Peer-review started: August 22, 2016
First decision: October 21, 2016
Revised: November 10, 2016
Accepted: November 27, 2016
Article in press: November 29, 2016
Published online: March 28, 2017
Processing time: 215 Days and 18.9 Hours
Sleep is essential for maintaining normal physiological processes. It has been broadly divided into rapid eye movement sleep (REMS) and non-REMS (NREMS); one spends the least amount of time in REMS. Sleep (both NREMS and REMS) disturbance is associated with most altered states, disorders and pathological conditions. It is affected by factors within the body as well as the environment, which ultimately modulate lifestyle. Noradrenaline (NA) is one of the key molecules whose level increases upon sleep-loss, REMS-loss in particular and it induces several REMS-loss associated effects and symptoms. The locus coeruleus (LC)-NAergic neurons are primarily responsible for providing NA throughout the brain. As those neurons project to and receive inputs from across the brain, they are modulated by lifestyle changes, which include changes within the body as well as in the environment. We have reviewed the literature showing how various inputs from outside and within the body integrate at the LC neuronal level to modulate sleep (NREMS and REMS) and vice versa. We propose that these changes modulate NA levels in the brain, which in turn is responsible for acute as well as chronic psycho-somatic disorders and pathological conditions.
Core tip: Sleep is affected by many internal factors as well as lifestyle changes and vice versa. Noradrenaline (NA) is one of the molecules affected by lifestyle as well as sleep-loss; rapid eye movement sleep-loss in particular. Many of the sleep-loss associated cellular-molecular-behavioral and patho-physiological changes are induced by NA. Therefore, we propose that disciplined sleep habit, which would maintain optimum level of NA, is essential for leading healthy life.
- Citation: Mehta R, Singh A, Mallick BN. Disciplined sleep for healthy living: Role of noradrenaline. World J Neurol 2017; 7(1): 6-23
- URL: https://www.wjgnet.com/2218-6212/full/v7/i1/6.htm
- DOI: https://dx.doi.org/10.5316/wjn.v7.i1.6
The classical proverb of wisdom…..“health is wealth”…..has been expressed in almost all cultures and ages in some form or the other. To enjoy life, one needs to be physically and mentally healthy. “Health” constitutes two aspects, the physical body and the mind; the substrate for the latter is the brain. Directly or indirectly the physical substrates, the body and the brain are constantly interacting with their immediate as well as distant surroundings, the environment. The interactions are complex; synthesis of one is often coupled with transformation of another; however they always remain in equilibrium for normal and healthy living. Disturbance or a shift in such equilibrium results in disease or altered state, which if gets rectified, cure or recovery may follow; however, in the absence of recovery, irreversible damage may precipitate/accumulate. In general, waking and sleep are among the fundamental instinct behaviors. Broadly and relative to each other, waking is considered to be energy consuming process (catabolic) while sleep (anabolic) as natural resuscitation. Until about mid-twentieth century sleep was largely considered to be a passive phenomenon; however, consistent research has proven that it is an active process regulated by the brain. Sleep researchers often face questions like why sleep is necessary, how much daily sleep is needed and how sleep loss exerts its effects and so on. In this review we will discuss the role of sleep, rapid eye movement sleep (REMS) in particular, in maintaining the level of a common factor, noradrenaline (NA), disturbance of which induces sleep-loss induced/associated effects.
Sleep is a spontaneous, reversible state of reduced sensitivity when the consciousness remains in a subdued state; during this state the body recuperates by replenishing the exhausted resources. Organisms have faced environmental and physiological challenges through evolution, which have impacted the quality and quantity of sleep-wake behavior in various species[1-4]. Also, the amount of sleep varies among different groups of individuals and populations depending on the lifestyle and environmental conditions. However, the modern lifestyle threatens the sleep behavior and pattern, which affect the health negatively. For example, the circadian misalignment usually seen in shift workers, truck drivers, frequent travelers, health support givers (nurses, etc.), those on special operation missions, etc., alters the natural sleep durations as well as cycle resulting in patho-physiological changes including fatigue, irritability, anxiety, restlessness, frequent daytime naps, lack of concentration, decreased performance at work[5]. These sleep-disturbances exert a global effect on body physiology leading to short (acute) - and long (chronic)-term pathological conditions. Further, these changes inflict significant hidden costs to the individual as well as to the society at large, which is not worth trading-off against a few hours of apparent immediate wakefulness. However, as we are constantly exposed to various psycho-social-environmental conditions which modulate sleep, with the best of efforts it is almost impossible to completely avoid sleep disturbance; however, we may attempt avoiding the effects if we know the reasons (etiology). Keeping this in mind, here we reviewed how even environmental and psycho-social factors modulate sleep and correlated them with changes in the level of a common physiological factor, NA, which is responsible for sleep-loss related effects.
Sleep is not a homogenous state; the least fraction of sleep time is spent in a unique state when one dreams. Based on electrophysiological signals recorded from the brain, eye- and neck-muscles sleep has been broadly divided into REMS and non-REMS (NREMS). REMS is a unique physiological process expressed in humans and most likely in other higher order vertebrates in evolution possessing evolved brain. Role of REMS has been implicated with several physiological processes including that it maintains brain excitability and thus maintains “house-keeping function of the brain”[6].
REMS disturbance has been reported to be associated with most physiological dysfunctions and pathological conditions including mood, mania, bipolar-disorders, Alzheimer’s (AD) and Parkinson’s (PD), epilepsy, narcolepsy, cognitive impairment, cardiovascular and respiratory disorders[7-13], infections, fever and trauma[14,15]. REMS is regulated by the interactions of neurons located in different brain regions forming complex neural network. Notably the NA-ergic neurons in brainstem are continuously active during wake as well as NREMS and cease activity during REMS, while they continue firing during REMS deprivation (REMSD). Upon REMS loss, the level of NA increases in the brain, which has been suggested to induce many REMS loss associated acute and chronic effects. Isolated studies have shown that several of the symptoms, e.g., hypertension, hyperglycemia, hyper-excitability, lack of concentration, memory loss, psycho-somatic disorders, etc., are reported to be modulated by increased NA[16-24]. Thus, there are enough convincing reasons to accept that disciplined sleep, which includes REMS, maintains optimum levels of NA in the brain and therefore, it is necessary for healthy living, which we would elaborate in this review. However, for complete understanding, it is necessary we understand how REMS is regulated by the brain and hence, first, we would discuss in short the essential basic mechanism(s) of REMS regulation.
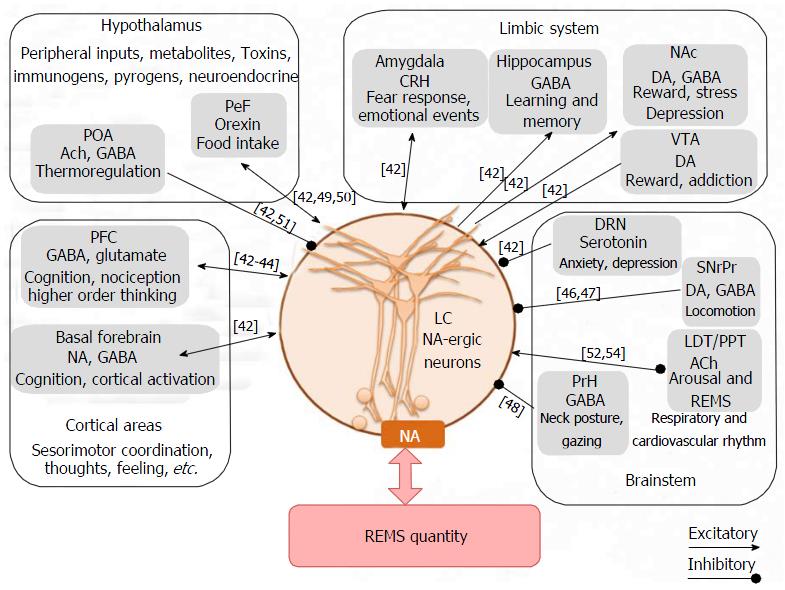
Aserinsky first objectively identified the REMS state in humans as having desynchronized EEG, phasic eye movements in the EOG and complete loss of muscle tone in the EMG recorded from the antigravity muscles[25]. Later, it was identified in rat, cat and many other mammalian species. By the time REMS was identified it was known that rostral brain stem reticular formation is important for EEG desynchronization and waking, while the caudal part is responsible for EEG synchronization and sleep (NREMS and REMS were not classified until then). Transection and lesion studies identified that the areas responsible for REMS regulation are also located in the brain stem[26,27]. Some studies reported that neurons in the medial[28] and the lateral[29,30] pontine reticular structure were critical for REMS. Transection made rostral or caudal to the pons showed that signs identifying REMS were expressed in the portion of the brain which remained connected with the pons[31,32]. The pons includes two major nuclei, the LC and the laterodorsal and pedunculopontine-tegmentum (LDT/PPT), which have been reported to be important for REMS regulation[33-35]. Non-pontine brain regions like perifornical area[36,37], preoptic area in hypothalamus[38-40], basal ganglia[41], nucleus accumbens, ventral tegmental area, amygdala[42], basal forebrain, prefrontal cortex[43,44], dorsal raphe nucleus[45], substantia nigra[46,47], prepositus hypoglossus[48], etc., have been reported to modulate REMS.
Based on the firing patterns of neurons associated with waking-NREMS-REMS, those neurons almost exclusively active during REMS were classified as REM-ON type, while those shut-off during REMS were termed as REM-OFF neurons. The former were identified primarily in the LDT/PPT, while the latter in the LC[49-54]. Interaction between LC-NA-ergic and PPT-acetylcholine (ACh)-ergic neurons has been proposed to regulate REMS and that has been the focus of several reviews[49,55]. Briefly, REM-OFF neurons are inactivated for activation of the REM-ON neurons and initiation of REMS. It has been proposed that cessation of LC neurons is a pre-requisite condition for REMS generation[56]. Therefore, the behavior of the LC neurons, their afferents (inputs) and efferents (outputs) for REMS regulation will be discussed in brief.
The LC neurons are normally silent during REMS and continue to remain active during REMSD[57]. Stimulation and inactivation of the LC neurons had opposite effects on REMS. For instance, inactivation of LC neurons by local cooling, 6-OHDA induced selective loss of NA-ergic neurons in LC or lesion of LC neurons increased REMS[58-61], whereas stimulation of LC neurons decreased REMS[62-64]. Tyrosine hydroxylase (TH) mRNA, TH as well as NA levels in brain were higher in REMS deprived animals[65]. NA concentration has been reported to be lower in brain regions[66,67] and blood[68] during REMS as compared to NREMS and wakefulness, while NA levels were elevated during REMSD[65]. Expression profiles of NA-ergic receptor density have been inversely correlated with ontogenetic development of REMS[69]. Therefore, it has been proposed that one of the functions of REMS is to maintain NA-level in the brain which in turn maintains brain excitability and thus serves house-keeping function of the brain[6].
Many studies investigated influence of various neurotransmitters and their diverse effects on LC neurons. Agonist and antagonist of various neurotransmitters injected into the LC modulated REMS. It was observed in cats that infusion of NA into the LC decreased REMS while adrenergic antagonist increased REMS[70]. Administration of ACh into LC decreased REMS[71]. Infusion of GABA and its agonist, muscimol into the LC increased[35,72], while GABA antagonist, picrotoxin[73], bicuculine[74] and baclophen[72] decreased REMS. Orexin (Orx)-ergic agonist injection into the LC has been shown to reduce REMS[75], whereas knockdown of Orx-ergic receptors in the LC increased REMS[76]. Electrical or pharmacological stimulation of Orx-ergic neurons reduced REMS, whereas the effects on REMS were abolished by simultaneously blocking Orx action in LC[75,77,78]. Infusion of a somatostatin antagonist into LC also resulted in marked decrease in REMS[79]. Application of serotonin (5-HT) into LC inhibited basal neuronal discharge rate; however, the effects on REMS was not studied[45,80]. Various studies have shown that although dopamine (DA) may modulate REMS, detailed mechanism and site of action, particularly on the LC neurons, are not known[81]. The projections from the GABA-ergic neurons from substantia nigra onto LC-NA-ergic terminals have been suggested to act pre-synaptically and fine tune NA release over PPT ACh-ergic neurons and initiate REMS[47,55]. Thus, the LC neurons are modulated by many of the neurotransmitters in the brain. Further, LC neurons project to various areas of the brain including on the ACh-ergic and Orx-ergic neurons and modulate physiological processes and behaviors including REMS[42]. Thus, complex communications among various neurons containing different neurotransmitters affect the LC neuronal activities, which would modulate release of NA and regulate REMS (Figure 1).
Therefore, we have sufficient evidence that REMS and NA-level in the brain are closely linked and they modulate each other; also REMS tends to maintain NA-level in the brain. Notwithstanding, it is also known that NA affects many other physiological processes and NA is modulated in many pathological conditions; further, REMS as well as many of the pathophysiological conditions are associated with lifestyle changes[11,22,82-84]. Hence, we propose that environmental and lifestyle processes and its associated changes might affect either NA levels or REMS and thereby may be responsible for many of the acute as well as chronic patho-physiological conditions.
Modernity and stormy lifestyle have resulted into reduced sleep. Changes in lifestyle which include shorter sleeping hours, electricity and artificial lights at night, long television viewing and low physical activity have precipitated various health disorders/problems. Several studies have reported the effects of changes in lifestyle, ambient as well as body temperature and diet on NREMS and REMS. Therefore, loss of REMS cannot be overlooked and the factors affecting REMS merit attention. Physical fitness, nutritious food, stress reduction, exercises, lifestyle changes motivating positive thinking are essential for maintenance of quality sleep including REMS. Even excess sleep may be harmful; as the Greek physician Hippocrates wrote “Disease exists if either sleep or watchfulness be excessive”. Thus, monitoring quality as well as quantity of sleep for the maintenance of healthy living beckons serious attention. Disturbance/loss of REMS is often associated with multiple symptoms, which might be an influence of complex circuitry involved in REMS regulation, NA being one of them.
In mammals sleep is strongly associated with thermoregulation and temperature maintenance is a key determinant of sleep[85]. The core body temperature varies along with the sleep-wake rhythm. In healthy individuals and under normal environmental conditions, sleep propensity and body temperature vary inversely across day and night[86]. As the body and brain temperatures decrease during NREMS, while it tends to increase during REMS, it has been proposed that one of the functions of REMS is to maintain the brain temperature by “warming the CNS”[87].
Among the sleep stages, REMS is more sensitive to changes in ambient temperature; its cycle length significantly decreases with an increase in ambient temperature from 13 °C to 25 °C[88]. In humans, sensitivity to hot or cold stimulation is reduced during REMS compared to NREMS and wakefulness[89,90]; however, it is not completely abolished. It has been observed in animal studies that thermoregulatory response as well as thermo-sensitivity of most of the hypothalamic preoptic neurons decrease during REMS[91], which suggested a causal relationship between them. Medial preoptic area in the brain is responsible for thermoregulation[92,93]; the thermosensitive neurons in the medial preoptic area possess α1-ARs and they are modulated by stimulation of brainstem ascending reticular activating system[94]. NA is involved in the neural regulation of REMS as well as body temperature[69,95]. Increased turnover of NA in specific nerve terminals of hypothalamus was observed in rats upon exposure to mild thermal stress[95]. As NA stimulates metabolic rate[96,97], it could elevate body temperature and trigger heat dissipation for thermoregulation. It has been suggested that one of the reasons for reduced core temperature during sleep is reduced NA-ergic peripheral vasoconstrictor tone resulting in increased peripheral blood flow and dissipation of heat[98,99]. Also, NA has been shown to induce hypothermia by acting on α1-ARs in the medial preoptic area[100,101]. Abnormalities in the body temperature rhythm are associated with insomnia and associated symptoms[102]. In fact, in a study the body temperature was monitored every 24 h during 10 d REMSD in rats. It was found that there was hyperthermia during initial about 4 d of REMSD; thereafter there was hypothermia if the deprivation continued. The authors explained the findings in relation to the REMSD associated elevated levels of NA[103]. Thus, REMS and associated change in NA level play crucial role in thermoregulation and as a corollary their disturbance would affect the body physiology.
“The sleep of a laboring man is sweet” is a beautiful phrase from biblical times written in the Holy book Ecclesiastes. Several research groups have examined the effects of exercise on sleep. Important relationship has been found between sleep and exercise although both the behaviors are mediated by physiologically different mechanisms. Regular physical activity promotes sleep, improves sleep quality and reduces day time sleepiness[104]. It is important to note that moderate amount of exercise is beneficial to health and upon exposure to severe acute exercise a reduction and a delay in REMS onset latency as well as an increase in slow wave sleep was observed[105-108]. These effects could be due to the stress associated with intense exercise. The observed increased latency for the onset of REMS was due to the significant increase in NA[109]. Several other studies have also reported significantly increased concentration of NA after exercise[110,111]. These results support that elevated levels of NA might reduce REMS and vice versa and that in turn might affect physiological processes.
Extensive research towards understanding the effects of exercise has shown that several brain areas receive various feedback inputs from peripheral structures such as muscles and joints which then influence the brain functions[112]. Exercise is beneficial for synaptic plasticity which could be due to molecules like brain derived neurotrophic factor that favors neuronal growth and plasticity[113,114]. It increases long term potentiation[115,116], neurogenesis[117] and thus has beneficial effects on learning and memory[118]. As a mechanism of action it may be said that sleep including REMS and NA[119] have been shown to enhance hippocampus dependent memory[120]. In support, sleep and REMS loss has been reported to reduce learning and memory formation[121,122]. Exercised sleep deprived rats learn and perform normally in comparison to sedentary/control sleep deprived rats in whom the learning and memory were severely impaired[123]. Regular exercise protocol prevents long term potentiation deficits and memory impairment induced by sleep deprivation (SD)[124]. As REMS-loss has been shown to elevate NA level in the brain[65] and that NA has been shown to induce apoptosis and neuronal loss[125,126], it appears that a critical level of NA in the brain is essential for normal healthy brain functioning including memory and plasticity. As REMS plays a critical role in maintenance of brain level of NA to its optimum levels, we propose that optimum REMS is essential for healthy living.
Several epidemiological studies have shown that regular physical activity, such as running, has favorable physiological effects. It reduces the risk of neurodegenerative disorders like AD, dementia which are also associated with REMS disorder; exercise also promotes functional recovery from brain injury[127-129]. Isolated studies in humans have shown exercise in combination with mood stabilizers as an adjunctive therapy improves manic symptoms of bipolar disorder, which is strongly influenced by environmental and genetic factors as well as inappropriate amount of sleep[130-132]. Moderate aerobic exercise also improves sleep quality, anti-depressive response and immune function in patients with chronic insomnia[133]. Thus, one of the possibilities is that the exercise mediates its beneficial effect on the body physiology by maintaining sleep, NREMS and REMS.
Yoga, an ancient Indian practice based knowledge, is apparently a holistic set of mind-body exercise. Of late it has become popular due to its benefits on physical and mental health and for amelioration of symptoms associated with many altered and patho-physiological conditions including insomnia[134,135]. For example, Yoga has been shown to reduce the severity of restless leg syndrome; it improved sleep in women with restless leg syndrome[136,137]. Yogic exercises have been reported to improve sleep qualitatively in cancer patients[138]. One of the possibilities is that at least some of the benefits of the Yoga could be by modulating the quality of sleep of an individual.
Sleep plays an important role in the metabolic control of the body[139,140]. Recently, a relationship between circadian clock and nutrition has been referred to as “chrono-nutrition”[141]. Not only nutrients quality and quantity, the meal timings are also important and thus affect the biological (circadian) clock.
Amino acids like tryptophan, glutamate, and tyrosine are the precursor molecules for the biosynthesis of neurotransmitters like serotonin, GABA, NA and DA respectively and they all are involved in sleep-wake regulation. Therefore, diet can influence the rate of biosynthesis and functions of these neurotransmitters and affect physiological processes including sleep-wake cycle. Neurotransmitter Orx regulates feeding behavior[142] and also enhances wakefulness which could be by activating the LC-NAergic neurons[77]. GABA promotes sleep and is also a food ingredient[143]. Loss of GABA and other sleep related nutrients from whole grains as in polished grain in diet is considered to be one of the key factors for insomnia[144]. Certain other dietary nutrients like calcium, magnesium and potassium are also associated with improving sleep quality[143].
Body mass index is closely associated with sleep since obese and overweight individuals mostly have shorter sleep duration compared with normal subjects[145]. This association between sleep duration and body mass index has also been reported in patients with sleep disorders like obstructive sleep apnea, narcolepsy, insomnia, restless leg syndrome or periodic limb movements during sleep[146]. Also, habitual short/long sleep duration as well as intervening sleep restriction have been suggested as a risk factor for weight gain, obesity[147], insulin resistance, type 2 diabetes[148,149] and hypertension[150,151]. Obese adults show high amount of NREMS though very low REMS percentage[145]. A low carbohydrate diet with high fat content increases NREMS while REMS is reduced[152,153]. This might be due to higher metabolic demand of REMS[154] because greater glucose utilization occurs during this stage as compared to NREMS[155]. Different studies have shown that consuming food closer to the bed time negatively influences sleep; it increases REMS latency and decreases REMS percentage in healthy individuals[156]. Dietary constituents influence sleep and adequate sleep protects against several nutritional and metabolic disorders including insulin resistance, diabetes[157-159], obesity[160], dyslipidemia[146]. Thus, maintenance of proper diet and sleep patterns is a necessity for healthy living.
Light is one of the most important external factors for maintaining normal healthy living. Depending on the intensity, light affects sleep directly by preventing us from falling asleep and indirectly by altering the circadian clock. Light not only regulates sleep timing but also elicits acute changes in many behaviors. As night approaches reduced light intensity is detected by the photoreceptors; in higher animals they are primarily located in the retina. The retina projects to the suprachiasmatic nucleus of the hypothalamus, which is the biological master clock and the site for homeostatic regulation of circadian functions[161]. Removal of suprachiasmatic nucleus abolishes the circadian rhythm including that of sleep-waking in individuals[162]. The effects of circadian phase shift and SD were more pronounced on NREMS[163]. The NREMS was selectively enhanced during short light periods while REMS was elevated during short dark periods[164].
Exposure to light in late night hours resets the internal clock and makes it difficult to return to sleep. For example, in shift workers or travelers across time zones, the internal clock adjusts to the altered day-night cycle which mostly predisposes these individuals to insomnia when trying to sleep outside their internal temporal phase. Definitely this is a serious concern for such individuals like pilots, physicians, nurses, public safety workers, police, etc. These individuals may fall asleep during work or driving and may cause threat to life in addition to other disorders. Sleep was recorded in humans with morning type and evening type sleep for 3 successive nights and then after shifting sleep during daytime for next 3 d. Although night time sleep was not significantly affected, the day time sleep was shortened; REMS episode was found to be longer without much difference in NREMS[163]. Thus, it appears that a homeostatic mechanism operates to regulate REMS quantity and it suggests that circadian rhythm related periodic appearance of REMS is necessary to avoid sleep related disorders.
Different neurotransmitters are involved to regulate the sleep response to light. For instance, the levels of melatonin, NA, ACh decrease, while serotonin increases under the influence of light[165,166]; NA exhibits a clear circadian variation[5]. NA levels were highest in the brain during night in rat, a nocturnal animal while during the day in rabbit and cat[167]. Notwithstanding, isolated studies have shown that exposure of light to sites other than eyes (extra-ocular light) during sleep also significantly increased REMS[168]. This suggests that light exposed to sites other than eyes may also influence brain functions although the mechanism of such action needs further investigation[169]. Thus, the light-dark is likely to affect the sleep-waking rhythm, affecting various neurotransmitters, or vice versa, which then affect other physiological processes.
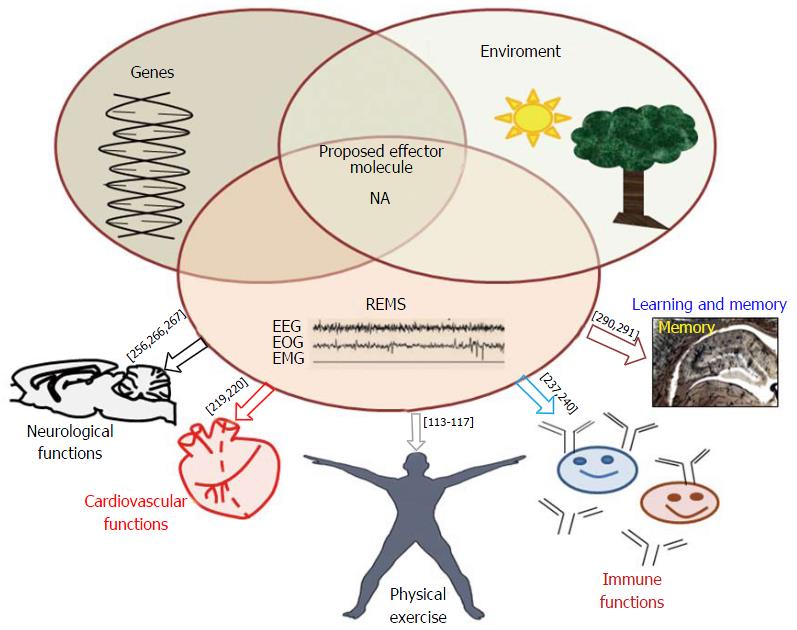
As discussed above, the brain modulates various behaviors including waking, NREMS and REMS by releasing biomolecules, the neurotransmitters. The latter are directly or indirectly modulated by gene expression, which are affected by modifications on the DNA per se or at the epigenetic level.
The susceptibility and vulnerability to diseases are strongly influenced by genetic makeup of individual as well as environmental conditions[170-173]. However, it is neither the genetic make-up nor the environment alone but their interactions, which decide the phenotype of an organism and expression of behaviors in health and diseases. Here, we would discuss these events with particular reference to NA and REMS in acute and chronic conditions in health and diseases.
Point mutation of prion protein-gene was possibly the first to be linked to human sleep disorder, fatal familial insomnia[174]. Later in 1999 through genetic studies, Orx was shown to be involved in human narcolepsy[175,176]. Sleep-wakefulness is associated with widespread changes in gene expressions in the mammalian brain[177]. REMS is a complex phenomenon and its regulation is multifactorial, therefore, many genes and their interactions are likely to contribute to its regulation and pathologies associated to REMS disorders, which are increasingly gaining recognition[178]. However, presently most of the studies have correlated gene expressions with loss of total sleep, which includes loss of both NREMS as well as REMS; not many studies have correlated changes in gene expressions upon exclusive loss of REMS. In 1970s and 1980s it was known that transcription is accelerated during sleep[179] and sleep promotes mRNA translation. Microarray experiments have shown that the patterns of gene expressions vary during sleep and wakefulness[180,181]. Subsequently, it has been demonstrated that extended wakefulness, due to SD, hinders the expression of several genes including those required for memory formation and learning[182-185].
During wakefulness, the NA-ergic system induces increased expression of genes encoding BDNF, NGF-1 and phosphorylated cAMP response element binding (pCREB) protein (required for neurogenesis and memory among other functions), c-fos (immediate early gene expression for several proteins), Arc and BiP[181]. Inactivity of NA-ergic system during sleep prevents the expressions of BDNF, Arc and pCREB, thus causing impairment of long-term memory formation during sleep[180]. Transcriptional regulatory molecule CREB, which is critical for synaptic plasticity and memory consolidation[186], also regulates the expression of TH gene which is essential for biosynthesis of NA and this factor in turn modulates REMS. Also, NA regulates the expression of transcription factors like CREB involved in memory formation and consolidation.
Sleep has been suggested to have an anabolic function by replenishing the wakefulness associated loss of energy (glycogen stores). This has been reported to be achieved by increasing protein targeting to glycogen, decreasing glycogen synthase and glycogen phosphorylase mRNA[187]. Further, this increased protein targeting to glycogen mRNA during wakefulness has been suggested to be due to increased level of NA during wakefulness.
In PD there is reduced expression of TH and dopamine β hydroxylase (required for biosynthesis of NA) resulting in reduced NA synthesis along with the loss of nigro-striatal DA-ergic and some other catecholamine neurons. These have been proposed to be caused by unknown exogenous environmental factors and by endogenous genetic factors suggesting interaction(s) between genes and environment[188]. However, although the role and mechanism of action of NA affecting wakefulness-NREMS-REMS have been investigated, their genetic regulation in health and diseases, particularly in relation to sleep and REMS disturbance needs to be studied.
Gene expression induces transcription and involves several processes including epigenetic modifications. Some of the important epigenetic changes are DNA methylation and chromatin remodeling through histone modifications. These regulate chromatin uncoiling and thus allow access to transcription factors and activation of the transcriptional machineries. Environmental as well as patho-physiological changes have been reported to modulate epigenetic machineries to regulate the genomic organizations in living organisms[189]. Increasing evidence (mostly indirect though) suggests that epigenetic changes induce chronic disorders including long term sleep-loss associated disorders and associated behavioral changes of sleep-wake states[190]. DNA methylation modulates the transcriptional and synaptic responses of neurons to sleep loss[191]. Genomic imprinting, which is established by epigenetic processes, also extends its effects to sleep-wake regulation[192]. Both REMS and NREMS are regulated by separate sets of imprinted genes which are differentially expressed in brain regions[193]. Maternally expressed imprinted gene, e.g., Gnas, has also been shown to modulate the expression of sleep-wake states[194]. These and similar other studies reinforce the concept of the role of epigenetic changes in sleep-REMS and their-loss associated sustained patho-physiological changes particularly for understanding the associated molecular circuitry underlying behavioral phenomena.
Independent studies have shown that the bio-molecules (factors) regulating levels of NA and molecules affecting NA in the brain, for instance TH[65,195], α1 adrenergic receptor[196,197] and monoamine oxidase[198] are transcriptionally regulated and they are modulated by REMSD[65,199-201]. These factors are encoded by one or more specific genes at the molecular level; however, our understanding about their transcriptional regulation in association with REMS and its loss in particular, are still lacking. Furthermore, many of the neurological disorders, including depression, AD, Schizophrenia, PD, cognition disorders, ageing, attention deficit/hyperactivity disorder, anxiety, post-traumatic stress disorder, etc., are associated with dysregulation of REMS as well as LC-NA-ergic system. As those disorders are generally chronic by nature, the component of those disorders modulated by NA is likely to be modulated directly or indirectly by epigenetic modulation of NA synthesis, or by the factors responsible for modulating the NA level at the synapse. However, direct evidences which can relate the role of epigenetic modifications of the NA-ergic system in these neurological disorders, especially in relation to REMS or its loss or dysfunction, are lacking. Recently we have proposed a model explaining the possible mechanism of REMS-loss associated epigenetic modifications of NA synthesis in LC neurons in the brain leading to sustained (chronic) associated symptoms[202]. The model explains how upon REMS-loss epigenetic modifications would regulate NA levels in the brain which in turn might modulate factors for transcriptional regulation of other bio-molecules in the brain. An understanding of these mechanisms is expected to provide insights into the detailed role of NA mediated regulation of REMS or its loss in health and diseases. Interactions among environment, genome and REMS for the regulation of the common mediator molecule, NA and their effects on physiological processes have been shown in Figure 2.
In humans, mean blood pressure was higher during REMS as compared to the remaining phases of sleep accompanied by progressive decrease in heart and respiratory rates[203,204]. During REMS, both hemispheric and brainstem blood flow increased even higher than during wakefulness[205,206]. REMSD may contribute to arterial hypertension and arthrosclerosis by altering blood parameters associated with cardiovascular disorder risk[207,208]; hypertensive patients have been reported to have significantly reduced REMS[209]. It has been observed that 96 h REMSD in young rats led to decrease in homocystein, an amino acid that is considered an independent risk factor for cardiovascular disease and stress[210]. Neves et al[211] reported that REMSD by platform method induced significant and sustained blood pressure elevation in rats with partial predisposition to developing hypertension. Association of REMS with considerable peripheral vasoconstriction has also been reported in humans[212]. Heart rate, cardiac pressure, cardiac output, arterial pressure, and breathing rate become irregular when one goes into REMS. In general, respiratory reflexes such as response to hypoxia diminish during REMS.
The NA is an important and common factor for the regulation of autonomic functions, e.g., cardio-vascular-respiratory systems[213], it increases heart rate, cardiac contractility and vascular tone[214]. Impaired neuronal NA reuptake transporter activity has been reported in hypertension and postural tachycardia syndrome[215]. Also, in common heart diseases, such as congestive heart failure, ischemic heart disease and stress-induced cardiomyopathy, NA transporter function seems to be reduced[214]. Patients with chronic sleep apnea associated with heart failure have been shown to be associated with higher urinary and plasma NA levels along with an increase in sympathetic activity[216,217]. As NA is an important factor to regulate both NREMS as well as REMS, a healthy sleep habit is likely to be very important to maintain overall physiological processes including the autonomic cardio-vascular responses.
Sleep is compromised in most infections and diseased conditions[218-223]. Immune responses also vary in relation to sleep conditions and quantity, while immune challenge alters sleep[220]. Shift workers and students studying overnight compromising sleep time have been seen to have propensity to suffer from cold or flu, suggesting sleep loss possibly enhances susceptibility to infections. A relationship between amount of sleep and number of white blood cells was observed across 26 mammalian species. Those with more sleep had more white blood cell count favoring better immuno-competency[220]. The amount of time spent in NREMS increases while REMS is reduced in cases of several infections[224]. REMSD has been reported to affect several hormones, metabolites[225], interleukins[221,226-228], enzymes[229], neuronal structural proteins and apoptosis[125,126] in the brain. REMS loss possibly initiates acute phase response. REMSD rats increased ceruloplasmin, an acute phase response protein[230,231]. A component of immune system like IL-1β is somnogenic, it has been shown to enhance NREMS[232,233]. The number and activity of phagocytes and natural killer cells, the white blood cells, decreased in the REMS deprived animals suggesting severely weakened immune system. In experimental rats, increased tendency of acquiring infection, lesions in foot paws and gastric mucosa after total SD and REMSD have been reported[234,235].
Supporting the general theme of this review, it has been reported that after REMSD the level of NA increases significantly in the blood[236] and the brain (Mehta et al, 2016 MS under revision), and NA is known to modulate the immune system[237]. NA has multiple roles in the body; it acts both as a hormone as well as neurotransmitter. Among the adrenoceptors, β2-subtypes are mostly expressed on immune cells[16]. NA is essential for the maintenance of normal level of antibody production in vivo and thus augments the CD4+-T cell and B-cell activity[237]. NA suppresses the expressions of pro-inflammatory molecules, such as TNF-α and IL-1β, while increasing the expressions of anti-inflammatory molecules, like IκB, by signaling through α1-, α2-, and β-adrenergic receptors on astrocytes and glia[24]. Thus, both NA levels and REMS contribute to optimum immune responses and their deficiencies predispose the body to compromised immunity.
Post-traumatic stress disorder: REMS disturbance is a hallmark symptom of post-traumatic stress disorder (PTSD)[238]; in some cases REMS is reduced, while it is increased in other cases[238-240]. NA-ergic involvement in PTSD is supported by the fact that pharmacologic stimulation of NA-ergic neurons evoked PTSD symptoms[241,242] and adrenoreceptors antagonist reduced nightmares and sleep disruption in patients with chronic PTSD[243]. Mellman et al[244] found that heart rate (LF/HF ratio) was higher during REMS of PTSD patient than non-PTSD group.
PD: PD is primarily due to loss of DA-ergic neurons. However, NA is also a catecholamine synthesized following the same pathway, and found to be involved in wide range of brain functions. It is important to note that in PD patients there is loss of the enzyme synthesizing NA, decreased NA level and there is some loss of LC-NA-ergic neurons[245,246]. PD patients show sleep disturbance, particularly reduced REMS[247]. In addition, PD patients show increased latency to sleep, fragmented sleep and symptoms like restless legs, daytime sleepiness which are usually associated with REMS disturbance and REMS behavior disorders[83]. Normally, REMS and NA level in brain are inversely related; REMSD induces elevated level of NA in brain. In case of diseases like PD the relationship between REMS and NA level in brain is lost and both are affected.
AD: Like other neurodegenerative diseases, AD patients also suffer from sleep disturbances. Polysomnography indicated the loss of REMS and increased REMS latency in AD patients[7,248,249]. REMS behavior disorder like REMS without atonia are distinguishing feature in AD[250]. Although the brain of the patients suffering from AD shows significant loss of cholinergic population in brain, loss of LC neurons has also been reported with progression of AD[251-253]. The surviving NA-ergic neurons are reported to be highly active possibly for maintenance of high NA level in the brain in aging and AD[254]. Prazosin that blocks the action of NA was found to improve aggression and agitation symptoms in AD[255]. Compensation of NA level in the brain by NA reuptake inhibitor are helpful in early stage of AD, possibly due to its anti- oxidant property[256,257] and neuroprotective role[258,259]. Although both REMS and NA-ergic mediators are affected in AD, the role of NA and REMS in pathogenesis of AD needs further investigation.
Depression: REMS loss is a characteristic symptom of depression; alterations in REMS have been observed in patients with depressive episodes[260]. An increase in total duration and density of REMS and decreased REMS latency have been observed in patients with major depressive disorder[261,262]. Depression is primarily associated with dysregulation of the LC NA-ergic system[263,264]. Also, disruptions in serotonin, NA and DA neurotransmissions are generally observed during major depression. In general, the monoaminergic hypo-function has been traditionally accepted as the cause of depression[265,266]. Anti-depressants inhibit re-uptake of the monoamine neurotransmitters, inhibits monoamine oxidase (which degrades NA), or antagonize the inhibitory presynaptic NA-ergic auto-receptors[23]. These are likely to enhance availability of NA at the synapse resulting in facilitation of NA-mediated neurotransmission and amelioration of the symptoms of depression.
Cognitive dysfunctions: Sleep has been implicated in the neuronal plasticity in the brain that underlie learning and memory[267]. Indications that sleep participates in the consolidation of fresh memory traces come from a wide range of experimental observations[268]. Sleep loss is associated with decreased concentration, attention, vagueness, longer reaction time, lack of coordination, disorientation and making mistakes[269,270]. Also, REMS has a positive effect on memory while its loss adversely affects memory[271,272]. NA is an essential modulator of memory formation because of its ability to regulate synaptic plasticity[119]. It is released during arousal and has a central role to play in the emotional regulation of memory[273]. The memory deficits observed during AD could be due to the loss of NA-ergic system reported during the disorder[251-253]. Thus, NA can be attributed to be a common molecule in most of the neurological disorders where REMS is also disrupted.
Obstructive sleep apnea: There are several sleep disorders where both REMS and NA are affected. Obstructive sleep apnea (OSA) is one such disorder characterized by sporadic collapse of upper airway during sleep leading to arousal. OSA patient suffers sleep loss, complains persistent drowsiness and daytime sleepiness. OSA events have been reported during both NREMS and REMS. During REMS the excitatory input to motoneurons regulating upper airway reduces due to cessation of NA-ergic neurons, consequently in OSA patients the REMS is often associated with higher propensity and frequency of obstructive events[274]. Epidemiologically observed such relationship is also known as “REMS related OSA”[275]. OSA patients show the multitude of the symptoms like hypertension, metabolic dysfunction, vascular irregularities and oxidative stress; these are associated with altered NA level in body. In fact, sympathetic activity and plasma NA is reported to be high in OSA patients[276]. Reduction in the number of catecholaminergic neurons is shown in OSA; there are also reports of activation of hypothalamic pituitary axis that may increase the NA-ergic activity in brain. The remarkable association of REMS and NA with OSA indicates their therapeutic importance of the disease.
Both quantity as well as quality of sleep (NREMS and REMS) is essential for healthy living. Interactions among various intracellular and extracellular factors viz. nutrition, light, temperature, exercise, genetic as well as epigenetic mechanisms affect/contribute to the regulation of sleep which affects health in short and long term. The NA system plays a significant role in regulating NREMS as well as REMS. The role of NA in relation to REMS and REMSD has been investigated in more detail; NA level decreases during REMS while it increases during REMSD. Broadly, it has been observed that NA is a key molecule which induces REMSD associated changes from molecule to behavior. As sleep is affected by lifestyle changes, we propose that many of the lifestyle related pathophysiological conditions could be due to dysregulation of sleep (NREMS and REMS) and the effects are mediated by elevated levels of NA. Thus, sleep discipline plays a key role in maintenance of good health.
Rachna Mehta received DST-WOSA fellowship and Abhishek Singh received UGC senior research fellowship. Research funding to BNM through Institutional support under BUILDER (DBT); PURSE (DST); FIST (DST); UPE, Networking and SAP-DRS (UGC); and grants to BNM from UGC, DBT, DST and J C Bose fellowship are acknowledged.
Manuscript source: Invited manuscript
Specialty type: Clinical neurology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Skobel E, Takahashi H S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Tobler I. Is sleep fundamentally different between mammalian species? Behav Brain Res. 1995;69:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 4. | Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 580] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 5. | Akerstedt T. Psychological and psychophysiological effects of shift work. Scand J Work Environ Health. 1990;16 Suppl 1:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Mallick BN, Singh A. REM sleep loss increases brain excitability: role of noradrenaline and its mechanism of action. Sleep Med Rev. 2011;15:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6 Suppl 1A:S16-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Petit D, Lorrain D, Gauthier S, Montplaisir J. Regional spectral analysis of the REM sleep EEG in mild to moderate Alzheimer’s disease. Neurobiol Aging. 1993;14:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16:622-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 331] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Malik S, Boeve BF, Krahn LE, Silber MH. Narcolepsy associated with other central nervous system disorders. Neurology. 2001;57:539-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 598] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 12. | Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Poryazova RG, Zachariev ZI. REM sleep behavior disorder in patients with Parkinson’s disease. Folia Med (Plovdiv). 2005;47:5-10. [PubMed] |
| 14. | Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Breslau N. Neurobiological research on sleep and stress hormones in epidemiological samples. Ann N Y Acad Sci. 2006;1071:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Cosentino M, Marino F. Nerve Driven Immunity: Noradrenaline and Adrenaline. In: Levite M, editor Nerve Driven Immunity: Neurotransmitters and Neuropeptides in the Immune System: Springer Vienna 2012; 47-96. |
| 17. | Francis BM, Yang J, Hajderi E, Brown ME, Michalski B, McLaurin J, Fahnestock M, Mount HT. Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37:1934-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | González MM, Debilly G, Valatx JL. Noradrenaline neurotoxin DSP-4 effects on sleep and brain temperature in the rat. Neurosci Lett. 1998;248:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Iimori K, Tanaka M, Kohno Y, Ida Y, Nakagawa R, Hoaki Y, Tsuda A, Nagasaki N. Psychological stress enhances noradrenaline turnover in specific brain regions in rats. Pharmacol Biochem Behav. 1982;16:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Adolfsson R, Gottfries CG, Roos BE, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry. 1979;135:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 334] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Berridge CW, Arnsten AF, Foote SL. Noradrenergic modulation of cognitive function: clinical implications of anatomical, electrophysiological and behavioural studies in animal models. Psychol Med. 1993;23:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Bryson G. Biogenic amines in normal and abnormal behavioral states. Clin Chem. 1971;17:5-26. [PubMed] |
| 23. | Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7:9-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson’s disease. Biochem Pharmacol. 2007;74:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1136] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1-165. [PubMed] |
| 27. | Siegel J. Brainstem mechanisms generating REM sleep. Principles and practice of sleep medicine. Philadelphia: Saunders 1989; 104-120. |
| 28. | Jones BE. Elimination of paradoxical sleep by lesions of the pontine gigantocellular tegmental field in the cat. Neurosci Lett. 1979;13:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Drucker-Colín R, Pedraza JG. Kainic acid lesions of gigantocellular tegmental field (FTG) neurons does not abolish REM sleep. Brain Res. 1983;272:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Friedman L, Jones BE. Computer graphics analysis of sleep-wakefulness state changes after pontine lesions. Brain Res Bull. 1984;13:53-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Siegel JM, Tomaszewski KS, Nienhuis R. Behavioral states in the chronic medullary and midpontine cat. Electroencephalogr Clin Neurophysiol. 1986;63:274-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Siegel JM, Nienhuis R, Tomaszewski KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 1983;268:344-348. [PubMed] |
| 33. | Jones BE. The role of noradrenergic locus coeruleus neurons and neighboring cholinergic neurons of the pontomesencephalic tegmentum in sleep-wake states. Prog Brain Res. 1991;88:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Mallick BN, Kaur S, Jha SK, Siegel JM. Possible role of GABA in the regulation of REM sleep with special reference to REM-OFF neurons. Mallick BN, Inoue S, editors. Rapid Eye Movement Sleep New York: Marcel Dekker 1999; 153-166. |
| 35. | Mallick BN, Kaur S, Saxena RN. Interactions between cholinergic and GABAergic neurotransmitters in and around the locus coeruleus for the induction and maintenance of rapid eye movement sleep in rats. Neuroscience. 2001;104:467-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3086] [Cited by in RCA: 2882] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 37. | Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911-10916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 902] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 38. | Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Lin JS, Sakai K, Jouvet M. [Role of hypothalamic histaminergic systems in the regulation of vigilance states in cats]. C R Acad Sci III. 1986;303:469-474. [PubMed] |
| 40. | Mallick BN, Thankachan S, Islam F. Influence of hypnogenic brain areas on wakefulness- and rapid-eye-movement sleep-related neurons in the brainstem of freely moving cats. J Neurosci Res. 2004;75:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Takakusaki K, Saitoh K, Harada H, Okumura T, Sakamoto T. Evidence for a role of basal ganglia in the regulation of rapid eye movement sleep by electrical and chemical stimulation for the pedunculopontine tegmental nucleus and the substantia nigra pars reticulata in decerebrate cats. Neuroscience. 2004;124:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6:254-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 43. | Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 225] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 45. | Aston-Jones G, Akaoka H, Charléty P, Chouvet G. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci. 1991;11:760-769. [PubMed] |
| 46. | Pal D, Mallick BN. Role of noradrenergic and GABA-ergic inputs in pedunculopontine tegmentum for regulation of rapid eye movement sleep in rats. Neuropharmacology. 2006;51:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Pal D, Mallick BN. GABA in pedunculopontine tegmentum increases rapid eye movement sleep in freely moving rats: possible role of GABA-ergic inputs from substantia nigra pars reticulata. Neuroscience. 2009;164:404-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Kaur S, Saxena RN, Mallick BN. GABAergic neurons in prepositus hypoglossi regulate REM sleep by its action on locus coeruleus in freely moving rats. Synapse. 2001;42:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | McCarley RW, Hobson JA. Discharge patterns of cat pontine brain stem neurons during desynchronized sleep. J Neurophysiol. 1975;38:751-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 153] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 771] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 51. | Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986;371:324-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 204] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Chu NS, Bloom FE. Activity patterns of catecholamine-containing pontine neurons in the dorso-lateral tegmentum of unrestrained cats. J Neurobiol. 1974;5:527-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 169] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Sakai K, Jouvet M. Brain stem PGO-on cells projecting directly to the cat dorsal lateral geniculate nucleus. Brain Res. 1980;194:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Steriade M, McCarley RW. Brain control of wakefulness and sleep. first ed. New York: Kluwer Acedemic/Plenum 2005; . |
| 55. | Kumar R, Bose A, Mallick BN. A mathematical model towards understanding the mechanism of neuronal regulation of wake-NREMS-REMS states. PLoS One. 2012;7:e42059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Pal D, Madan V, Mallick BN. Neural mechanism of rapid eye movement sleep generation: Cessation of locus coeruleus neurons is a necessity. Shengli Xuebao. 2005;57:401-413. [PubMed] |
| 57. | Mallick BN, Siegel JM, Fahringer H. Changes in pontine unit activity with REM sleep deprivation. Brain Res. 1990;515:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Cespuglio R, Gomez ME, Faradji H, Jouvet M. Alterations in the sleep-waking cycle induced by cooling of the locus coeruleus area. Electroencephalogr Clin Neurophysiol. 1982;54:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Caballero A, De Andrés I. Unilateral lesions in locus coeruleus area enhance paradoxical sleep. Electroencephalogr Clin Neurophysiol. 1986;64:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Laguzzi RF, Adrien J, Bourgoin S, Hamon M. Effects of intraventricular injection of 6-hydroxydopamine in the developing kitten. 1. On the sleepwaking cycles. Brain Res. 1979;160:445-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Farber J, Miller JD, Crawford KA, McMillen BA. Dopamine metabolism and receptor sensitivity in rat brain after REM sleep deprivation. Pharmacol Biochem Behav. 1983;18:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Singh S, Mallick BN. Mild electrical stimulation of pontine tegmentum around locus coeruleus reduces rapid eye movement sleep in rats. Neurosci Res. 1996;24:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Kaitin KI, Bliwise DL, Gleason C, Nino-Murcia G, Dement WC, Libet B. Sleep disturbance produced by electrical stimulation of the locus coeruleus in a human subject. Biol Psychiatry. 1986;21:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 822] [Cited by in RCA: 675] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 65. | Porkka-Heiskanen T, Smith SE, Taira T, Urban JH, Levine JE, Turek FW, Stenberg D. Noradrenergic activity in rat brain during rapid eye movement sleep deprivation and rebound sleep. Am J Physiol. 1995;268:R1456-R1463. [PubMed] |
| 66. | Léna I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, Suaud-Chagny MF, Gottesmann C. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep--wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 67. | Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 154] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 167] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Mallick BN, Singh A, Khanday MA. Activation of inactivation process initiates rapid eye movement sleep. Prog Neurobiol. 2012;97:259-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Masserano JM, King C. Effects on sleep of phentolamine and epinephrine infused into the locus coeruleus of cats. Eur J Pharmacol. 1982;84:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Masserano JM, King C. Effects on sleep of acetylcholine perfusion of the locus coeruleus of cats. Neuropharmacology. 1982;21:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Kaur S, Saxena RN, Mallick BN. GABA in locus coeruleus regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats. Neurosci Lett. 1997;223:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Pollock MS, Mistlberger RE. Rapid eye movement sleep induction by microinjection of the GABA-A antagonist bicuculline into the dorsal subcoeruleus area of the rat. Brain Res. 2003;962:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Bourgin P, Huitrón-Résendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760-7765. [PubMed] |
| 76. | Chen L, McKenna JT, Bolortuya Y, Winston S, Thakkar MM, Basheer R, Brown RE, McCarley RW. Knockdown of orexin type 1 receptor in rat locus coeruleus increases REM sleep during the dark period. Eur J Neurosci. 2010;32:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Choudhary RC, Khanday MA, Mitra A, Mallick BN. Perifornical orexinergic neurons modulate REM sleep by influencing locus coeruleus neurons in rats. Neuroscience. 2014;279:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Alam MA, Mallick BN. Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci Lett. 2008;439:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Toppila J, Niittymäki P, Porkka-Heiskanen T, Stenberg D. Intracerebroventricular and locus coeruleus microinjections of somatostatin antagonist decrease REM sleep in rats. Pharmacol Biochem Behav. 2000;66:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Segal M. Serotonergic innervation of the locus coeruleus from the dorsal raphe and its action on responses to noxious stimuli. J Physiol. 1979;286:401-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 215] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 81. | Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 284] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 82. | Cuturic M, Abramson RK, Vallini D, Frank EM, Shamsnia M. Sleep patterns in patients with Huntington’s disease and their unaffected first-degree relatives: a brief report. Behav Sleep Med. 2009;7:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson’s disease. Mov Disord. 1990;5:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 205] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 84. | Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T. Sleep in Alzheimer’s disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep. 1995;18:145-148. [PubMed] |
| 85. | Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J Physiol Anthropol. 2012;31:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 86. | Gilbert SS, van den Heuvel CJ, Ferguson SA, Dawson D. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Wehr TA. A brain-warming function for REM sleep. Neurosci Biobehav Rev. 1992;16:379-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Muzet A, Ehrhart J, Candas V, Libert JP, Vogt JJ. REM sleep and ambient temperature in man. Int J Neurosci. 1983;18:117-126. [PubMed] |
| 89. | Jennings JR, Reynolds CF, Bryant DS, Berman SR, Buysse DJ, Dahl RE, Hoch CC, Monk TH. Peripheral thermal responsivity to facial cooling during sleep. Psychophysiology. 1993;30:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | Candas V, Libert JP, Muzet A. Heating and cooling stimulations during SWS and REM sleep in man. J Thermal Biol. 1982;7:155-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Parmeggiani PL. Interaction between sleep and thermoregulation: an aspect of the control of behavioral states. Sleep. 1987;10:426-435. [PubMed] |
| 92. | Boulant JA, Demieville HN. Responses of thermosensitive preoptic and septal neurons to hippocampal and brain stem stimulation. J Neurophysiol. 1977;40:1356-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Jha SK, Mallick BN. Presence of alpha-1 norepinephrinergic and GABA-A receptors on medial preoptic hypothalamus thermosensitive neurons and their role in integrating brainstem ascending reticular activating system inputs in thermoregulation in rats. Neuroscience. 2009;158:833-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Simmonds MA. Effect of environmental temperature on the turnover of noradrenaline in hypothalamus and other areas of rat brain. J Physiol. 1969;203:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Nemoto EM, Klementavicius R, Melick JA, Yonas H. Norepinephrine activation of basal cerebral metabolic rate for oxygen (CMRO2) during hypothermia in rats. Anesth Analg. 1996;83:1262-1267. [PubMed] |
| 97. | Ratheiser KM, Brillon DJ, Campbell RG, Matthews DE. Epinephrine produces a prolonged elevation in metabolic rate in humans. Am J Clin Nutr. 1998;68:1046-1052. [PubMed] |
| 98. | Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Functional link between distal vasodilation and sleep-onset latency? Am J Physiol Regul Integr Comp Physiol. 2000;278:R741-R748. [PubMed] |
| 99. | Lack L, Gradisar M. Acute finger temperature changes preceding sleep onsets over a 45-h period. J Sleep Res. 2002;11:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Mallick BN, Alam MN. Different types of norepinephrinergic receptors are involved in preoptic area mediated independent modulation of sleep-wakefulness and body temperature. Brain Res. 1992;591:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Poole S, Stephenson JD. Effects of noradrenaline and carbachol on temperature regulation of rats. Br J Pharmacol. 1979;65:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Lack LC, Gradisar M, Van Someren EJ, Wright HR, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. 2008;12:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 103. | Jaiswal MK, Mallick BN. Prazosin modulates rapid eye movement sleep deprivation-induced changes in body temperature in rats. J Sleep Res. 2009;18:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | O’Connor PJ, Youngstedt SD. Influence of exercise on human sleep. Exerc Sport Sci Rev. 1995;23:105-134. [PubMed] |
| 105. | Youngstedt SD. Effects of exercise on sleep. Clin Sports Med. 2005;24:355-365, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 106. | Horne JA, Moore VJ. Sleep EEG effects of exercise with and without additional body cooling. Electroencephalogr Clin Neurophysiol. 1985;60:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Kubitz KA, Landers DM, Petruzzello SJ, Han M. The effects of acute and chronic exercise on sleep. A meta-analytic review. Sports Med. 1996;21:277-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Youngstedt SD, Kline CE. Epidemiology of exercise and sleep. Sleep Biol Rhythms. 2006;4:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 109. | Netzer NC, Kristo D, Steinle H, Lehmann M, Strohl KP. REM sleep and catecholamine excretion: a study in elite athletes. Eur J Appl Physiol. 2001;84:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Hagberg JM, Seals DR, Yerg JE, Gavin J, Gingerich R, Premachandra B, Holloszy JO. Metabolic responses to exercise in young and older athletes and sedentary men. J Appl Physiol (1985). 1988;65:900-908. [PubMed] |
| 111. | Greiwe JS, Hickner RC, Shah SD, Cryer PE, Holloszy JO. Norepinephrine response to exercise at the same relative intensity before and after endurance exercise training. J Appl Physiol (1985). 1999;86:531-535. [PubMed] |
| 112. | Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 219] [Reference Citation Analysis (0)] |
| 113. | Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus. 2009;19:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 115. | O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 116. | Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 600] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 117. | van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2696] [Cited by in RCA: 2752] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 118. | Xu WP, Shan LD, Gong S, Chen L, Zhang YJ, Yin QZ, Hisamitsu T, Jiang XH, Guo SY. Forced running enhances neurogenesis in the hippocampal dentate gyrus of adult rats and improves learning ability. Sheng Li Xue Bao. 2006;58:415-420. [PubMed] |
| 119. | Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 120. | Cai DJ, Shuman T, Gorman MR, Sage JR, Anagnostaras SG. Sleep selectively enhances hippocampus-dependent memory in mice. Behav Neurosci. 2009;123:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 537] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 122. | Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Sleep deprivation prevents stimulation-induced increases of levels of P-CREB and BDNF: protection by caffeine. Mol Cell Neurosci. 2011;46:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 123. | Zagaar M, Alhaider I, Dao A, Levine A, Alkarawi A, Alzubaidy M, Alkadhi K. The beneficial effects of regular exercise on cognition in REM sleep deprivation: behavioral, electrophysiological and molecular evidence. Neurobiol Dis. 2012;45:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 124. | Zagaar MA, Dao AT, Alhaider IA, Alkadhi KA. Prevention by Regular Exercise of Acute Sleep Deprivation-Induced Impairment of Late Phase LTP and Related Signaling Molecules in the Dentate Gyrus. Mol Neurobiol. 2016;53:2900-2910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Somarajan BI, Khanday MA, Mallick BN. Rapid Eye Movement Sleep Deprivation Induces Neuronal Apoptosis by Noradrenaline Acting on Alpha1 Adrenoceptor and by Triggering Mitochondrial Intrinsic Pathway. Front Neurol. 2016;7:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 126. | Biswas S, Mishra P, Mallick BN. Increased apoptosis in rat brain after rapid eye movement sleep loss. Neuroscience. 2006;142:315-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 127. | Bohannon RW. Physical rehabilitation in neurologic diseases. Curr Opin Neurol. 1993;6:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 128. | Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1432] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 129. | Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA. Ageing, fitness and neurocognitive function. Nature. 1999;400:418-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 855] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 130. | Ng F, Dodd S, Berk M. The effects of physical activity in the acute treatment of bipolar disorder: a pilot study. J Affect Disord. 2007;101:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 131. | Kucyi A, Alsuwaidan MT, Liauw SS, McIntyre RS. Aerobic physical exercise as a possible treatment for neurocognitive dysfunction in bipolar disorder. Postgrad Med. 2010;122:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 132. | Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ. 2012;345:e8508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 133. | Passos GS, Poyares D, Santana MG, Teixeira AA, Lira FS, Youngstedt SD, dos Santos RV, Tufik S, de Mello MT. Exercise improves immune function, antidepressive response, and sleep quality in patients with chronic primary insomnia. Biomed Res Int. 2014;2014:498961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 134. | Chen KM, Chen MH, Lin MH, Fan JT, Lin HS, Li CH. Effects of yoga on sleep quality and depression in elders in assisted living facilities. J Nurs Res. 2010;18:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 135. | Halpern J, Cohen M, Kennedy G, Reece J, Cahan C, Baharav A. Yoga for improving sleep quality and quality of life for older adults. Altern Ther Health Med. 2014;20:37-46. [PubMed] |
| 136. | Innes KE, Selfe TK. The Effects of a Gentle Yoga Program on Sleep, Mood, and Blood Pressure in Older Women with Restless Legs Syndrome (RLS): A Preliminary Randomized Controlled Trial. Evid Based Complement Alternat Med. 2012;2012:294058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 137. | Innes KE, Selfe TK, Agarwal P, Williams K, Flack KL. Efficacy of an eight-week yoga intervention on symptoms of restless legs syndrome (RLS): a pilot study. J Altern Complement Med. 2013;19:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 138. | Mustian KM, Janelsins M, Peppone LJ, Kamen C. Yoga for the Treatment of Insomnia among Cancer Patients: Evidence, Mechanisms of Action, and Clinical Recommendations. Oncol Hematol Rev. 2014;10:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 1541] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 140. | Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1543] [Cited by in RCA: 1569] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 141. | Tahara Y, Shibata S. Chrono-biology, chrono-pharmacology, and chrono-nutrition. J Pharmacol Sci. 2014;124:320-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 142. | Belanger-Willoughby N, Linehan V, Hirasawa M. Thermosensing mechanisms and their impairment by high-fat diet in orexin neurons. Neuroscience. 2016;324:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 143. | Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 144. | Zeng Y, Yang J, Du J, Pu X, Yang X, Yang S, Yang T. Strategies of Functional Foods Promote Sleep in Human Being. Curr Signal Transduct Ther. 2014;9:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Afaghi A, O’Connor H, Chow CM. Acute effects of the very low carbohydrate diet on sleep indices. Nutr Neurosci. 2008;11:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 146. | Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 147. | Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, Woods NF, Chen Z. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring). 2014;22:E55-E61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 148. | Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985). 2005;99:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 722] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 149. | Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 517] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 150. | Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 876] [Article Influence: 46.1] [Reference Citation Analysis (1)] |
| 151. | Moreno CR, Louzada FM, Teixeira LR, Borges F, Lorenzi-Filho G. Short sleep is associated with obesity among truck drivers. Chronobiol Int. 2006;23:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 152. | Phillips F, Chen CN, Crisp AH, Koval J, McGuinness B, Kalucy RS, Kalucy EC, Lacey JH. Isocaloric diet changes and electroencephalographic sleep. Lancet. 1975;2:723-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 153. | Porter JM, Horne JA. Bed-time food supplements and sleep: effects of different carbohydrate levels. Electroencephalogr Clin Neurophysiol. 1981;51:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 154. | Howorka K, Heger G, Schabmann A, Anderer P, Tribl G, Zeitlhofer J. Severe hypoglycaemia unawareness is associated with an early decrease in vigilance during hypoglycaemia. Psychoneuroendocrinology. 1996;21:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 155. | Boyle PJ, Scott JC, Krentz AJ, Nagy RJ, Comstock E, Hoffman C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J Clin Invest. 1994;93:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 156. | Crispim CA, Zimberg IZ, dos Reis BG, Diniz RM, Tufik S, de Mello MT. Relationship between food intake and sleep pattern in healthy individuals. J Clin Sleep Med. 2011;7:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 157. | Mikuni E, Ohoshi T, Hayashi K, Miyamura K. Glucose intolerance in an employed population. Tohoku J Exp Med. 1983;141 Suppl:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 158. | Padilha HG, Crispim CA, Zimberg IZ, Folkard S, Tufik S, de Mello MT. Metabolic responses on the early shift. Chronobiol Int. 2010;27:1080-1092. [PubMed] |
| 159. | Knutsson A. Relationships between serum triglycerides and gamma-glutamyltransferase among shift and day workers. J Intern Med. 1989;226:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 160. | Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rössler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661-666. [PubMed] |
| 161. | Mitchell HA, Weinshenker D. Good night and good luck: norepinephrine in sleep pharmacology. Biochem Pharmacol. 2010;79:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 162. | Satinoff E, Prosser RA. Suprachiasmatic nuclear lesions eliminate circadian rhythms of drinking and activity, but not of body temperature, in male rats. J Biol Rhythms. 1988;3:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 163. | Lancel M, van Riezen H, Glatt A. Effects of circadian phase and duration of sleep deprivation on sleep and EEG power spectra in the cat. Brain Res. 1991;548:206-214. [PubMed] |
| 164. | Alföldi P, Franken P, Tobler I, Borbély AA. Short light-dark cycles influence sleep stages and EEG power spectra in the rat. Behav Brain Res. 1991;43:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 165. | Héry F, Rouer E, Glowinski J. Daily variations of serotonin metabolism in the rat brain. Brain Res. 1972;43:445-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 204] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 166. | Roberts JE. Light and immunomodulation. Ann N Y Acad Sci. 2000;917:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 167. | Ziegler MC, Lake CR, Wood JH, Ebert MH. Circadian rhythm in cerebrospinal fluid noradrenaline of man and monkey. Nature. 1976;264:656-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 47] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Murphy PJ, Campbell SS. Enhancement of REM sleep during extraocular light exposure in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1606-R1612. [PubMed] |
| 169. | Campbell SS, Murphy PJ. Extraocular circadian phototransduction in humans. Science. 1998;279:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 170. | Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA. 1996;93:1619-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 171. | Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2263] [Cited by in RCA: 2116] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 172. | Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1171] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 173. | Seckl JR, Meaney MJ. Early life events and later development of ischaemic heart disease. Lancet. 1993;342:1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 174. | Medori R, Tritschler HJ, LeBlanc A, Villare F, Manetto V, Chen HY, Xue R, Leal S, Montagna P, Cortelli P. Fatal familial insomnia, a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med. 1992;326:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 370] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 175. | Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1856] [Cited by in RCA: 1754] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 176. | Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2247] [Cited by in RCA: 2158] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 177. | Jones S, Pfister-Genskow M, Benca RM, Cirelli C. Molecular correlates of sleep and wakefulness in the brain of the white-crowned sparrow. J Neurochem. 2008;105:46-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 178. | Dauvilliers Y, Maret S, Tafti M. Genetics of normal and pathological sleep in humans. Sleep Med Rev. 2005;9:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 179. | Giuditta A, Rutigliano B, Vitale-Neugebauer A. Influence of synchronized sleep on the biosynthesis of RNA in neuronal and mixed fractions isolated from rabbit cerebral cortex. J Neurochem. 1980;35:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 180. | Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187-9194. [PubMed] |
| 181. | Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 182. | Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 450] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 183. | Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 184. | Vecsey CG, Peixoto L, Choi JH, Wimmer M, Jaganath D, Hernandez PJ, Blackwell J, Meda K, Park AJ, Hannenhalli S. Genomic analysis of sleep deprivation reveals translational regulation in the hippocampus. Physiol Genomics. 2012;44:981-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 185. | Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 186. | Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23:R774-R788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 187. | Petit JM, Tobler I, Allaman I, Borbély AA, Magistretti PJ. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur J Neurosci. 2002;16:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 188. | Nagatsu T, Ichinose H. Molecular Biology of Catecholamine Systems: Multiple Tyrosine Hydroxylases in Different Simian Species, and in Humans in Relation to Parkinson’s Disease. Alzheimer’s and Parkinson’s Diseases: Recent Developments. New York: Plenum Press 1995; 655-659. |
| 189. | Powledge TM. Behavioral Epigenetics: How Nurture Shapes Nature. Bioscience. 2011;61:588-592. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 190. | Qureshi IA, Mehler MF. Epigenetics of sleep and chronobiology. Curr Neurol Neurosci Rep. 2014;14:432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 191. | Massart R, Freyburger M, Suderman M, Paquet J, El Helou J, Belanger-Nelson E, Rachalski A, Koumar OC, Carrier J, Szyf M. The genome-wide landscape of DNA methylation and hydroxymethylation in response to sleep deprivation impacts on synaptic plasticity genes. Transl Psychiatry. 2014;4:e347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 192. | McNamara P. Genomic Imprinting and Neurodevelopmental Disorders of Sleep. Sleep and Hypnosis. 2004;82-90. |
| 193. | Tucci V, Nolan PM. Toward an understanding of the function of sleep: New insights from mouse genetics. Evolution of Sleep: Phylogenetic and Functional Perspectives: Cambridge University Press 2009; 218-237. |
| 194. | Lassi G, Ball ST, Maggi S, Colonna G, Nieus T, Cero C, Bartolomucci A, Peters J, Tucci V. Loss of Gnas imprinting differentially affects REM/NREM sleep and cognition in mice. PLoS Genet. 2012;8:e1002706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 195. | Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 517] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 196. | Michelotti GA, Brinkley DM, Morris DP, Smith MP, Louie RJ, Schwinn DA. Epigenetic regulation of human alpha1d-adrenergic receptor gene expression: a role for DNA methylation in Sp1-dependent regulation. FASEB J. 2007;21:1979-1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 197. | Razik MA, Lee K, Price RR, Williams MR, Ongjoco RR, Dole MK, Rudner XL, Kwatra MM, Schwinn DA. Transcriptional regulation of the human alpha1a-adrenergic receptor gene. Characterization Of the 5’-regulatory and promoter region. J Biol Chem. 1997;272:28237-28246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 198. | Zhu QS, Chen K, Shih JC. Bidirectional promoter of human monoamine oxidase A (MAO A) controlled by transcription factor Sp1. J Neurosci. 1994;14:7393-7403. [PubMed] |
| 199. | Thakkar M, Mallick BN. Effect of rapid eye movement sleep deprivation on rat brain monoamine oxidases. Neuroscience. 1993;55:677-683. [PubMed] |
| 200. | Majumdar S, Mallick BN. Increased levels of tyrosine hydroxylase and glutamic acid decarboxylase in locus coeruleus neurons after rapid eye movement sleep deprivation in rats. Neurosci Lett. 2003;338:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 201. | Basheer R, Magner M, McCarley RW, Shiromani PJ. REM sleep deprivation increases the levels of tyrosine hydroxylase and norepinephrine transporter mRNA in the locus coeruleus. Brain Res Mol Brain Res. 1998;57:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 202. | Mehta R, Singh A, Bókkon I, Nath Mallick B. REM sleep and its Loss-Associated Epigenetic Regulation with Reference to Noradrenaline in Particular. Curr Neuropharmacol. 2016;14:28-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 203. | Snyder F, Hobson JA, Goldfrank F. Blood pressure changes during human sleep. Science. 1963;142:1313-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 204. | Snyder F, Hobson JA, Morrison DF, Goldfrank F. Changes in respiration, heart rate, and systolic blood pressure in human sleep. J Appl Physiol. 1964;19:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 205. | Sakai F, Meyer JS, Karacan I, Derman S, Yamamoto M. Normal human sleep: regional cerebral hemodynamics. Ann Neurol. 1980;7:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 206. | Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2433] [Cited by in RCA: 3212] [Article Influence: 267.7] [Reference Citation Analysis (0)] |
| 207. | Martín B, Fernández B, Domínguez FJ. [Sleep apnea-hypopnea syndrome and cardiovascular diseases]. An Sist Sanit Navar. 2007;30 Suppl 1:89-95. [PubMed] |
| 208. | Andersen ML, Martins PJ, D’Almeida V, Santos RF, Bignotto M, Tufik S. Effects of paradoxical sleep deprivation on blood parameters associated with cardiovascular risk in aged rats. Exp Gerontol. 2004;39:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 209. | Friedman O, Bradley TD, Ruttanaumpawan P, Logan AG. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. Am J Hypertens. 2010;23:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 210. | de Oliveira AC, D’Almeida V, Hipólide DC, Nobrega JN, Tufik S. Sleep deprivation reduces total plasma homocysteine levels in rats. Can J Physiol Pharmacol. 2002;80:193-197. [PubMed] |
| 211. | Neves FA, Marson O, Baumgratz RP, Bossolan D, Ginosa M, Ribeiro AB, Kohlmann O, Ramos OL. Rapid eye movement sleep deprivation and hypertension. Genetic influence. Hypertension. 1992;19:II202-II206. [PubMed] |
| 212. | Lavie P, Schnall RP, Sheffy J, Shlitner A. Peripheral vasoconstriction during REM sleep detected by a new plethysmographic method. Nat Med. 2000;6:606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 213. | El Fazaa S, Somody L, Gharbi N, Kamoun A, Gharib C, Gauquelin-Koch G. Effects of acute and chronic starvation on central and peripheral noradrenaline turnover, blood pressure and heart rate in the rat. Exp Physiol. 1999;84:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 214. | Schroeder C, Jordan J. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol. 2012;303:H1273-H1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 215. | Esler M, Alvarenga M, Pier C, Richards J, El-Osta A, Barton D, Haikerwal D, Kaye D, Schlaich M, Guo L. The neuronal noradrenaline transporter, anxiety and cardiovascular disease. J Psychopharmacol. 2006;20:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 216. | van de Borne P, Oren R, Abouassaly C, Anderson E, Somers VK. Effect of Cheyne-Stokes respiration on muscle sympathetic nerve activity in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;81:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 217. | Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 324] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 218. | Krueger JM, Karnovsky ML. Sleep and the immune response. Ann N Y Acad Sci. 1987;496:510-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 471] [Cited by in RCA: 585] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 219. | Moldofsky H, Lue FA, Davidson JR, Gorczynski R. Effects of sleep deprivation on human immune functions. FASEB J. 1989;3:1972-1977. [PubMed] |
| 220. | Opp MR. Sleeping to fuel the immune system: mammalian sleep and resistance to parasites. BMC Evol Biol. 2009;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 221. | Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597-3603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 222. | Ruiz FS, Andersen ML, Martins RC, Zager A, Lopes JD, Tufik S. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immun. 2012;18:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 223. | Schiffelholz T, Lancel M. Sleep changes induced by lipopolysaccharide in the rat are influenced by age. Am J Physiol Regul Integr Comp Physiol. 2001;280:R398-R403. [PubMed] |
| 224. | Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 466] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 225. | Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2332] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 226. | Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 227. | Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res. 2009;29:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 228. | Kang WS, Park HJ, Chung JH, Kim JW. REM sleep deprivation increases the expression of interleukin genes in mice hypothalamus. Neurosci Lett. 2013;556:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 229. | Mallick BN, Madan V, Faisal M. Biochemical Change. Kushida CA, editor Sleep deprivation Basic Science, Physiology and behavior. NewYork: Marcel Dekker 2005; 339-357. |
| 230. | Andersen M, Guindalini C, Alvarenga TA, Egydio F, Tufik S. Expression of ceruloplasmin in cavernosal tissue of paradoxical sleep deprived rats. Sleep Sci. 2012;5:40-44. |
| 231. | Pandey AK, Kar SK. REM sleep deprivation of rats induces acute phase response in liver. Biochem Biophys Res Commun. 2011;410:242-246. [PubMed] |
| 232. | Tobler I, Borbély AA, Schwyzer M, Fontana A. Interleukin-1 derived from astrocytes enhances slow wave activity in sleep EEG of the rat. Eur J Pharmacol. 1984;104:191-192. [PubMed] |
| 233. | Opp MR, Krueger JM. Anti-interleukin-1 beta reduces sleep and sleep rebound after sleep deprivation in rats. Am J Physiol. 1994;266:R688-R695. [PubMed] |
| 234. | Kushida CA. Sleep Deprivation; basic science, physiology and behavior. newyork: marcel-dekker. Marcel Dekker. 2005;. |
| 235. | Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. 1989. Sleep. 2002;25:68-87. [PubMed] |
| 236. | Bergmann BM, Seiden LS, Landis CA, Gilliland MA, Rechtschaffen A. Sleep deprivation in the rat: XVIII. Regional brain levels of monoamines and their metabolites. Sleep. 1994;17:583-589. [PubMed] |
| 237. | Kohm AP, Sanders VM. Norepinephrine: a messenger from the brain to the immune system. Immunol Today. 2000;21:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 238. | Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 228] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 239. | Mellman TA. Psychobiology of sleep disturbances in posttraumatic stress disorder. Ann N Y Acad Sci. 1997;821:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 240. | Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 241. | Southwick SM, Bremner JD, Rasmusson A, Morgan CA, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 242. | Southwick SM, Morgan CA, Charney DS, High JR. Yohimbine use in a natural setting: effects on posttraumatic stress disorder. Biol Psychiatry. 1999;46:442-444. [PubMed] |
| 243. | Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Tröster K. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 363] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 244. | Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psychiatry. 2004;55:953-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 245. | Hurst JH, LeWitt PA, Burns RS, Foster NL, Lovenberg W. CSF dopamine-beta-hydroxylase activity in Parkinson’s disease. Neurology. 1985;35:565-568. [PubMed] |
| 246. | Greenfield JG, Bosanquet FD. The brain-stem lesions in Parkinsonism. J Neurol Neurosurg Psychiatry. 1953;16:213-226. [PubMed] |
| 247. | Friedman A. Sleep pattern in Parkinson’s disease. Acta Med Pol. 1980;21:193-199. [PubMed] |
| 248. | Christos GA. Is Alzheimer’s disease related to a deficit or malfunction of rapid eye movement (REM) sleep? Med Hypotheses. 1993;41:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 249. | Kundermann B, Thum A, Rocamora R, Haag A, Krieg JC, Hemmeter U. Comparison of polysomnographic variables and their relationship to cognitive impairment in patients with Alzheimer’s disease and frontotemporal dementia. J Psychiatr Res. 2011;45:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 250. | Gagnon JF, Petit D, Fantini ML, Rompré S, Gauthier S, Panisset M, Robillard A, Montplaisir J. REM sleep behavior disorder and REM sleep without atonia in probable Alzheimer disease. Sleep. 2006;29:1321-1325. [PubMed] |
| 251. | Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 434] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 252. | Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. Neuropathology of aminergic nuclei in Alzheimer’s disease. Prog Clin Biol Res. 1989;317:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 263] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 253. | Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D, Jardanhazi-Kurutz D, Walter J, Kirchhoff F, Hanisch UK. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci USA. 2010;107:6058-6063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 254. | Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 255. | Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, Raskind MA, Peskind ER. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009;17:744-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 256. | Traver S, Salthun-Lassalle B, Marien M, Hirsch EC, Colpaert F, Michel PP. The neurotransmitter noradrenaline rescues septal cholinergic neurons in culture from degeneration caused by low-level oxidative stress. Mol Pharmacol. 2005;67:1882-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 257. | García CR, Angelé-Martínez C, Wilkes JA, Wang HC, Battin EE, Brumaghim JL. Prevention of iron- and copper-mediated DNA damage by catecholamine and amino acid neurotransmitters, L-DOPA, and curcumin: metal binding as a general antioxidant mechanism. Dalton Trans. 2012;41:6458-6467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 258. | Troadec JD, Marien M, Darios F, Hartmann A, Ruberg M, Colpaert F, Michel PP. Noradrenaline provides long-term protection to dopaminergic neurons by reducing oxidative stress. J Neurochem. 2001;79:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 259. | Patri M, Singh A, Mallick BN. Protective role of noradrenaline in benzo[a]pyrene-induced learning impairment in developing rat. J Neurosci Res. 2013;91:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 260. | Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 261. | Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 747] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 262. | Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol Psychiatry. 2011;70:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 263. | Aston-Jones G, Gonzalez M, Doran S. Role of the locus coeruleus-norepinephrine system in arousal and circadian regulation of the sleep–wake cycle. Cambridge Cambridge University Press. 2007;157-195. |
| 264. | Varghese FP, Brown ES. The Hypothalamic-Pituitary-Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians. Prim Care Companion J Clin Psychiatry. 2001;3:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 265. | Hensler JG, Artigas F, Bortolozzi A, Daws LC, De Deurwaerdère P, Milan L, Navailles S, Koek W. Catecholamine/Serotonin interactions: systems thinking for brain function and disease. Adv Pharmacol. 2013;68:167-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 266. | Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1480] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 267. | Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 430] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 268. | Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 621] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 269. | Orzeł-Gryglewska J. Consequences of sleep deprivation. Int J Occup Med Environ Health. 2010;23:95-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 270. | Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 744] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 271. | Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1702] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 272. | Smith C. Sleep states and learning: a review of the animal literature. Neurosci Biobehav Rev. 1985;9:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 202] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 273. | Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 274. | Conwell W, Patel B, Doeing D, Pamidi S, Knutson KL, Ghods F, Mokhlesi B. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 275. | Mokhlesi B, Punjabi NM. “REM-related” obstructive sleep apnea: an epiphenomenon or a clinically important entity? Sleep. 2012;35:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |