension, diabetes mellitus and cardiovascular disease: These conditions are found in over 64% of RVO patients over 50 years old. They tend to appear more in BRVO patients than in CRVO ones[23]. Hypertension is a significant risk factor and accelerates arterial stiffness[32]. In diabetic patients, the prevalence of CRVO is equal to that of the general population, but following CRVO, diabetic patients have more disc neovascularization and are more likely to require panretinal photocoagulation (PRP) laser treatment[33].
Hyperlipidemia and hypercholesterolemia: Hyperlipidemia and hypercholesterolemia are found in over 70% of RVO patients and more prominent in RVO patients under the age of 50[24,34,35].
Obesity and smoking: These two risk factors are associated with RVO but in a lesser degree than the prior risk factors[34,36].
Thrombophilia: The findings of high levels of homocysteine in RVO patients led to the idea that thrombophilia has a role in the pathogenesis[8,12]. This role is particularly interesting in young RVO patients, in whom the pathogenesis of the disease may differ from patients with atherosclerosis, usually older[37]. A big meta-analysis of more than 500000 patient’s files indicated a RR of nearly 2.5 times for CRVO in the presence of a hypercoagulable state including homocysteinemia[32].
Two other meta-analyses showed an increased risk for RVO by 50%-60% in patients carrying the factor V Laiden mutation. Other disorders such as disturbances in antithrombin, protein C or S or the G21201a mutation were not found to be in association with RVO[15].
The relation between other conditions such as lupus anticoagulant or anticardiolipin antibodies and RVO is still not clear[8,9,38-47]. Changes in platelets reactivity may be a predisposing factor.
In a recent study on the levels of intravitreal thrombin in RVO patients compared to control eyes, a significant elevated thrombin activity and VEGF levels were found in RVO patients compared to control eyes. Higher levels were found in CRVO patients compared to BRVO ones. This led Bertelman , 2014, to the conclusion that thrombin plays a role in RVO and direct treatment should be evaluated[48].
Inflammatory disease: Inflammatory diseases can cause retinal vasculitis or inflammation and may be associated with a nearby RVO. These diseases mostly affect younger individuals, under the age of 50, and include infectious diseases such as toxoplasmosis, syphilis and tuberculosis, systemic inflammatory diseases such as sarcoidosis, Behcet’s disease and systemic lupus erythematosus, and vascular diseases such as polyarteritis nodosa, Wegner’s granulomatosis and Goodpasture’s syndrome[49]. Therefore in patients under the age of 50, a systemic investigation is warranted for these conditions, whereas is patients over 50 years arteriosclerosis is the main cause[49].
Other risk factors: Oral contraceptives and optic disc vasculitis are a debatable risk factors and evidence is available for both sides[49-53]. The risk for RVO is reduced by 70% in women in the post-menopausal period when treated with estrogen replacing therapy, consistent with reduced cardiovascular risk profile associated with this treatment[54].
Association to obstructive sleep apnea was also reported[55] and it may double the incidence of RVO[56]. Myeloprolipherative disorders are found in 1% of RVO patients[49,57]. No relation to gender was found in RVO[14], but ethnicity plays a role with a prevalence of 3.7 per 1000 population in whites, 3.9 in blacks, 5.7 in Asians and 6.9 in Hispanics[32,58].
Visual acuity: In BRVO patients the initial VA is generally found to be worse than 20/40 and although it tends to improve, a final VA better than 20/40 is seldom seen[59,60]. In most patients the improvement in VA was found to be up to 28 letters[59].
In the BVOS[30,31] a significant deterioration of vision was found in 20% of untreated eyes, and in 25% of cases final VA was worse than 20/200.
NV: The incidence is believed to be relatively low but there is no meaningful data on BRVO in relation to NV and neovascular glaucoma (NVG)[59].
It is believed that with severe and extensive area of ischemia of over one-third of the retina, there is a higher incidence of NV[15].
Macular edema: Macular edema in BRVO patients develops in 5%-15% of eyes in 12 mo[59]. The GENEVA clinical trial showed an improvement in both treatment and sham groups, although the treatment group had a bigger decrease in central retinal thickness of 208 μm compared to only 85 μm in the sham group. In a sub-analysis, eyes with a shorter duration of ME had a better VA outcome after treatment[61].
Fellow-eye involvement: The BVOS reported bilateral involvement in 9%[30,31]. In several other studies, bilateral involvement was reported in 4.5%-6.5% of patients at baseline[2,38,62]. Some publications indicated a similar 5%-10% bilateral involvement[15].
Two objectives are to be simultaneously managed by the physician in RVO patients: (1) identification and management of the risk factors leading to RVO; and (2) the diagnosis and treatment of sight-threatening complication associated with the disease, mainly macular edema and neovascularisation.
Risk factors management: The first goal in the management of RVO is the prevention of the disease and its complications by reducing and controlling systemic risk factors. Those risk factors mentioned above are to be treated and monitored closely. Management of these factors may diminish the severity of the disease and risk of complications including fellow eye involvement. (1) systemic risk factor management: When findings of RVO are clinically present [(engorgement and dilatation of retinal veins, hemorrhages and increased retinal circulation time on fluorescein angiography (FA)] in asymptomatic patients, initiation of treatment for systemic medical risk factors, may slow or even prevent the disease progression; (2) many studies including the CVOS have shown an association between arterial hypertension or glaucoma and RVO. The physician should exclude those conditions, or if present, treat them. All though prompt treatment is recommended in these cases, no clear evidence was found regarding the benefits of the management of glaucoma and/or reduction of arterial hypertension in regard to the visual outcome in RVO patients[14]; (3) anticoagulants, antiplatelet medications and fibrinolytic medications: Though the use of such medication can help resolve RVO or lower complication rate, several studies using those drugs (Aspirin, Heparin, Streptokinase and Warfarin) showed little to no benefit, and in patients over 55 years, a greater tendency towards vascular adverse effects[63,64]. The use of Aspirin in the management of RVO is controversial and could only be suggested, yet no proven, in the prevention of cardio vascular events[15]; and (4) hemodilution: Hemodilution was suggested by several studies as a therapy in RVO. The rationale is to lower the blood viscosity thus preventing the slowdown of blood circulation and its developing complications. The studies showing benefits of Hemodilution were mono-center studies and conducted in the 1980s and 1990s but showed significant results.
A more recent multicenter, prospective study using the recent method of hemodilution, showed some limited but positive results, and recommended the use of hemodilution in the early stage of RVO. This was shown in cases when there were no contraindications such as ischemic CRVO requiring panretinal laser photocoagulation (PRP), cardiovascular conditions such as diabetes mellitus, uncontrolled hypertension and severe cardiac or renal failure, or haematological disease such as anemia or sickle cell disease[65].
The ophthalmological RVO management: The systemic investigation and treatments as mentioned above are identical for all types of RVO. Several types of management are available today for treating patients with RVO: The dexamethasone intravitreal implant (Ozurdex) that is based on the GENEVA trial, anti-VEGF treatments as with Ranibizumab, based on the Bravo and Cruise trials.
Management of BRVO is not that different from the management of CRVO regarding the systemic cardio-vascular risk factors, but the differences are that in BRVO there is a limited risk of progression, conversion to ischemic type and neovascularization.
Several targets should be held by the physician in the management of a BRVO patient: (1) systemic risk factors management; (2) localization of the area of lesion (major or minor branch); (3) assessment of the degree of nonperfusion and ischemia of the macula; and (4) treatment according to eventual complications, mainly ME and NV.
Patients with BRVO, who has a good baseline vision acuity of 20/40 or better with perfused periphery, have a favourable prognosis yet monitoring should be maintained even without intervention. The follow-up should consist of a VA examination, biomicroscopy and optical coherence tomography (OCT) in order to detect the development of ME. If necessary or when in doubt, FA should be obtained.
The follow-up should begin with monthly visits for the first 3 mo, followed by a visit every other month for a year. Patients should be instructed to seek medical assistance if they notice a VA decline which may be an early sign of ME formation.
In patients with BRVO and a deterioration of vision, physician should initiate an assessment for the presence of ME. This assessment should be done with biomicroscopy and OCT. Treatment should be initiated promptly in cases of ME.
The BVOS, a prospective, randomized, controlled clinical trial on BRVO patients, set the criteria for the use of laser photocoagulation in BRVO in order to “stabilize VA”, and included patients with VA of 20/40 or less, who had ME of 4 mo or more, and absorption of macular haemorrhages[30,31].
The SCORE study, a prospective double-masked, randomized trial, concluded that grid laser photocoagulation should be used in eyes with vision deterioration due to ME secondary to BRVO[66,67]. No difference in 12 mo for VA outcome between the laser treated group and the triamcinolone treated groups (4.2 letters compared to 5.7 and 4.0 letters respectively) was seen[10]. The proportion of patients with a ≥ 15 letters VA improvement was 28.9%, 25.6% and 27.2% in the standard care group and both treatment groups with a non significant difference[10]. To add was the fact that the 4 mg triamcinolone treated group had a worse safety profile (cataract and elevated IOP).
The use of paracentral laser coagulation may lead to paracentral scotomas which can cause visual field defect which may decrease the quality of vision. The central vision field was not tested in the SCORE BRVO study, thus the conclusions are still controversial[66,67]. The new navigated pattern laser (NAVILAS) and patterned scanning laser (PASCAL) systems allow for a more accurate and effective laser treatment with less pain and treatment time[68,69].
Nowadays it is custom by practitioners to use sectorial laser photocoagulation in cases of an extensive area of nonperfusion in the peripheral retina with the development of neovascularization.
Triamcinolone Acetonide is a known treatment, from several studies on RVO, and was shown to decrease edema and angiogenesis. The visual improvement is transient because of its limited duration of intraocular availability.
The SCORE clinical trial compared the efficacy and safety of intravitreal triamcinolone in two doses, 1 mg and 4 mg, to the standard of care (grid laser photocoagulation). The drug used in the SCORE trial was Trivaris which is a sterile preservative free, intravitreal injection. In the trial no difference was seen regarding VA after 12 mo between the standard care group and the treatment groups with gaining 15 letters or more in 28.9% of the standard care group compared to 25.6% and 27.2% in the treatment groups. However, more IOP elevations and higher percentage of cataract were found in the 4 mg treatment group with 35% and 33% respectively compared to 8% and 18% in the standard care group[66,67].
The SCORE study suggested that grid laser photocoagulation should still act as the standard care for BRVO patients with VA deterioration due to ME.
Since the duration of ME is of great significance, in a subgroup analysis of the SCORE BRVO trial, it was shown that patients had greater benefit with classical treatment if disease duration was < 3 mo.
Of the patients with ME over 3 mo, a third showed a 15 letter or more gain in VA in the 4 mg treatment group compared to only 15% in the laser treatment group. These findings were not found to be statistically significant but indicated the importance of duration of ME in choosing treatment.
Dexamethasone is a corticosteroid that decreases inflammatory mediators which cause ME. Dexamethasone has a short half-life and is highly soluble therefore an intravitreal implant of dexamethasone (Ozurdex) was developed so it can deliver a sustained level of the drug during up to 6 mo. This drug was studied in the Ozurdex GENEVA study, a multicenter, masked, randomized, sham-controlled, clinical trial of RVO patients with ME[61]. A prefilled single use applicator, containing 0.7 mg of dexamethasone in a sustained-release biodegradable implant (Ozurdex) was used.
Patients in this trial were treated with a first masked treatment at baseline and another treatment in as needed after 180 d. In this prospective, multicenter study, two randomized, parallel groups of the same number of patients showed a statistically significant effect on VA which persisted up to 180 d and was maximal after 60 d. The second OZURDEX injection showed a better effectiveness than the first one. Adverse effects were low rates of cataract formation and elevations in IOP. No injection related adverse effects were noted.
The primary endpoint of the GENEVA study was set to be the time to achieve an improvement in best corrected VA (BCVA) of ≥ 15 letters, and secondary endpoints were BCVA over 180 d, and central retinal thickness as measured by OCT.
Duration of ME was similar in both study groups with 16.4% of patients with ME duration of under 3 mo, 51.3% with a duration of 3-6 mo and 32.3% with a duration of over 6 mo. An important fact to notice in comparison between trials related to RVO is that the proportion of patients with ME under 3 mo duration was 16.4%, in comparison with 50%-60% in the SCORE BRVO[67], the BRAVO[70] and the CRUISE[71] trials. This fact is thought to have affected the results and made it difficult to compare trials, since resolution is thought to be higher in patients with ME of shorter duration.
A significant larger percentage of patients achieving a VA improvement of ≥ 15 letters was seen in the 0.7 mg treatment group after 30 d and throughout day 90 rather than the sham group. The best response was at day 60 with 29.6% of the 0.7 mg treatment group achieving the desired improvement and only 12.5% in the sham treatment group. In achieving at least 10 letters improvement in VA from baseline the rates were 52% for the treatment group vs 29.4% in the sham group. By day 180, 41% of patients in the treatment group had an improvement of at least 10 letters from baseline compared to 33% in the sham treated group[61].
The differences in BCVA between the 0.7 mg treatment group and the sham group were significant for all time points throughout the study for patients with BRVO. Mean BCVA in the treatment group improved by 10 letters at 60 d, and then declined towards 180 d with only 7 letters improvement. In the sham treatment group mean VA improved by 5 letters by 60 d and did not change up to 180 d. Patients receiving sham at baseline demonstrated a lower improvement in VA even after receiving the open-label injection of dexamethasone than patients who were treated with the drug from the beginning.
At day 60 the percentage of patients in the treatment group which had an increased IOP peaked, was 2%-3% with an IOP over 35 mmHg, 15% with over 25 mmHg, and 15% with a rise of 10 mmHg and more. Patients returned to normal by day 180. After 12 mo of study and two injections only about 1% had a pressure lowering procedure, and only 0.9% had cataract surgery. All patients with adverse effects were in the 0.7 mg/0.7 mg treatment group.
A recent post-hoc study on the GENEVA results[61] showed that treatment of ME associated with BRVO of short duration is more effective than delaying treatment. The percentage of reduced odds of gaining 15 letters with treatment at day 180 was 54% in patients with ME duration of 6 mo, 32% for ME duration was 3 mo and only 12% for the duration of 1 mo.
Retreatment with Ozurdex was studied in several studies with special emphasize on the time frame between injections. In a multi-center retrospective study[72] of 128 patients, 70 of them (54.7%) with BRVO, the mean time between dexamethasone injections was 5.9 mo after the first injection and 8.7 mo after the second injection. A ≥ 15 letter gain was seen in 28% of eyes with a central macular thickness reduction of 214 μm. Some of the patients had decreases VA before 6 mo which leads to the conclusion that patients should be monitored for the chance of deterioration before the 6-mo period.
The SHASTA study[73], a retrospective study showed similar results with a mean reinjection interval of 5.6 mo and a significant improvement both in VA and central retinal thickness (CRT). Retreatment was also studied in several other studies with a mean time between injections of 4.7-5.3 mo, all with a favourable VA outcome[74-76].
The 0.7 mg dexamethasone implant (Ozurdex) is United States food and drug administration (FDA) and European Union (EU) approved for the treatment of BRVO patients with ME.
Ranibizumab is a pan-VEGF blocker (Lucentis) which its efficacy and safety were studied in the BRAVO trial. The trial was a multicenter, randomized, double-blinded, sham controlled, phase III study on patients with ME secondary to BRVO.
The 3 parallel groups of the trial were: standard treatment group with grid laser, 0.5 mg Ranibizumab, and a combination of grid laser and 0.3 mg or 0.5 mg Ranibizumab. According to the study’s design, patients were injected on a monthly basis for the first 6 mo, followed by another 6 mo with treatment when needed. At 6 mo the treatment groups showed a better visual acuity recovery than the control group with an 18.3 letters gained in the 0.5 mg treatment group and 16.6 letters min the 0.3 mg group, compared to 7.3 letters in the sham group. This was achieved with an average of 5.7 injections. The proportion of patients achieving a ≥ 15 letters VA gain was 61.1% in the 0.5 mg group and 55% in the 0.3 mg group compared to 28.8% in the sham group[10].
In the second 6 mo period, patients were treated pro re nata (PRN), and visual improvement was maintained with an addition of only 2.7 injections. Though the change in VA was the largest in the treatment groups, the patients in the sham group also gained in VA. The BRAVO trial had ME of a short duration in about half of the patients (51.5%-53.8%)[70].
The recent RETAIN study followed the BRAVO patients in an open-label, single arm, multicenter long-term extension trial[77]. In a mean follow-up of 49.0 mo, 50% had edema resolution (no intraretinal fluid for 6 mo or more after last injection), with 76% receiving their last injection within 2 years of the first one. Final VA of 20/40 or better was seen in 80%. The mean central foveal thickness (CFT) remained under 200 μm with 88.5% with a CFT under 250 μm.
Ranibizumab 0.5 mg (Lucentis) is approved by the FDA and EU for the treatment of BRVO patients with ME.
A study conducted on Bevacizumab, in an off-label fashion off for the treatment of exudative age-related macular degeneration[78], made it commonly used medication for patients with RVO[79-85].
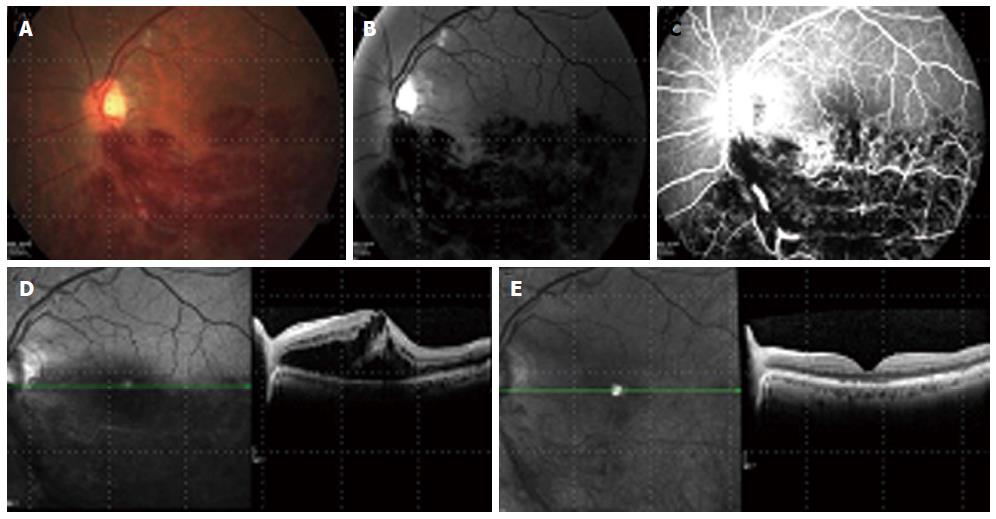
In a large open-label, single arm trial on the 2-year outcomes of Bevacizumab for the treatment of ME in eyes with BRVO, an improvement in VA of 0.31 logMAR was seen, with a decrease in foveal thickness of 361 μm. The response to Bevacizumab was fast and lasted throughout the year with a mean of 3.8 injections[86] (Figure 1).
No data is available on the use of pegaptanib in BRVO.
In patients with peripheral nonperfusion, assessment of the perfusion status of the macula must be done. If macula is well perfused, carrying out treatment should be as above mentioned, with grid laser photocoagulations for areas with extensive nonperfusion[60].
Even if macula is not perfused, still the treatment should be carried out in the same way, but the physician should inform the patient on the poor prognosis as related to VA.
In cases of BRVO with peripheral NV, a combined treatment of intravitreal therapy along with grid laser photocoagulation to the area of occluded vein should be promptly initiated[31].
The randomized controlled studies on RVO have provided much information regarding treatment. An important lesson from those studies is that the duration of the disease before initiation of treatment is an important factor influencing outcome. Treatment is beneficial in any stage of the disease, including in the late stages. It has been shown that in patients with a shorter duration of the disease, the rapid initiation of treatment may be more beneficial and the outcome is better.
Patients with BRVO should be primary screened for systemic and ocular risk factors and in case of any found, the family physician should be notified regarding the disease. The management and control of these risk factors should be fast and aggressive. Patients should be evaluated by vision assessment, biomicroscopy, measurements of IOP, and OCT. Examining the patient with FA should be done in order to find the location of the occlusive vein and to evaluate areas of nonperfusion in the periphery and the macular area. FA can also evaluate for the existence of ME or NV of the disk or retina. NV of iris or angle should be determined in a gonioscopy examination.
When periphery is perfused, and even in the face of a perfect vision, still a monthly evaluation is warranted, at least for the first 3 mo. In stable patients, the follow-up can be continued every 3 mo. All follow-up visits should include VA assessment, OCT and biomicroscopy.
In cases with decreased VA of 20/40 and under, the existence of ME should be evaluated. If ME is present, treatment should be initiated promptly. First line treatment should be with a properly approved drug, either Ozurdex with re-treatment decision according to follow-up, or with injections of an anti-VEGF drug every month for the first 3-6 mo, with additional injections depending on the progression or regression of ME.
Several characteristics of the patient may help the physician in deciding on the initial treatment. Mobility of the patient is of importance due to the necessity of a monthly visit for injections if treated with anti-VEGF. The socioeconomic status should be considered for the cost of the treatments. Pseudophakic patients can be treated with steroids with less concern. The presence of glaucoma can exclude steroids as first line treatment. Patients after vitrectomy are better treated with steroids due to their pharmacokinetics, non-compliant patients are better treated with steroids because of the need or less office visits. Younger patients should be considered for anti-VEGF because of the lens status. Patients with systemic disease such as MI or stroke are to be handled carefully with anti-VEGF. Adding to that is the physician’s experience and treatment availability of the various treatments which are factors to consider.
The ongoing COMO trial is an interventional, randomized, single blind, comparison of Ozurdex vs ranibizumab for the treatment of BRVO. Patients with ME secondary to BRVO are randomized 1:1 to receive one of the drugs with assessments at day 7 and monthly for the first year. The hypothesis is that the effect of Ozurdex is non-inferior to that of ranibizumab in BRVO patients as assessed by change in BCVA after 1 year.
When there is non-perfusion of the periphery, and if ME is present, a rapid initiation of treatment is mandatory. First line treatment should be with an approved drug, with Ozurdex and decision about re-treatment according to follow-up, or with injections of an anti-VEGF drug every month for 3-6 mo, with additional injections based on the progression or regression of ME.
Laser treatment can still be considered for the ischemic areas in the periphery. In cases of macular ischemia the prognosis for VA improvement is generally poor even in cases of prompt treatment and ME resolution.
The development of neovascularization anywhere in the posterior or anterior chamber, no matter at what point during follow-up, should prompt the immediate treatment with sectorial laser photocoagulation to the ischemic areas. The addition of an intravitreal drug, steroids or anti-VEGF should be considered, although not proven in the trials available.
Visual acuity: Several studies including the Central Vein Occlusion Study (CVOS) show a poor visual outcome in patients with CRVO[7,29]. Baseline VA for CRVO is usually less than 20/40 and in most ischemic CRVO (10 disk areas or more of capillary non-perfusion), it is less than 20/200[87].
VA loss is usually more accentuated in ischemic CRVO, although VA is also poor in the non-ischemic type with more than 60% of non-ischemic CRVO patients had VA of less than 20/40 in the CVOS[6,87]. In the Ozurdex GENEVA study 92.5% of the observation group had no improvement or mild improvement (< 15 letters) after 30 d[61]. The SCORE study (Standard Care vs Corticosteroid for Retinal Vein Occlusion) reported 75% of CRVO eyes (both types) in the observation group with a final VA of 20/40 or worse after 12 mo[66,87].
In most studies the mean decline in VA ranged from 1 to 75 letters, although a mean improvement in VA of 1.5-12.5 letters was seen in several studies. No studies showed an improvement above 20/40[87]. In a meta-analysis of over 50 studies, the mean decrease in VA was 10 letters from baseline in 6 mo and 3 letters from baseline in 1 year for non-ischemic CRVO. In the ischemic group the decrease was of 15 letters and 35 letters accordingly[87].
In many prospective studies such as the GENEVA, CRUISE, COPERNICUS, GALILEO and others, the use of treatment for macular edema improved visual outcome largely, mainly in the non-ischemic type and changed the visual outcome of the disease in a large scale[61,71,88,89].
Alternative blood drainage formation: In BRVO, the process of venous collaterals formation is a way to facilitate the flow of blood from the vein which was obstructed to a close-by vein which is open and have normal flow. The collateral formation is the result of pressure and flow changes within the retinal veins after the obstruction[90]. Collateral formation allows reversibility of the circulation interruption and inflammation formation, and was correlated to better visual outcome. In one study VA improved from 0.22 logMAR to 0.59 in patients with collateral formation compared to an improvement from 0.24 log of the minimum angle of resolution (logMAR) to only 0.31 in eyes with no collateral formation[91].
In regards to CRVO, retino-choroidal collateral veins, also known as optico-ciliary veins tend to develop on the optic disc. They act as an alternative drainage route for retinal blood. Some researchers believe these shunts are formatted de novo, while others hypothesise that the vessels only enlarge in the face of CRVO[92]. In a study regarding the formation of those shunts, the mean time to develop them was 6.7 mo, and most patients with those shunts did not develop anterior segment NV. The conclusion of Fuller et al[92] (2003), was that optico-ciliary veins are protective in CRVO patients from the development of anterior segment NV.
Conversion from well perfused to ischemic CRVO: The recognition of well perfused (non ischemic) retina and ischemic areas is best done by FA.
Conversion rates were reported in several studies to be up to 27%[87,93,94] in CRVO, after up to a 13 mo period. The ischemic conversion was described in the CVOS[6] where a total of 34% of patients converted to ischemia in the 3 year follow-up period. Of them 15% converted in the first 4 mo since disease developed[87]. Since conversion to the ischemic type can occur in up to third of CRVO patients in up to 3 years time, a long-duration, close follow-up is warranted in these cases with high clinical suspicion and performing FA and initiating treatment when conversion is suspected.
NV: NV of retina or disk secondary to an initially non-ischemic CRVO was found in up to 33% over a period of up to 15 mo[87]. As for ischemic CRVO, the incidence of NV was up to 20% over a period of 9 mo[87,95]. In some studies with no sub-division, NV was seen in up to 50% of patients after a 6 mo period[87].
The strongest predictors for NV of iris or angle were found to be visual acuity and extend of ischemic areas as seen on FA. 35% of ischemic eyes in the CVOS, developed NV of the iris or angle, compared to only 10% developing anterior chamber NV in non-ischemic eyes[29].
Ischemic CRVO is associated with neovascular glaucoma in 23%-60% of cases, and is first detected by gonioscopy. The primary finding is of a vascular network located in the trabecular meshwork and causing blockage[87]. Gonioscopy is useful and should be part of the examination regularly in all CRVO patients, but mostly in the patients with the ischemic subtype, in order to detect NV of the angle as soon as possible and allow immediate treatment with PRP for the prevention of neovascular glaucoma as proposed by the CVOS[7].
Macular edema: Most studies on CRVO enrolled patients already diagnosed with having ME at baseline. Only 2 studies reported the development of ME over time but both had only 3 eyes[87]. ME is a major complication of CRVO and associated with poor visual prognosis without treatment. Early treatment is essential since the longer the edema exists, the worse is the structural damage to the fovea[87], but even late treatment could improve VA.
In cases of ischemic CRVO resolution of ME ranged up to 73% in up to 15 mo, compared to the non-ischemic type where the corresponding proportion was about 30% by 15 mo[96,97].
Fellow-eye involvement: Systemic risk factors of CRVO patient make the fellow eye as vulnerable as the effected eye. Both eyes involvement at baseline was described in 9 studies and showed a rate of 0.4%-43% of CRVO cases[87]. 5%-10% of CRVO cases will develop RVO of any type in the fellow eye in a 3 year period[87,98-100].
Vitreous haemorrhage: The incidence of vitreous hemorrhage (VH): in CRVO patients was described in one study and was 10% in a 9 mo follow-up[87].
Two objectives are to be simultaneously managed by the physician in RVO patients: (1) identification and management of the risk factors leading to RVO; and (2) the diagnosis and treatment of sight-threatening complication associated with the disease, mainly macular edema and neovascularisation.
Risk factors management: The first goal in the management of RVO is the prevention of the disease and its complications by reducing and controlling systemic risk factors. Those risk factors are to be treated and monitored closely. The management of these factors may diminish the severity of the disease and risk of complications including fellow eye involvement.
When findings of RVO are clinically present (engorgement and dilatation of retinal veins, hemorrhages, increased retinal circulation time on FA) in asymptomatic patients, initiation of treatment for systemic medical risk factors, may slow or even prevent the disease progression.
Many studies including the CVOS have shown an association between arterial hypertension or glaucoma and RVO. The physician should exclude those conditions, or if present, treat them. All though prompt treatment is recommended in these cases, no clear evidence was found regarding the benefits of the management of glaucoma and/or reduction of arterial hypertension in regard to the visual outcome in RVO patients[14].
Though the use of such medication can help resolve RVO or lower complication rate, several studies using those drugs (Aspirin, Heparin, Streptokinase and Warfarin) showed little to no benefit, and in patients over 55 years, a greater tendency towards vascular adverse effects[63,64].
The use of Aspirin in the management of RVO is controversial and could only be suggested, yet no proven, in the prevention of cardiovascular events[15].
Hemodilution was suggested by several studies as a therapy in RVO. The rationale is to lower the blood viscosity thus preventing the slowdown of blood circulation and its developing complications. The studies showing benefits of Hemodilution were mono-center studies and conducted in the 1980’s and 1990’s but showed significant results.
A more recent multicenter, prospective study using the recent method of hemodilution, showed some limited but positive results, and recommended the use of hemodilution in the early stage of RVO. This was shown in cases when there were no contraindications such as ischemic CRVO requiring panretinal laser photocoagulation, cardiovascular conditions such as diabetes mellitus, uncontrolled hypertension and severe cardiac or renal failure, or haematological disease such as anemia or sickle cell disease[65].
Ophthalmological management of CRVO: The systemic investigation and treatments as mentioned above are identical for all RVO patients. The ophthalmological management differs between BRVO and CRVO. Several types of management are available today for the treatment of RVO: The dexamethasone intravitreal implant (Ozurdex) that is based on the GENEVA trial, anti-VEGF treatments as with Ranibizumab, based on the BRAVO and CRUISE trials, the recent Aflibercept, based on the COPERNICUS and GALILEO trials, and the laser treatments in specific indications.
When managing a CRVO patient it is crucial to classify it into well perfused (or non-ischemic) or non-perfused (ischemic). This classification is based upon the evaluation of capillary non-perfusion areas both at the posterior pole and at the periphery of the retina by fluorescein angiography. This classification is the basis for the treatment indications of sight-threatening complications.
In the well perfused, non-ischemic CRVO, the major sight-threatening complications are ME and the conversion into the ischemic subtype.
In the non-perfused, ischemic CRVO the major sight-threatening complications are again macular edema, usually in a more severe way, but also and mainly, neovascularization of the posterior pole [neovascularization of disc (NVD) or elsewhere (NVE)], or of the anterior segment of the eye [iris or angle neovascularization (NVI or NVA accordingly)].
The differentiation between ischemic and non ischemic CRVO subtypes can be difficult, especially at an early stage of the disease[3,5,7,29,38]. There are several clinical and functional findings that are typically found more in ischemic CRVO: acute onset of the disease, a very poor baseline VA, relative afferent papillary defect, the presence of deep and extensive intraretinal hemorrhages, the rapid formation of multiple cotton wool spots, and, as seen on FA, an extensive retinal capillary non-perfusion (more than 10 disc areas) both in the periphery and the macular region. The enlargement on FA of the foveal avascular zone is an indication of macular ischemia, and those patients usually carry a less favourable VA outcome[7].
Electroretinogram is another clinical tool to aid differentiate ischemic to non-ischemic CRVO as showed by Hayreh in 1989. In Ischemic CRVO a subnormal b-wave amplitude of < 60% of the mean value for normal individuals, or < 64%-69% of the patient’s normal eye amplitude is usually found[101].
The use of spectral domain optical coherence tomography (SD-OCT) is essential in the evaluation and quantification of the amount of cystoid macular edema in RVO patients. It also provides further information regarding the location and amount of fluid in the retinal layers or in the sub-retinal space. SD-OCT showing hyper-reflective dots, especially in the outer layers of the retina, is suggestive of an inflammatory reaction and may represent disease activity[11].
Following absorption of fluid, severe ischemia is shown on SD-OCT by a decrease in retinal thickness, atrophy of the macular area and disruption of the outer retinal layers (external limiting membrane, Ellipsoid zone and photoreceptors)[11].
The integrity of several of the retinal layers, including the external limiting membrane as well as the inner segment and outer segment of the photoreceptors, is indicative of the visual prognosis. The existence of the ischemic component is shown by thinning of the retinal nerve fiber layer that can be discovered during follow up[102].
Patients with non-ischemic CRVO which have a favourable baseline VA (better than 20/40) have a good prognosis and observation only policy is acceptable. No ophthalmological treatment is compulsory, due to the lack of complications. Yet this situation does warrant a systemic investigation for hypertension, hyperlipidemia and diabetes mellitus, with risk factor management, in order to decrease the likelihood for complications such as ischemic conversion, or fellow eye involvement. Ophthalmological risk factors such as glaucoma should be ruled out or treated. Close and prolonged follow up must be suggested in order to detect progression to the ischemic subtype as early as possible[25,27].
Monitoring these patients closely with OCT, VA assessments and biomicroscopy is essential for early identification of ME and/or conversion to ischemic CRVO. An addition of FA is warranted when progression cannot be assessed properly, or it is doubtful, and when the physician needs to assess the amount of retinal ischemia.
Monitoring these patients is suggested be done every month for the first 3 mo, followed by every other month for the first year. Gonioscopy has been suggested during this follow-up. Patients should be informed to be aware of their vision, and return promptly for an examination with every deterioration of visual acuity, which may be a sign of macular edema.
In patients with non-ischemic CRVO and poor VA, physician should assess the macula for the presence of ME, and in case of its presence, an immediate treatment should be initiated.
Treatment nowadays is indicated in eyes with non-ischemic CRVO with macular edema and a VA of 20/40 or worse[38].
The CVOS showed that no statistically significant VA benefit was seen with laser photocoagulation treatment, though improvement of the macular edema was seen. This finding was with the exception of the younger patient population[103]. Because of these findings, grid laser photocoagulation is no longer indicated for that purpose.
Corticosteroids are used in CRVO with ME due to their ability to decrease capillary permeability and inhibit inflammatory reaction and expression of inflammatory mediators, and affect the metabolism of most of inflammatory mediators including VEGF.
Triamcinolone Acetonide in a corticosteroid preparation containing benzyl alcohol and was used to treat CRVO patients in an off label fashion (Kenalog*; Squibb). Several studies have showed the benefits of Kenalog for the treatment of patients with ME secondary to non-ischemic CRVO[104]. Kenalog* is known to have some side effects including cataract development and progression and raised IOP. The benzyl alcohol component in the preparation was also associated with sterile endophthalmitis.
The multicenter SCORE CRVO study[67] showed the beneficial effects of a preparation of preservative free intravitreal triamcinolone acetonide, (Trivaris; Allergan), for the treatment of patients with ME secondary to non-ischemic CRVO[105]. This study showed that the odds of reaching a ≥ 15 letters gain in VA, were 5 times better in both the 1 mg dosage group and the 4 mg dosage group than the observational arm (26.5%, 25.6% and 6.8% respectively)[10]. No difference was seen between both treatment groups. The 1 mg regiment had a better safety profile rather than the 4 mg group in regards to cataract formation, IOP elevation, disease progression and the necessity for surgery. Trivaris is nowadays FDA approved.
The use of Triamcinolone acetonide is rare nowadays as newer better treatments are available.
Dexamethasone is a potent corticosteroid that is known to decrease the expression of inflammatory mediators exhibited in ME including VEGF. Dexamethasone is intravitrealy injected as a slow release, biodegradable implant (Ozurdex; Allergan), allowing up to 6 mo of medication in the vitreous. The use of an implant is mainly due to dexamethasone being highly soluble and with a short half-life when in the vitreous. The effect of Ozurdex on RVO with ME was studied in a multicenter, randomized, sham-controlled clinical trial (the GENEVA study)[61]. A disposable applicator prefilled with 0.7 mg of dexamethasone in a polyglycolate-acetate implant to induce slow release of the drug, is used for the insertion of the drug into the vitreous cavity.
The GENEVA study was a prospective, multicenter, sham-controlled study which included 3 identical, randomized, parallel groups treated with either 0.35 mg or 0.7 mg dexamethasone or sham treatment (needless applicator). In the second 6 mo of the study, the open-label treatment (second injection), all patients eligible for treatment received the 0.7 mg implant. The primary endpoint of the study was the time to achieve a ≥ 15 letter improvement on BCVA. The secondary endpoints of the study included the BCVA over the whole 6-mo period, the central retinal thickness and the safety profile of both dosages.
The study resulted in that the 0.7 mg dexamethasone implant (Ozurdex) showed an improvement in VA with a peak effect after 60 d, followed by a decline towards the baseline VA after 180 d. After 60 d the proportion of patients achieving the primary endpoint was 29.3% and 28.5% in the 0.7 mg and 0.35 mg treatment groups, compared to only 11.3% in the sham treatment group. At 180 d the proportions were 26.4%, 19.4% and 17.0% respectively. Over a 1 year follow-up, VA improvement was achieved with a second injection after 180 d. OCT demonstrated an anatomical improvement in macular edema[61].
In regards to safety issues cataract rate was low with 7.3% in the treatment group, and so was the rate of IOP increases, 4% with a peak over 2 mo. In all cases pressure declined throughout the follow-up period, especially if treated with anti-glaucomatous topical treatment. Treatment, if given, was ceased by 180 d after implant injection. No adverse effects, regarding the injection, were noted[61].
A major conclusion from the GENEVA study was that early treatment of ME is much better than delayed treatment in regards to vision improvement. A retrospective review of the study groups has shown that eyes treated within 3 mo from onset of ME showed a better improvement of VA than eyes treated after more than 90 d[15].
A more recent post-hoc analysis of the GENEVA study, regarding the onset and duration of BCVA improvement in eyes treated with Ozurdex, showed an improvement of ≥ 15 letters in 10% of the treatment group as soon as 7 d post treatment. The duration of a ≥ 3 lines improvement was 60-90 d[106].
Retreatment with Ozurdex was studied in several studies with special emphasize on the time frame between injections. In a multi-center retrospective study[72] of 128 patients, 58 of them (45.2%) with CRVO, mean interval between Ozurdex injections was 5.9 mo following the first injection and 8.7 mo following the second injection. A ≥ 15 letter gain was seen in 48.8% of eyes with a central macular thickness reduction of 355 μm. Some of the patients had decreases VA before 6 mo which leads to the conclusion that patients should be monitored closely for the chance of deterioration before the 6-mo period.
The SHASTA study[73], a retrospective study showed similar results with a mean reinjection interval of 5.6 mo and a significant improvement both in VA and CRT. Retreatment was also investigated in several other small studies with a mean time between injections of 4.7-5.3 mo, all with a favourable VA outcome[74,75].
A retrospective study on 15 eyes with ME secondary to CRVO compared the efficacy of Ozurdex treatment in vitrectomized vs non-vitrectomized eyes[107]. The study demonstrated both groups had a significant improvement with no significant difference between groups in regards to VA improvement and CMT reduction. Conclusion is to be made that Ozurdex is effective in both vitrectomized and non-vitrectomized eyes, and the absence of vitreous does not alter the pharmacodynamics of the drug, therefore making it suitable even in eyes after pars plana vitrectomy.
Ozurdex has the FDA and EU approval and is licensed for the treatment of patients with ME secondary to non-ischemic CRVO. The GENEVA study suggests that the implant may be considered a first-line choice in the treatment of ME secondary to CRVO.
Ranibizumab is a pan-VEGF blocker (Lucentis; Novartis) which showed effectiveness in patients with ME secondary to CRVO in the CRUISE trial[71]. In the first 6 mo of the study, ranibizumab was injected every month in two doses (0.3 and 0.5 mg) and yielded a VA gain of 12.7 and 14.9 letters, respectively, compared to 0.8 letters gained in the sham injections group. The proportion of patients achieving a ≥ 15 letters VA gain was 47.7% in the 0.5 mg group compared to 16.9% in the sham group. The effect of ranibizumab was noticed as soon as 7 d post first injection with a 9 letter improvement in the treatment group which was significantly better than the sham group[65]. In relation to the anatomical change mean CFT was significantly reduced at 6 mo in 433-452 μm in the treatment groups compared to only 162 μm in the sham group[71].
Following treatment in the first 6 mo, all patients continued in an extension for another 6 mo of monitoring and therapy PRN. The 12 mo results of the study concluded that the VA improvement showed in the first 6 mo could be maintained. Earlier treatment after ME diagnosis may bring a better functional improvement in retinal thickness than delayed therapy.
The recent RETAIN study followed the CRUISE patients in an open-label, single arm, multicenter long-term extension trial[77]. In a mean follow-up of 49.7 mo, 44% had edema resolution (no intraretinal fluid for 6 mo or more after last injection), with 71% receiving their last injection within 2 years of the first one. VA improved in 15 letters or more in 53.1%, with 43.8% having a final VA of 20/40 or better. The CMT remained as it was in the end of the CRUISE trial with a mean of 420 μm reduction.
Ranibizumab is FDA and EU approved for the treatment of ME in patients with CRVO.
Bevacizumab is a pan-VEGF blocker (Avastin; Roche) which is not licensed for intraocular use. A prospective, randomized, double-masked clinical study on Bevacizumab compared to sham in patients with ME secondary to CRVO was conducted on 60 eyes with a 1:1 randomization[108]. At the 6-mo follow-up time 60% of the study group gained ≥ 15 letters compared to only 20% in the sham group. The BCVA improved by 14.1 letters compared to a decrease in 2 letters in the sham group and the decrease in CRT was 426 μm compared to 102 μm. No residual edema was found in 86.7% of the treatment group compared to 20% in the sham group. No rubeosis was developed in the treatment group and no safety concerns were detected.
In a 6 mo extension of the study all patients received Bevacizumab every 6 wk. The percentage of patients with a ≥ 15 letters gain did not change in the primary treatment group, but in the sham group it rose to 33%. The mean VA improved in both groups to 16 letters in the treatment group compared to 4.6 letters in the sham group. In the latter, a further decrease in CRT was noticed to a total reduction of 404 μm. No rubeosis or safety issues were found in both groups in the extension trial[109].
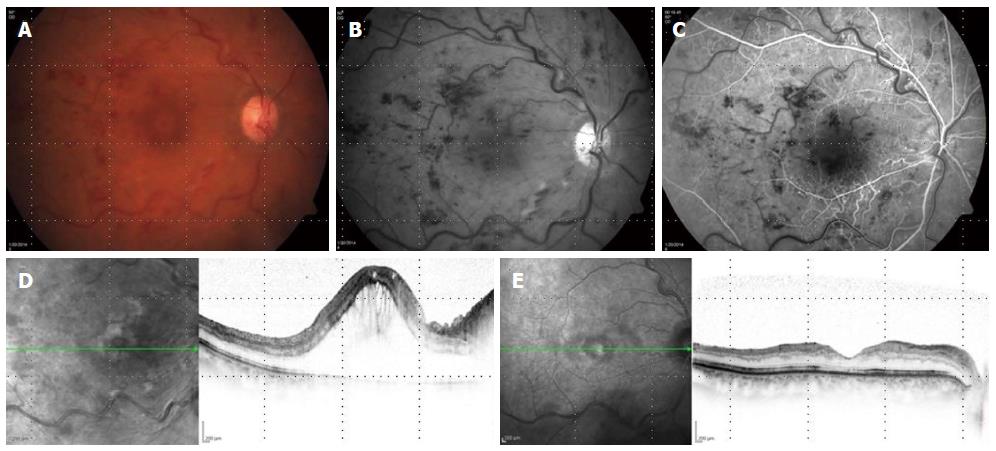
Many other uncontrolled studies have reported that the intravitreal injection of bevacizumab may lead to VA improvement and regression of ME[110,111]. Long term outcomes and safety data is non-conclusive because of the variations in treatment regiments between those studies. Bevacizumab is less expensive than other anti-VEGF treatments making is widely used (Figure 2).
Pegaptanib is a selective anti-VEGF blocker (MACUGEN; Pfizer) which was investigated in a multicenter randomized study as treatment in RVO. Patients with ME associated with CRVO were randomized to receive either a sham injection or 0.3 mg or 1 mg of pegaptanib sodium. The phase II trial showed that 0.3 mg pegaptanib administered every 6 wk caused an improvement in VA of 7 letters, over a 6 mo follow-up[112]. Due to the vast use of bevacizumab and ranibizumab, pegaptanib is not frequently used.
Aflibercept is a VEGF Trap-Eye (Eylea; Regeneron) protein comprising of the second domain of VEGF receptor 1 and the third domain of the VEGF receptor 2 fused to the Fc domain of immunoglobulin G1. Its binding affinity for VEGF is greater than that of either Ranibizumab or Bevacizumab.
Aflibercept was studied in the COPERNICUS which is a 2-year, phase 3, prospective, randomized, double-masked, multi-center study of aflibercept compared to sham injection[88]. Patients were assigned randomly in a 3:2 ratio to receive aflibercept 2 mg or sham injection every 4 wk for a 24 wk period. Between weeks 24 and 52 patients were treated according to specific retreatment criteria based on VA and CRT on OCT. The second year treatment was PRN based.
VA outcome was significantly better in the treatment group with 56.1% of eyes treated achieving a ≥ 15 letter VA improvement compared to 12.3% in the sham group. 93.9% of treated eyes gained 10 letters or more compared to 52.1% in the sham group.
The improvement in VA was seen in the treatment group as soon as 4 wk post first injection. By week 24 treated eyes had a mean improvement of 17 letters compared to a loss of 4 letters in the sham group. BCVA improved steadily from week 4 to week 24 in the treatment group. A sub-group analysis of perfused and non-perfused eyes showed a significant better VA improvement in both groups in the treated eyes.
Reduction in CRT was noticed even after 4 wk with a 457 μm reduction in the treatment group compared to a 144 μm reduction in the sham group. Reduction in CRT was significantly better in treated eyes both in perfused and non-perfused sub-groups.
No progression to NV was seen in the treatment group compared to 6.8% in the sham group. Regarding nonperfusion, at week 12 the proportion was similar whereas at week 24 there was much less non-perfused eyes in the treatment group.
The 1-year results of the COPERNICUS trial[113] were similar. Patients were treated with 2 mg aflibercept as needed (PRN). At week 52, 55.3% of the primary treated group gained ≥ 15 letters in VA compared to only 30.1% in the primary sham group. The mean VA gain was 16.2 letters vs 3.8 letters in both groups. No adverse events were noted in the second treatment period.
The improvement in VA was significant in both perfused and non-perfused eyes compared to the sham group even after PRN treatment. Regarding CRT the reduction observed at week 24 in the treatment group was maintained in the PRN regiment. The sham treatment group showed great reduction in CRT during weeks 24-52 and in the end of the 1 year study both groups showed a similar reduction of CRT, around 400 μm.
During a second year follow-up on the COPERNICUS patients VA continued to be superior in the primary treatment group, but CRT reduction which was similar at week 52, continued to be similar at week 100 with a mean of 3 injections for both groups[114].
Another phase 3 randomized, double-masked, multi-center clinical study of aflibercept for CRVO, conducted in Europe, is the GALILEO[89]. The randomization was 3:2 to monthly intravitreal 2 mg aflibercept injections vs sham injections.
Results were similar to COPERNICUS with VA improvement of ≥ 15 letters at 6 mo 60.2% in the treatment group compared to 22.1% in the sham group. The mean change was 18 letters compared to 3.3. An important note is that the change between the treatment and sham groups was greater among patients with disease duration of up to 2 mo. The proportion of patients reaching the 15 letter gain endpoint at 6 mo among the treatment group was 70.9% among patients with disease duration of fewer than 2 mo and 50% in patients with disease duration of over 2 mo.
The anatomical outcome was also similar to that of the COPERNICUS with a different of 279 μm between treatment group and sham group in the CRT reduction. No significant ocular or non-ocular adverse events were noticed.
In the second year of the GALILEO study[115] the treatment group continued the 2 mg aflibercept treatment PRN and the sham group continued receiving sham injections. After 52 wk the percentage of patients with at least 15 letters VA improvement was 60.2% in the treatment group compared to 32.4% in the sham group. The VA improvement was of 16.9 letters compared to 3.8 letters and CRT reduction was of 423 μm compared to 219 μm in the sham group. The average in the PRN treatment was 2.5 injections during the second 6 mo.
The drug was found to be safe with no difference found in ocular and non-ocular adverse events between the two groups. Aflibercept is approved both by the FDA and EU for the treatment of ME secondary to CRVO.
Follow-up after the recommended treatment in the initial 6 mo as seen in the above mentioned studies is dependent on the treatment which was initiated for ME (corticosteroids or anti-VEGF). Follow-up is usually advised for up to 2 years, even in cases with no sight-threatening complications. Close monitoring should be held especially to detect conversion to ischemic CRVO and the occurrence or reoccurrence of ME. Development of collaterals of the optic disk or resolution of ME should bring the physician to lower the frequency of follow-up[15].
The recurrence or a persistent ME, diagnosed by means of decreased VA, biomicroscopy and OCT examination, should lead the physician to a decision to re-inject. The use of laser photocoagulation is to be suggested for several populations such as non-responders or partially responders, or patients who are non-compliant with multiple injections.
Ischemic CRVO is characterized by a peripheral area of non perfusion, initially defined in the CVOS Study, as greater than 10 disk diameters, as evaluated by FA, but more recently to a more extensive area as used in the ischemic index method, with the use of wide field retinal imaging[116].
In patients with ischemic CRVO, physician should primarily evaluate and asses both peripheral area of non perfusion and macular perfusion, the presence of ME and the existence of NV.
In a patient with ME, with FA that shows a relatively good perfusion to the macular area, treatment should be as outlined above as for non-ischemic CRVO. In cases where the macula is not perfused, the VA prognosis is very poor, yet immediate treatment with dexamethasone implants is reported to be effective.
In patients with a large non perfusion area, defined as more than 10 disc areas, an early and immediate PRP treatment is strongly suggested as an attempt to prevent the development of ocular NV, associated simultaneously with anti-VEGF intra vitreous injection[7].
In cases with less severe non perfusion, without any neovascularization (including on gonioscopy), scatter laser treatment aimed at the non perfused area may suffice with a very close monitoring and follow-up. Patients who pose a great difficulty to treat are noncompliant patients[31].
Patients with ischemic CRVO and (moderate) area of peripheral ischemia, and no ME nor NV should still be monitored monthly with a VA check, biomicroscopy, OCT and FA. In addition, the iris and corneal angle should be assessed regularly with gonioscopy[29].
Evidence nowadays supports the immediate PRP whenever an anterior segment NV (iris or angle) is found. An anterior segment NV which necessitates treatment is any degree of angle NV and/or iris NV in an area of 2 clock hours[7].
The complete PRP treatment can be carried out in one session or be divided into several sessions. The aim is to treat the retina completely from the periphery to the main vascular arcades. Typically the treatment in done on a slit lamp and consists of 1500-2000 burns (but usually more to 3000), 500 micron each, 0.1 seconds burn, and the space between burns should be 1 burn width. The burns should be in an energy level enough to produce a white burn in the retinal layers. PRP should begin in the inferior quadrants with avoidance of areas with retinal hemorrhages. Repeating treatment can be done whenever anterior segment NV does not regress. Today with the NAVILAS and PASCAL a great degree of accuracy is seen with lesser variations between burn size and a more uniform burn shape[117].
A treatment combination of PRP and anti-VEGF injection has not been tried in a randomized clinical trial, but has been suggested with favourable results in some publications and seems reasonable to attempt, in order to achieve faster regression of anterior segment NV and/or at least limit the evolution, hemorrhage and pain associated to NVG along with IOP reduction[68,118,119]. No indication in the studies mentioned about the timing of bevacizumab injection related to the PRP. Since those were all retrospective studies, the decision regarding timing is reserved to the physician as suited to each patient.
Posterior segment NV of the retina or disc can appear alone or along with anterior segment NV and needs to be actively detected during monitoring due to the risk of vitreous hemorrhages. In cases of posterior segment NV an immediate and non delayed PRP treatment is in order.
Anti-VEGF mono-therapy can only lead to a transient regression of NV[118,119]. No clinical data is available about the efficacy of anti-VEGF mono-therapy to stop NV, but repeated injections may be required to stop NV progression, probably without complete cessation.
A PRP treatment in conjunction with anti-VEGF may prove to be more effective even if still not tried in controlled studies.
In cases of severe NV, especially with vitreous hemorrhage, early PRP is strongly indicated (in all areas accessible). In these cases anti-VEGF injection may help in controlling the development of NV until the resolution of the vitreous hemorrhage allowing better visualization for complete PRP treatment.
In patients with neovascular glaucoma, the intravitreal injection of anti VEGF has been shown regress iris NV and improve the level of obstruction of the angle[120]. Some case series has shown that anti VEGF (Bevacizumab) with PRP induced a faster regression of iris NV than PRP alone[68].
Juvenile CRVO, defined as CRVO in patients under the age of 50, should be differentiated from the traditional CRVO because of a different pathogenesis and clinical course. In some patients the CRVO is related to a systemic disease and patients should be evaluated with a complete systemic workup for the underlying cause.
Juvenile CRVO could often present as benign, well perfused, with limited or no risk factors. Sometimes Juvenile CRVO could be preceded by inflammation as evident by cells in the vitreous[50]. The visual prognosis is usually better than the traditional CRVO, though the risk of complication may become the same, after one or more recurrence.
There is evidence showing that steroids treatment in a systemic administration can hasten the resolution of symptoms. It is custom to treat such patients that have ME with intraocular steroids, especially Ozurdex though little evidence exist.
The randomized controlled studies on RVO have provided much information regarding treatment. An important lesson from those studies is that the duration of the disease before initiation of treatment is an important factor influencing outcome. Treatment is beneficial in any stage of the disease, including in the late stages. It has been shown that in patients with a shorter duration of the disease, the rapid initiation of treatment may be more beneficial and the outcome is better.
Patients with CRVO should be first screened for known risk factors and in case any, the family physician should be notified regarding the disease. The management and control of the risk factors mentioned earlier should be fast and aggressive. The patient is evaluated by VA assessment, biomicroscopy, measurements of intra ocular pressure, and OCT. Examining the patient with fluorescein angiography should be done in order to evaluate areas of ischemia in the periphery and the macular area. FA can also evaluate for the existence of ME or NV of the disk or retina. NV of iris or angle should be determined in a gonioscopy examination. Distinguishing the subtype of CRVO should be done according to the extent of ischemia as seen on FA.
In cases of preserved VA of 20/40 and better, observation in a monthly fashion is advised, at least for the first 3 mo. If no sight-threatening complications are detected, the follow-up may be continued every other month for at least 1 year. All follow-up visits should include VA assessment, OCT and biomicroscopy and FA when needed.
In cases of decreased VA of less than 20/40, the physician should assess for the presence of ME. In the presence of ME, treatment should be initiated promptly. First line treatment should be with a properly approved drug, with Ozurdex and re-treatment decision based on the follow-up, or with injections of an anti-VEGF drug every month for 3-6 mo, with additional injections based on the progression of ME. As for BRVO, same considerations should be taken to account in deciding the first line treatment.
In cases of nonperfused CRVO, in the presence of ME, treatment is still warranted, but the prognosis is poor. First line treatment should be with a properly approved drug, with Ozurdex and re-treatment decision based on the follow-up, or with injections of an anti-VEGF drug every month for 3-6 mo, with additional injections based on the progression of ME. The addition of PRP treatment directed at areas of non-perfusion should also be considered by the physician in order to prevent NV. In cases of macular ischemia the prognosis for VA improvement is generally poor even in cases of prompt treatment and ME resolution. As mentioned before, same considerations should be taken in deciding the first line treatment.
At any visit during follow-up, the identification of neovascularization anywhere in the posterior or anterior chamber should prompt the treatment with scatter laser photocoagulation to the ischemic areas, guided by FA. The addition of an intravitreal drug, steroids or anti-VEGF should be considered, although not proven in the trials available.
The treatment of RVO patients has a long course. Multiple visits with examinations and intravitreal injections are the main course of action today. The common use of intravitreal injections is a burden on the patient and may cause further morbidity.
When treating patients with Ozurdex, a close monitoring should be advised and a reinjection should be done in cases of VA deterioration due to recurrence of ME usually after 5-6 mo.
When treating with anti-VEGF treatments, usually initiating treatment with Ranibizumab or Bevacizumab, two main methods can be employed. The first method is the monthly injection of anti-VEGF for the first 3 mo followed by a PRN approach. The patient is examined every month for the first 6 mo followed by an exam every other month for the rest of the first year. Injection is carried out only if recurrence is noted by VA worsening and ME presence on OCT.
The second method is rising among practitioners and is the treat and extend. After the primary 3 injections the patient is being injected in every visit until macula is dry. Since achieving a dry macula, the patient is evaluated by VA exam, biomicroscopy and OCT and is being injected in every visit. If macula is considered dry in the examination, the follow-up time increases by 2 wk and so on after every visit with no ME. If ME is presence in one of the visits, the follow-up decreases by 2 wk. This method allows the practitioner to discover the precise amount of time between injections for the individual patient with close enough monitoring and less injections than other methods.
In a review of intravitreal therapy for ME secondary to RVO, all anti-VEGF treatments showed a better improvement in VA than steroid treatment at month 12. The greatest gain in VA in CRVO patients after 12 mo was shown with the use of aflibercept and Bevacizumab with a gain of 16 letters compared to Ranibizumab with 14 letters improvement. In BRVO patients Ranibizumab showed to bring the greatest gain of 18.3 letters compared to Bevacizumab with 15 letter gain[121].
The use of longer-acting dexamethasone implant (Ozurdex) in conjunction with anti-VEGF therapy was examined in a small prospective, non comparative trial. VA gains were achieved up to 6 mo 14 letters, with 29% showing a ≥ 15 letters gain. CMT decreased by 200 μm. The mean time to re-treatment was 125.9 d, but it was unnecessary in 18.6% of patients[122]. The study demonstrated the synergy between both drugs with increasing VA and prolonging time between injections compared to each drug alone.
A recent prospective study on the effects of multiple anti-VEGF injections on IOP resulted in the conclusion that multiple injections were not found to be a risk factor for elevation in intra ocular pressure[123].
Few surgical approaches are utilized today in the treatment of RVO. These treatments target the vein occlusion itself or the macular edema. Most surgical treatments are abandoned and not in use nowadays due to the new and effective pharmacological treatments.
Radial optic neurotomy (RON) was used in the past for the treatment of CRVO[124]. However the benefit effect has not been studied enough and is still questionable[125]. In most places this technique is not used.
The surgical approach relied on the assumption that the radial incision will decompress the pressure on the vein.
Optic neuropathy leads to the development of opto-ciliary venous anastamosis (or retino-choroidal shunts), which increase the retinal venous outflow[126-130]. Incisions are made on the nasal side of the optic nerve, radial to the optic nerve itself and parallel to the nerve fiber layer. A study conducted on 11 CRVO patients, 73% had improved vision with an average gain of 5 lines.
Hayreh[125] raised the concerns about the location of the incision close to the central retinal artery which can cause optic nerve head ischemia and complete vision loss.
RON was associated with some serious complications in more than 71% including damaging central retinal artery, central retinal vein, optic nerve fiber, globe perforation, retinal detachment, cataract, choroidal neovascularization and anterior segment neovascularization[124,130,131].
Patient selection is important with more benefit for patients with CRVO of under 90 d and a pronounced peripapillary swelling[127].
RON is a very questionable procedure with controversial benefits and serious possible adverse events. Nowadays it is generally abandoned except for selected cases.
In this procedure, in order to bypass the occluded vein, a shunt is made between a retinal vein and the choroid. This procedure creates another route to for the retinal outflow and relieves the obstruction. The anastamosis can be induced by laser or surgery[132-134].
A successful anastamosis was first reported in 33% with various degrees of VA improvement[132]. However complications were described in several studies and included posterior vitreous detachment, hemorrhages, retinal fibrosis, NV of the choroid, retinal ischemia and retinal detachment[129,132,134].
The anastamosis, in all the techniques, does not re-perfuse areas of nonperfusion, however it leads to better perfusion of the perifoveal and parafoveal areas, reduce ischemia, increase venous return, decrease macular edema and improve VA.
This technique also is generally abandoned and not in use.
Pars plana vitrectomy (PPV) with ILM peeling can bring resolution of retinal damage and ME in CRVO patients[135-137]. The exact mechanism is unknown but 70% of RVO patients have shown decreases retinal thickness and increased VA after this operation[136,137], with an effect of up to 5 years[138].
This procedure is reserved to patients where other treatments, especially intravitreal injection have failed in achieving improvement, and the blood perfusion of the macula is sufficient to allows improvement of VA[136,139-146].
Successful results after PPV without ILM peeling have been described. PPV itself removes VEGF and other mediators from the vitreous and allows better oxygenation of the retina[147]. PPV with gas/air tamponade for ME showed a statistically significant improvement in BRVO patients[148,149]. In CRVO patients the benefit is questionable[150]. This procedure can also be combined with intravitreal injection of steroids which permits a more rapid and lasting action.
PPV is hardly used in most clinics for the purpose of RVO treatment.
A need for direct tissue type plasminogen activator (t-PA) injection had followed some unsuccessful attempts to treat RVO patients with t-PA systemically or intravitrealy[151]. The surgery includes PPV with removal of the posterior hyaloid, and injection of 200 μg/mL t-PA to the optic nerve head through a cannulation of a peri-papillary retinal vein.
This technique is favourable because: (1) t-PA is delivered directly to the site; (2) Allows the visualization of the drug reaching the thrombus; (3) Very small dose provides sufficient concentration near the thrombus; and (4) The injection can dislodge the thrombus and induce dilatation of the central retinal vein. VA was increased in about 50% of the CRVO patients. However in another study the results were poor with high complication rate[152].
This technique can cause some complications like vitreous hemorrhage, retinal tears or detachment, NVG formation, endophthalmitis and phthisis bulbi. Only retrospective data is at hand. This technique is also hardly ever used.
The surgical procedure, first introduces in 1988, included PPV with posterior hyaloids detachment and the opening of the adventitial sheath at the location of the arterio-venous block in BRVO patients and resulted in improved visual acuity[153]. The endpoint was the separation of arteriole from the venule. More recently, better results were seen using a bimanual technique followed by intravitreal recombinant t-PA[13].
Most studies regarding this surgical procedure failed to show an outcome justifying the risk of the surgery in BRVO patients[127,154]. In a study comparing this technique with intravitreal triamcinolone acetonide in BRVO patients, authors showed similar anatomical and functional improvement after 6 mo[155].
A recent match-control study compared 45 eyes with BRVO who underwent arteriovenous sheathotomy to 45 naïve eyes with BRVO. Improvement in VA was 0.42 logMAR in the treatment group compared to 0.22 logMAR in the control group. The mean postoperative CMT was significantly thinner than the control group at 1 mo, but not at 3, 6 and 12 mo[156]. This technique is not actually utilized in most clinics.
This is a new surgical treatment in which a retinal endovascular cannulation using a microneedle is done in eyes with CRVO, in order to flush the thrombus out of the central retinal vein and through the lamina cribrosa. Seventy-five percent of patients had a VA improvement of 15 letters or more 24 wk post surgery, mean BCVA improved by 16.3 letters and CFT decreased by 271 μm. No adverse events were noted[157].
This technique is not actually utilized in most clinics.
The prevention of recurrent RVO in the same eye, or fellow eye involvement has been discussed in several studies without any benefit shown, including in the use of anti-aggregates or anticoagulants[54,158].
The only available data about recurrence supports the medical treatment of underlying cardiovascular and other systemic and ocular risk factors as mentioned above.
Studies estimate that there are about 500 new RVO patients per million population[38], which can be divided to 85% BRVO and 15% CRVO.
Despite the large numbers, only 40%-50% require intervention, as the others have good vision not necessitating ophthalmological intervention[7,38].
RVO is a common disease and responsible for a large percentage of ocular morbidity and decreased vision. The modern imaging techniques allow fast diagnosis with detection of the ischemic forms if presence, and the complications, mainly macular edema with OCT and neovascularisation with biomicroscopy and gonioscopy. Studies have shown that fast diagnosis and treatment initiation brings better outcome in terms of visual acuity.
The treatment modalities have evolved over the last few years with the introduction of Ozurdex, anti-VEGF treatments including bevacizumab and ranibizumab and the new and promising aflibercept. The diversity allows the physician to switch between drugs according to patients characteristics and reaction to treatment. The recent extension studies for the large trials suggest benefit of both CS and anti-VEGF treatment after 36-48 mo. The ongoing COMO study will shed more light on the first choice for treatment in the comparison between Ozurdex and ranibizumab.
P- Reviewer: Campa C, Romero-Aroca P S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Hayreh SS. Occlusion of the central retinal vessels. Br J Ophthalmol. 1965;49:626-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 212] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 3. | Hayreh SS, Klugman MR, Beri M, Kimura AE, Podhajsky P. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Hayreh SS. Retinal vein occlusion. Indian J Ophthalmol. 1994;42:109-132. [PubMed] |
| 5. | Coscas G, Gaudric A. Natural course of nonaphakic cystoid macular edema. Surv Ophthalmol. 1984;28 Suppl:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol. 1993;111:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 239] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 249] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Frangieh GT, Green WR, Barraquer-Somers E, Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol. 1982;100:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Duker JS, Brown GC. Anterior location of the crossing artery in branch retinal vein obstruction. Arch Ophthalmol. 1989;107:998-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Brand CS. Management of retinal vascular diseases: a patient-centric approach. Eye (Lond). 2012;26 Suppl 2:S1-S16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Coscas G, Coscas F, Zucchiatti I, Glacet-Bernard A, Soubrane G, Souïed E. SD-OCT pattern of retinal venous occlusion with cystoid macular edema treated with Ozurdex®. Eur J Ophthalmol. 2011;21:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Zhao J, Sastry SM, Sperduto RD, Chew EY, Remaley NA. Arteriovenous crossing patterns in branch retinal vein occlusion. The Eye Disease Case-Control Study Group. Ophthalmology. 1993;100:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Christoffersen NL, Larsen M. Pathophysiology and hemodynamics of branch retinal vein occlusion. Ophthalmology. 1999;106:2054-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | MacDonald D. The ABCs of RVO: a review of retinal venous occlusion. Clin Exp Optom. 2014;97:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Coscas G, Loewenstein A, Augustin A, Bandello F, Battaglia Parodi M, Lanzetta P, Monés J, de Smet M, Soubrane G, Staurenghi G. Management of retinal vein occlusion--consensus document. Ophthalmologica. 2011;226:4-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Joffe L, Goldberg RE, Magargal LE, Annesley WH. Macular branch vein occlusion. Ophthalmology. 1980;87:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Haymore JG, Mejico LJ. Retinal vascular occlusion syndromes. Int Ophthalmol Clin. 2009;49:63-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. [PubMed] |
| 19. | Janssen MC, den Heijer M, Cruysberg JR, Wollersheim H, Bredie SJ. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Williamson TH. Central retinal vein occlusion: what’s the story? Br J Ophthalmol. 1997;81:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Gewaily D, Greenberg PB. Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2009;CD007324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Scholl S, Kirchhof J, Augustin AJ. Pathophysiology of macular edema. Ophthalmologica. 2010;224 Suppl 1:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1996;114:545-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 286] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Risk factors for branch retinal vein occlusion. The Eye Disease Case-control Study Group. Am J Ophthalmol. 1993;116:286-296. [PubMed] |
| 25. | Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 400] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Rath EZ, Frank RN, Shin DH, Kim C. Risk factors for retinal vein occlusions. A case-control study. Ophthalmology. 1992;99:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Hirota A, Mishima HK, Kiuchi Y. Incidence of retinal vein occlusion at the Glaucoma Clinic of Hiroshima University. Ophthalmologica. 1997;211:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Prager F, Michels S, Kriechbaum K, Georgopoulos M, Funk M, Geitzenauer W, Polak K, Schmidt-Erfurth U. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009;93:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 508] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 30. | Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984;98:271-282. [PubMed] |
| 31. | Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Branch Vein Occlusion Study Group. Arch Ophthalmol. 1986;104:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 263] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Santiago JG, Walia S, Sun JK, Cavallerano JD, Haddad ZA, Aiello LP, Silva PS. Influence of diabetes and diabetes type on anatomic and visual outcomes following central rein vein occlusion. Eye (Lond). 2014;28:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Dodson PM, Galton DJ, Hamilton AM, Blach RK. Retinal vein occlusion and the prevalence of lipoprotein abnormalities. Br J Ophthalmol. 1982;66:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Bertelsen M, Linneberg A, Rosenberg T, Christoffersen N, Vorum H, Gade E, Larsen M. Comorbidity in patients with branch retinal vein occlusion: case-control study. BMJ. 2012;345:e7885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Prisco D, Marcucci R, Bertini L, Gori AM. Cardiovascular and thrombophilic risk factors for central retinal vein occlusion. Eur J Intern Med. 2002;13:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Elman MJ. Thrombolytic therapy for central retinal vein occlusion: results of a pilot study. Trans Am Ophthalmol Soc. 1996;94:471-504. [PubMed] |
| 38. | Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, Kowalski JW, Nguyen H, Wong TY. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313-319.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 754] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 39. | Sofi F, Marcucci R, Fedi S, Giambene B, Sodi A, Menchini U, Gensini GF, Abbate R, Prisco D. High lipoprotein (a) levels are associated with an increased risk of retinal vein occlusion. Atherosclerosis. 2010;210:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Stojakovic T, Scharnagl H, März W, Winkelmann BR, Boehm BO, Schmut O. Low density lipoprotein triglycerides and lipoprotein(a) are risk factors for retinal vascular occlusion. Clin Chim Acta. 2007;382:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Turello M, Pasca S, Daminato R, Dello Russo P, Giacomello R, Venturelli U, Barillari G. Retinal vein occlusion: evaluation of “classic” and “emerging” risk factors and treatment. J Thromb Thrombolysis. 2010;29:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Rehak M, Krcova V, Slavik L, Fric E, Langova K, Ulehlova J, Rehak J. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Kuhli-Hattenbach C, Scharrer I, Lüchtenberg M, Hattenbach LO. Coagulation disorders and the risk of retinal vein occlusion. Thromb Haemost. 2010;103:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Lahey JM, Tunç M, Kearney J, Modlinski B, Koo H, Johnson RN, Tanaka S. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Ophthalmology. 2002;109:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Larsson J, Hillarp A, Olafsdottir E, Bauer B. Activated protein C resistance and anticoagulant proteins in young adults with central retinal vein occlusion. Acta Ophthalmol Scand. 1999;77:634-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Fegan CD. Central retinal vein occlusion and thrombophilia. Eye (Lond). 2002;16:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Bertram B, Remky A, Arend O, Wolf S, Reim M. Protein C, protein S, and antithrombin III in acute ocular occlusive diseases. Ger J Ophthalmol. 1995;4:332-335. [PubMed] |
| 48. | Bertelmann T, Stief T, Sekundo W, Mennel S, Nguyen N, Koss MK. Intravitreal thrombin activity is elevated in retinal vein occlusion. Blood Coagul Fibrinolysis. 2014;25:954-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Yau JW, Lee P, Wong TY, Best J, Jenkins A. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Dodson PM, Kritzinger EE. Underlying medical conditions in young patients and ethnic differences in retinal vein occlusion. Trans Ophthalmol Soc UK. 1985;104:114-119. [PubMed] |
| 51. | Kirwan JF, Tsaloumas MD, Vinall H, Prior P, Kritzinger EE, Dodson PM. Sex hormone preparations and retinal vein occlusion. Eye (Lond). 1997;11:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 52. | Ciardella AP, Yannuzzi LA, Freund KB, DiMichele D, Nejat M, De Rosa JT, Daly JR, Sisco L. Factor V Leiden, activated protein C resistance, and retinal vein occlusion. Retina. 1998;18:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Cruciani F, Moramarco A, Curto T, Labate A, Recupero V, Conti L, Gandolfo GM, Balacco Gabrieli C. MTHFR C677T mutation, factor II G20210A mutation and factor V Leiden as risks factor for youth retinal vein occlusion. Clin Ter. 2003;154:299-303. [PubMed] |
| 54. | Koizumi H, Ferrara DC, Bruè C, Spaide RF. Central retinal vein occlusion case-control study. Am J Ophthalmol. 2007;144:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Glacet-Bernard A, Leroux les Jardins G, Lasry S, Coscas G, Soubrane G, Souied E, Housset B. Obstructive sleep apnea among patients with retinal vein occlusion. Arch Ophthalmol. 2010;128:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Chou KT, Huang CC, Tsai DC, Chen YM, Perng DW, Shiao GM, Lee YC, Leu HB. Sleep apnea and risk of retinal vein occlusion: a nationwide population-based study of Taiwanese. Am J Ophthalmol. 2012;154:200-205.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Dodson PM, Kritzinger EE, Clough CG. Diabetes mellitus and retinal vein occlusion in patients of Asian, west Indian and white European origin. Eye (Lond). 1992;6:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Zhou JQ, Xu L, Wang S, Wang YX, You QS, Tu Y, Yang H, Jonas JB. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing eye study. Ophthalmology. 2013;120:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, Nguyen HP, Wang JJ, Wong TY. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1094-1101.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 60. | Hayreh SS, Zimmerman MB. Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol. 2014;132:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Haller JA, Bandello F, Belfort R, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jacques ML, Jiao J. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134-1146.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 755] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 62. | Clemett RS, Kohner EM, Hamilton AM. The visual prognosis in retinal branch vein occlusion. Trans Ophthalmol Soc UK. 1973;93:523-535. [PubMed] |
| 63. | Sartori MT, Barbar S, Donà A, Piermarocchi S, Pilotto E, Saggiorato G, Prandoni P. Risk factors, antithrombotic treatment and outcome in retinal vein occlusion: an age-related prospective cohort study. Eur J Haematol. 2013;90:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Hayreh SS, Podhajsky PA, Zimmerman MB. Central and hemicentral retinal vein occlusion: role of anti-platelet aggregation agents and anticoagulants. Ophthalmology. 2011;118:1603-1611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Glacet-Bernard A, Atassi M, Fardeau C, Romanet JP, Tonini M, Conrath J, Denis P, Mauget-Faÿsse M, Coscas G, Soubrane G. Hemodilution therapy using automated erythrocytapheresis in central retinal vein occlusion: results of a multicenter randomized controlled study. Graefes Arch Clin Exp Ophthalmol. 2011;249:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Scott IU, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA, Jumper JM, Figueroa M. SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 67. | Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, Singerman LJ, Tolentino M, Chan CK, Gonzalez VH. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 68. | Ehlers JP, Spirn MJ, Lam A, Sivalingam A, Samuel MA, Tasman W. Combination intravitreal bevacizumab/panretinal photocoagulation versus panretinal photocoagulation alone in the treatment of neovascular glaucoma. Retina. 2008;28:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Kernt M, Cheuteu RE, Cserhati S, Seidensticker F, Liegl RG, Lang J, Haritoglou C, Kampik A, Ulbig MW, Neubauer AS. Pain and accuracy of focal laser treatment for diabetic macular edema using a retinal navigated laser (Navilas). Clin Ophthalmol. 2012;6:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102-1112.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 652] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 71. | Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, Rundle AC, Rubio RG, Murahashi WY. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 634] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 72. | Coscas G, Augustin A, Bandello F, de Smet MD, Lanzetta P, Staurenghi G, Parravano MC, Udaondo P, Moisseiev E, Soubrane G. Retreatment with Ozurdex for macular edema secondary to retinal vein occlusion. Eur J Ophthalmol. 2014;24:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Capone A, Singer MA, Dodwell DG, Dreyer RF, Oh KT, Roth DB, Walt JG, Scott LC, Hollander DA. Efficacy and safety of two or more dexamethasone intravitreal implant injections for treatment of macular edema related to retinal vein occlusion (Shasta study). Retina. 2014;34:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Querques L, Querques G, Lattanzio R, Gigante SR, Del Turco C, Corradetti G, Cascavilla ML, Bandello F. Repeated intravitreal dexamethasone implant (Ozurdex®) for retinal vein occlusion. Ophthalmologica. 2013;229:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Matonti F, Meyer F, Guigou S, Barthelemy T, Dumas S, Gobert F, Hajjar C, Merite PY, Parrat E, Rouhette H. Ozurdex in the management of the macular edema following retinal vein occlusion in clinical practice. Acta Ophthalmol. 2013;91:e584-e586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Querques G, Lattanzio R, Querques L, Triolo G, Cascavilla ML, Cavallero E, Del Turco C, Casalino G, Bandello F. Impact of intravitreal dexamethasone implant (Ozurdex) on macular morphology and function. Retina. 2014;34:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Campochiaro PA, Sophie R, Pearlman J, Brown DM, Boyer DS, Heier JS, Marcus DM, Feiner L, Patel A. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 2014;121:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 275] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 78. | Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331-335. [PubMed] |
| 79. | Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, Sorenson J, Slakter JS, Freund KB, Cooney M. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 80. | Jaissle GB, Ziemssen F, Petermeier K, Szurman P, Ladewig M, Gelisken F, Völker M, Holz FG, Bartz-Schmidt KU. Bevacizumab for treatment of macular edema secondary to retinal vein occlusion. Ophthalmologe. 2006;103:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Costa RA, Jorge R, Calucci D, Melo LA, Cardillo JA, Scott IU. Intravitreal bevacizumab (avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina. 2007;27:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Matsumoto Y, Freund KB, Peiretti E, Cooney MJ, Ferrara DC, Yannuzzi LA. Rebound macular edema following bevacizumab (Avastin) therapy for retinal venous occlusive disease. Retina. 2007;27:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Rabena MD, Pieramici DJ, Castellarin AA, Nasir MA, Avery RL. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007;27:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 84. | Spandau UH, Ihloff AK, Jonas JB. Intravitreal bevacizumab treatment of macular oedema due to central retinal vein occlusion. Acta Ophthalmol Scand. 2006;84:555-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336-339. [PubMed] |
| 86. | Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, Ohtsuka H, Kitamei H, Shioya S. Two-year outcomes of intravitreal bevacizumab therapy for macular oedema secondary to branch retinal vein occlusion. Br J Ophthalmol. 2014;98:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 87. | McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, Mitchell P, Kowalski JW, Nguyen HP, Wong TY. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1113-1123.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 88. | Boyer D, Heier J, Brown DM, Clark WL, Vitti R, Berliner AJ, Groetzbach G, Zeitz O, Sandbrink R, Zhu X. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 89. | Holz FG, Roider J, Ogura Y, Korobelnik JF, Simader C, Groetzbach G, Vitti R, Berliner AJ, Hiemeyer F, Beckmann K. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol. 2013;97:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 90. | Weinberg DV, Wahle AE, Ip MS, Scott IU, VanVeldhuisen PC, Blodi BA. Score Study Report 12: Development of venous collaterals in the Score Study. Retina. 2013;33:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Im CY, Lee SY, Kwon OW. Collateral vessels in branch retinal vein occlusion. Korean J Ophthalmol. 2002;16:82-87. [PubMed] |
| 92. | Fuller JJ, Mason JO, White MF, McGwin G, Emond TL, Feist RM. Retinochoroidal collateral veins protect against anterior segment neovascularization after central retinal vein occlusion. Arch Ophthalmol. 2003;121:332-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Giuffrè G, Randazzo-Papa G, Palumbo C. Central retinal vein occlusion in young people. Doc Ophthalmol. 1992;80:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Hansen LL, Wiek J, Schade M, Müller-Stolzenburg N, Wiederholt M. Effect and compatibility of isovolaemic haemodilution in the treatment of ischaemic and non-ischaemic central retinal vein occlusion. Ophthalmologica. 1989;199:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Hayreh SS, Zimmerman MB. Ocular neovascularization associated with central and hemicentral retinal vein occlusion. Retina. 2012;32:1553-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Laatikainen L, Kohner EM, Khoury D, Blach RK. Panretinal photocoagulation in central retinal vein occlusion: A randomised controlled clinical study. Br J Ophthalmol. 1977;61:741-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | May DR, Klein ML, Peyman GA, Raichand M. Xenon arc panretinal photocoagulation for central retinal vein occlusion: a randomised prospective study. Br J Ophthalmol. 1979;63:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 99. | Fong AC, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol. 1993;37:393-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Walters RF, Spalton DJ. Central retinal vein occlusion in people aged 40 years or less: a review of 17 patients. Br J Ophthalmol. 1990;74:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Hayreh SS, Klugman MR, Podhajsky P, Kolder HE. Electroretinography in central retinal vein occlusion. Correlation of electroretinographic changes with pupillary abnormalities. Graefes Arch Clin Exp Ophthalmol. 1989;227:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Shin HJ, Shin KC, Chung H, Kim HC. Change of retinal nerve fiber layer thickness in various retinal diseases treated with multiple intravitreal antivascular endothelial growth factor. Invest Ophthalmol Vis Sci. 2014;55:2403-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995;102:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 361] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 104. | Jonas J, Paques M, Monés J, Glacet-Bernard A. Retinal vein occlusions. Dev Ophthalmol. 2010;47:111-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 105. | Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, Chan CK, Gonzalez VH, Singerman LJ, Tolentino M. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127:1115-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 106. | Kuppermann BD, Haller JA, Bandello F, Loewenstein A, Jiao J, Li XY, Whitcup SM. Onset and duration of visual acuity improvement after dexamethasone intravitreal implant in eyes with macular edema due to retinal vein occlusion. Retina. 2014;34:1743-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Shaikh AH, Petersen MR, Sisk RA, Foster RE, Riemann CD, Miller DM. Comparative effectiveness of the dexamethasone intravitreal implant in vitrectomized and non-vitrectomized eyes with macular edema secondary to central retinal vein occlusion. Ophthalmic Surg Lasers Imaging Retina. 2013;44:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Epstein DL, Algvere PV, von Wendt G, Seregard S, Kvanta A. Bevacizumab for macular edema in central retinal vein occlusion: a prospective, randomized, double-masked clinical study. Ophthalmology. 2012;119:1184-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 109. | Epstein DL, Algvere PV, von Wendt G, Seregard S, Kvanta A. Benefit from bevacizumab for macular edema in central retinal vein occlusion: twelve-month results of a prospective, randomized study. Ophthalmology. 2012;119:2587-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 110. | Ferrara DC, Koizumi H, Spaide RF. Early bevacizumab treatment of central retinal vein occlusion. Am J Ophthalmol. 2007;144:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 111. | Figueroa MS, Contreras I, Noval S, Arruabarrena C. Results of bevacizumab as the primary treatment for retinal vein occlusions. Br J Ophthalmol. 2010;94:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Wroblewski JJ, Wells JA, Adamis AP, Buggage RR, Cunningham ET, Goldbaum M, Guyer DR, Katz B, Altaweel MM. Pegaptanib sodium for macular edema secondary to central retinal vein occlusion. Arch Ophthalmol. 2009;127:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Brown DM, Heier JS, Clark WL, Boyer DS, Vitti R, Berliner AJ, Zeitz O, Sandbrink R, Zhu X, Haller JA. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 114. | Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ, Kazmi H, Ma Y, Stemper B, Zeitz O. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014;121:1414-1420.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 115. | Korobelnik JF, Holz FG, Roider J, Ogura Y, Simader C, Schmidt-Erfurth U, Lorenz K, Honda M, Vitti R, Berliner AJ. Intravitreal Aflibercept Injection for Macular Edema Resulting from Central Retinal Vein Occlusion: One-Year Results of the Phase 3 GALILEO Study. Ophthalmology. 2014;121:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 116. | Singer M, Tan CS, Bell D, Sadda SR. Area of peripheral retinal nonperfusion and treatment response in branch and central retinal vein occlusion. Retina. 2014;34:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 117. | Chhablani J, Mathai A, Rani P, Gupta V, Arevalo JF, Kozak I. Comparison of conventional pattern and novel navigated panretinal photocoagulation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55:3432-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 118. | Beutel J, Peters S, Lüke M, Aisenbrey S, Szurman P, Spitzer MS, Yoeruek E, Grisanti S. Bevacizumab as adjuvant for neovascular glaucoma. Acta Ophthalmol. 2010;88:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 119. | Moraczewski AL, Lee RK, Palmberg PF, Rosenfeld PJ, Feuer WJ. Outcomes of treatment of neovascular glaucoma with intravitreal bevacizumab. Br J Ophthalmol. 2009;93:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 120. | Wittström E, Holmberg H, Hvarfner C, Andréasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. Eur J Ophthalmol. 2012;22:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 121. | Pielen A, Feltgen N, Isserstedt C, Callizo J, Junker B, Schmucker C. Efficacy and safety of intravitreal therapy in macular edema due to branch and central retinal vein occlusion: a systematic review. PLoS One. 2013;8:e78538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 122. | Singer MA, Bell DJ, Woods P, Pollard J, Boord T, Herro A, Porbandarwalla S. Effect of combination therapy with bevacizumab and dexamethasone intravitreal implant in patients with retinal vein occlusion. Retina. 2012;32:1289-1294. [PubMed] |
| 123. | Kim YJ, Sung KR, Lee KS, Joe SG, Lee JY, Kim JG, Yoon YH. Long-term effects of multiple intravitreal antivascular endothelial growth factor injections on intraocular pressure. Am J Ophthalmol. 2014;157:1266-1271.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 124. | Arevalo JF, Garcia RA, Wu L, Rodriguez FJ, Dalma-Weiszhausz J, Quiroz-Mercado H, Morales-Canton V, Roca JA, Berrocal MH, Graue-Wiechers F. Radial optic neurotomy for central retinal vein occlusion: results of the Pan-American Collaborative Retina Study Group (PACORES). Retina. 2008;28:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Hayreh SS. Management of central retinal vein occlusion. Ophthalmologica. 2003;217:167-188. [PubMed] |
| 126. | Spaide RF, Klancnik JM, Gross NE. Retinal choroidal collateral circulation after radial optic neurotomy correlated with the lessening of macular edema. Retina. 2004;24:356-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Hasselbach HC, Ruefer F, Feltgen N, Schneider U, Bopp S, Hansen LL, Hoerauf H, Bartz-Schmidt U, Roider J. Treatment of central retinal vein occlusion by radial optic neurotomy in 107 cases. Graefes Arch Clin Exp Ophthalmol. 2007;245:1145-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 128. | Opremcak EM, Bruce RA, Lomeo MD, Ridenour CD, Letson AD, Rehmar AJ. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina. 2001;21:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 129. | Williamson TH, Poon W, Whitefield L, Strothidis N, Jaycock P. A pilot study of pars plana vitrectomy, intraocular gas, and radial neurotomy in ischaemic central retinal vein occlusion. Br J Ophthalmol. 2003;87:1126-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 130. | Nomoto H, Shiraga F, Yamaji H, Kageyama M, Takenaka H, Baba T, Tsuchida Y. Evaluation of radial optic neurotomy for central retinal vein occlusion by indocyanine green videoangiography and image analysis. Am J Ophthalmol. 2004;138:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 131. | Martínez-Jardón CS, Meza-de Regil A, Dalma-Weiszhausz J, Leizaola-Fernández C, Morales-Cantón V, Guerrero-Naranjo JL, Quiroz-Mercado H. Radial optic neurotomy for ischaemic central vein occlusion. Br J Ophthalmol. 2005;89:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | McAllister IL, Douglas JP, Constable IJ, Yu DY. Laser-induced chorioretinal venous anastomosis for non-ischemic central retinal vein occlusion: evaluation of the complications and their risk factors. Am J Ophthalmol. 1998;126:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 133. | Peyman GA, Kishore K, Conway MD. Surgical chorioretinal venous anastomosis for ischemic central retinal vein occlusion. Ophthalmic Surg Lasers. 1999;30:605-614. [PubMed] |
| 134. | Sharma A, D’Amico DJ. Medical and surgical management of central retinal vein occlusion. Int Ophthalmol Clin. 2004;44:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Furino C, Ferrari TM, Boscia F, Cardascia N, Sborgia L, Reibaldi M, Ferreri P, Sborgia C. Combined radial optic neurotomy, internal limiting membrane peeling, and intravitreal triamcinolone acetonide for central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:422-425. [PubMed] |
| 136. | Liang XL, Chen HY, Huang YS, Au Eong KG, Yu SS, Liu X, Yan H. Pars plana vitrectomy and internal limiting membrane peeling for macular oedema secondary to retinal vein occlusion: a pilot study. Ann Acad Med Singapore. 2007;36:293-297. [PubMed] |
| 137. | Mandelcorn MS, Nrusimhadevara RK. Internal limiting membrane peeling for decompression of macular edema in retinal vein occlusion: a report of 14 cases. Retina. 2004;24:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 138. | Park DH, Kim IT. Long-term effects of vitrectomy and internal limiting membrane peeling for macular edema secondary to central retinal vein occlusion and hemiretinal vein occlusion. Retina. 2010;30:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Kumagai K, Furukawa M, Ogino N, Uemura A, Larson E. Long-term outcomes of vitrectomy with or without arteriovenous sheathotomy in branch retinal vein occlusion. Retina. 2007;27:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Kumagai K, Furukawa M, Ogino N, Larson E, Uemura A. Long-term visual outcomes after vitrectomy for macular edema with foveal hemorrhage in branch retinal vein occlusion. Retina. 2007;27:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 141. | Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 142. | DeCroos FC, Shuler RK, Stinnett S, Fekrat S. Pars plana vitrectomy, internal limiting membrane peeling, and panretinal endophotocoagulation for macular edema secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147:627-633.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 143. | Lu N, Wang NL, Wang GL, Li XW, Wang Y. Vitreous surgery with direct central retinal artery massage for central retinal artery occlusion. Eye (Lond). 2009;23:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Uemura A, Yamamoto S, Sato E, Sugawara T, Mitamura Y, Mizunoya S. Vitrectomy alone versus vitrectomy with simultaneous intravitreal injection of triamcinolone for macular edema associated with branch retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2009;40:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 145. | Arai M, Yamamoto S, Mitamura Y, Sato E, Sugawara T, Mizunoya S. Efficacy of vitrectomy and internal limiting membrane removal for macular edema associated with branch retinal vein occlusion. Ophthalmologica. 2009;223:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 146. | Oh IK, Kim S, Oh J, Huh K. Long-term visual outcome of arteriovenous adventitial sheathotomy on branch retinal vein occlusion induced macular edema. Korean J Ophthalmol. 2008;22:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 148. | Saika S, Tanaka T, Miyamoto T, Ohnishi Y. Surgical posterior vitreous detachment combined with gas/air tamponade for treating macular edema associated with branch retinal vein occlusion: retinal tomography and visual outcome. Graefes Arch Clin Exp Ophthalmol. 2001;239:729-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 149. | Noma H, Funatsu H, Sakata K, Mimura T, Hori S. Macular microcirculation before and after vitrectomy for macular edema with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2010;248:443-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 150. | Radetzky S, Walter P, Fauser S, Koizumi K, Kirchhof B, Joussen AM. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004;242:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 151. | Weiss JN, Bynoe LA. Injection of tissue plasminogen activator into a branch retinal vein in eyes with central retinal vein occlusion. Ophthalmology. 2001;108:2249-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 152. | Feltgen N, Junker B, Agostini H, Hansen LL. Retinal endovascular lysis in ischemic central retinal vein occlusion: one-year results of a pilot study. Ophthalmology. 2007;114:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 153. | Osterloh MD, Charles S. Surgical decompression of branch retinal vein occlusions. Arch Ophthalmol. 1988;106:1469-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Avci R, Inan UU, Kaderli B. Evaluation of arteriovenous crossing sheathotomy for decompression of branch retinal vein occlusion. Eye (Lond). 2008;22:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 155. | Chung EJ, Lee H, Koh HJ. Arteriovenous crossing sheathotomy versus intravitreal triamcinolone acetonide injection for treatment of macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2008;246:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 156. | Yamane S, Kamei M, Sakimoto S, Inoue M, Arakawa A, Suzuki M, Matsumura N, Kadonosono K. Matched control study of visual outcomes after arteriovenous sheathotomy for branch retinal vein occlusion. Clin Ophthalmol. 2014;8:471-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 157. | Kadonosono K, Yamane S, Arakawa A, Inoue M, Yamakawa T, Uchio E, Yanagi Y, Amano S. Endovascular cannulation with a microneedle for central retinal vein occlusion. JAMA Ophthalmol. 2013;131:783-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 158. | McIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions for branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:835-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |