Revised: December 3, 2013
Accepted: April 9, 2014
Published online: March 28, 2014
Processing time: 222 Days and 2.2 Hours
Coenzyme Q10 (CoQ10) is an essential cofactor in the mitochondrial respiratory pathway and also functions as a lipid-soluble antioxidant. CoQ10 deficiency has been implicated in many clinical disorders and aging. Primary CoQ10 deficiency is a group of recessively inherited diseases caused by mutations in any gene involved in the CoQ10 biosynthesis pathway. Although primary CoQ10 deficiency is rare, its diagnosis is important because it is potentially treatable with exogenous CoQ10. Multiple system atrophy (MSA) was recently shown to be linked to mutations in the COQ2 gene, one of the genes involved in the CoQ10 biosynthesis pathway. MSA is relatively common in adult-onset neurodegenerative diseases characterized by Parkinsonism, cerebellar ataxia and autonomic failures. Because COQ2 mutations are associated with an increased risk of MSA, oral CoQ10 supplementation may be beneficial for MSA, as for other primary CoQ10 deficiencies. Statins are 3-hydroxy-3-methylglutaryl coenzyme A inhibitors that inhibit the biosynthesis of cholesterol, as well as the synthesis of mevalonate, a critical intermediate in cholesterol synthesis. Statin therapy has been associated with a variety of muscle complaints from myalgia to rhabdomyolysis. Statin treatment carries a potential risk of CoQ10 deficiency, although no definite evidence has implicated CQ10 deficiency as the cause of statin-related myopathy.
Core tip: Recently, multiple system atrophy (MSA), relatively common in adult-onset neurodegenerative diseases, was shown to be linked to mutations in the COQ2 gene, one of the genes involved in the Coenzyme Q10 (CoQ10) biosynthesis pathway. Neurologists so far have not paid much attention to CoQ10 because primary CoQ10 deficiency caused by mutations in the CoQ10 synthesizing genes is very rare. The most important message is that primary CoQ10 deficiency is treatable with exogenous CoQ10 and that oral CoQ10 supplementation might be also beneficial for patients with MSA.
- Citation: Takahashi H, Shimoda K. Coenzyme Q10 in neurodegenerative disorders: Potential benefit of CoQ10 supplementation for multiple system atrophy. World J Neurol 2014; 4(1): 1-6
- URL: https://www.wjgnet.com/2218-6212/full/v4/i1/1.htm
- DOI: https://dx.doi.org/10.5316/wjn.v4.i1.1
Coenzyme Q10 (CoQ10), or ubiquinone, is a lipophilic molecule present in cell membranes that functions as an essential cofactor for electron transport in the mitochondrial respiratory chain and as an endogenous antioxidant[1]. CoQ10 is synthesized in all cells from tyrosine (or phenylalanine) and mevalonate, with its highest levels found in tissues with high energy turnover, including the heart, brain, liver and kidneys[2]. Levels of CoQ10 are known to decrease with age in various tissues of healthy normal humans and rats[1,3,4].
CoQ10 may be used to improve mitochondrial dysfunction and act as an antioxidant in various clinical conditions[5]. Although CoQ10 has been approved in some countries to treat conditions such as congestive heart failure, it is generally classified as a dietary supplement in most countries and can be purchased over the counter. Interest is particularly keen in the United States where CoQ10 is available in more than 100 single and combination ingredient products, although it has not been approved by the Food and Drug Administration for the medical treatment of any condition.
Primary CoQ10 deficiency is a group of rare, recessively inherited diseases. Its recognition is very important because it is potentially treatable with exogenous CoQ10[6,7]. A study by the multiple system atrophy (MSA) Research Collaboration, published in a recent issue of the New England Journal of Medicine, reported a link between MSA and mutations in the COQ2 gene, which encodes one of the proteins involved in the CoQ10 biosynthesis pathway[8]. MSA is relatively common in adult-onset neurodegenerative diseases characterized by Parkinsonism, cerebellar ataxia and autonomic failures. This discovery prompted the reconsideration of the roles of mitochondrial function and oxidative stress in the pathogenesis of these neurodegenerative diseases and the potential benefit of CoQ10 supplementation in patients with MSA and related diseases. In this mini review, we also discuss the potential risk in these patients of statins, a group of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, because statin inhibition of mevalonate synthesis not only inhibits the biosynthesis of cholesterol, but may also inhibit the biosynthesis of CoQ10.
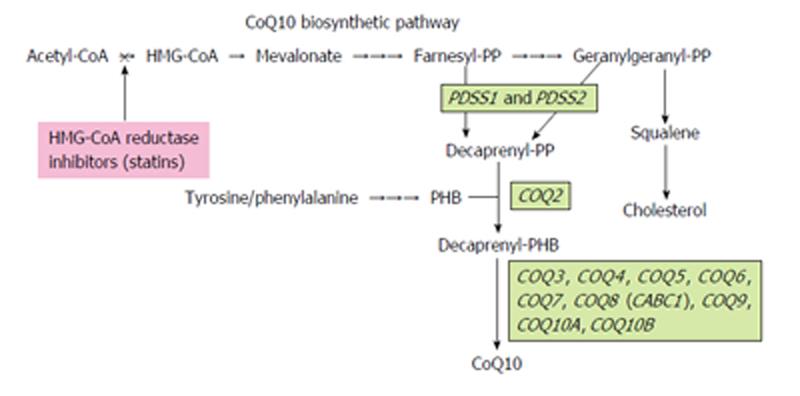
CoQ10 is a vital component of the mitochondrial respiratory chain, with de novo biosynthesis of CoQ10 occurring mainly in the mitochondria. CoQ10 biosynthesis is a complex biological process that is not completely understood in humans[9]. Therefore, its biosynthesis pathway has been elucidated in other organisms, including yeasts and bacteria. CoQ10 consists of a benzoquinone ring and a polyprenyl side chain; the benzoquinone ring is synthesized from tyrosine or phenylalanine and the polyprenyl side chain from intermediates in the mevalonate pathway[9].
As shown in Figure 1, decaprenyl diphosphate (decaprenyl-PP) is synthesized from mevalonate by the PDSS1-PDSS2 enzyme complex via the intermediates farnesyl-PP and geranylgeranyl-PP. Para-hydroxybenzoate-polyprenyl transferase, or COQ2, subsequently catalyzes the condensation of decaprenyl-PP with para-hydroxybenzoate, synthesized from tyrosine or phenylalanine. At least eight more COQ enzymes (COQ3-COQ10A, B), which catalyze methylation, decarboxylation and hydroxylation reactions, are required to produce functional CoQ10.
Primary CoQ10 deficiency is caused by mutations in any of the COQ genes, whereas secondary COQ10 deficiency is caused by genetic defects independent of the CoQ10 biosynthesis pathway or by other inhibitors of the CoQ10 biosynthesis pathway[6,10,11].
Ten genes implicated in the biosynthesis of CoQ10 have been characterized in yeast and 16 human homologs of these genes have been identified in the human genome database[10]. To date, primary CoQ10 deficiency has been linked to 7 of the 16 human genes, PDSS1, PDSS2, COQ2, COQ4, COQ6, COQ8 (ADCK3/CABC1) and COQ9[12-24] (Table 1). CoQ10 deficiency has been associated with five major clinical phenotypes: (1) encephalomyopathy; (2) severe infantile multisystemic disease; (3) cerebellar ataxia; (4) isolated myopathy; and (5) nephropathy[25]. Primary CoQ10 deficiency is clinically and molecularly heterogeneous and phenotypes differ even in patients with the same gene mutation. Importantly, primary CoQ10 deficiency is generally responsive to CoQ10 supplementation (Table 1).
| Clinical features | Age at onset | Response to CoQ10 supplementation | Ref. | |
| PDSS1 | Severe infantile multi-systemic disease | 1-2 year | Improved and alive | [12] |
| PDSS2 | Severe infantile multi-systemic disease | 3 mo | No clinical response | [13] |
| Leigh syndrome | ||||
| COQ2 | Nephropathy Severe infantile multi-systemic disease | Infantile-or early childhood-onset | Dramatic improvement of neurological manifestations and nephritic syndrome | [12,14-17] |
| Multiple system atrophy | Adult-onset | Unknown | [8] | |
| COQ4 | Encephalomyopathy | < 3 yr | Significant improvement of neuromuscular symptoms | [18] |
| COQ6 | Nephropathy with sensorineural deafness | Infantile-or early childhood-onset | Improvement of nephritic syndrome and hearing loss | [19] |
| Severe infantile multi-systemic disease | < 3 yr | |||
| COQ8 (CABC1) | Cerebellar ataxia | Juvenile-or adult-onset | Severe neurological deficit with epilepsy ~mild improvement of ataxia | [20-23] |
| COQ9 | Severe infantile multi-systemic disease | Birth | No clinical response | [24] |
Primary CoQ10 deficiency is unique among mitochondrial diseases because an effective therapy is available, at least for some patients. Early and sufficient administration of primary CoQ10 is considered important for good outcomes[25]. Early administration of CoQ10 was found to resolve renal symptoms and prevent neurological damage in a patient with a COQ2 mutation[26]. In contrast, a patient with a COQ9 mutation and severe infantile multisystemic disease did not respond to CoQ10 treatment[24]. The reason that patients with a CoQ10 deficiency vary in response to CoQ10 treatment is not completely understood. Insufficient improvement may be due, however, to the occurrence of irreversible disease manifestations prior to diagnosis and treatment. Therefore, correct and timely diagnosis allows prompt treatment with exogenous CoQ10 and may improve the outcome of these otherwise devastating and potentially fatal disorders.
The amount of CoQ10 in the diet is not sufficient to significantly increase the serum CoQ10 level[9,27]. Rather, a significant increase in its serum level requires supplementation with about 100 mg/d CoQ10[5]. Patients with CoQ10 deficiency have been treated with 100-3000 mg/d of this agent[25]. High doses and long-term administration of exogenous CoQ10 are considered necessary for patient benefit. The bioavailability of different CoQ10 formulations should also be considered[28]. Although CoQ10 is present as both oxidized and reduced forms in the body and both forms are commercially available, the absorption rate of the reduced form is higher than that of the oxidized form because the oxidized form must be reduced upon absorption from the gastrointestinal tract[29].
CoQ10 content in various tissues increases after CoQ10 supplementation. Oral administration of CoQ10 was found to increase CoQ10 levels in both the brain and brain mitochondria[30]. Transfer of exogenous CoQ10 across the blood-brain barrier may require higher CoQ10 doses, perhaps explaining why cerebellar ataxia in patients with primary CoQ10 deficiency shows variable responses to exogenous CoQ10 treatment.
No absolute contraindications are known for CoQ10. Adverse effects with CoQ10 are rare, with fewer than 1% of patients reporting mild gastrointestinal discomfort[29]. Even oral administration of 3000 mg/d for 8 mo was reported to be safe and well tolerated in patients with amyotrophic lateral sclerosis[31].
MSA is an adult-onset, progressive, neurodegenerative disorder that clinically presents as autonomic failure and cerebellar ataxia and/or parkinsonism[32,33]. To date, few symptomatic therapies are available. L-dopa therapy has been shown to be effective for motor symptoms of Parkinsonism for a limited period and several drugs have been used to treat autonomic failure, such as orthostatic hypotension and urinary bladder disturbance[34]. Symptoms in MSA progress rather rapidly and its prognosis is relatively poor; overall survival after disease onset is less than 10 years on average[35,36].
MSA is generally considered a sporadic disease but several familial cases have been reported, suggesting that some genetic factors are associated with susceptibility to MSA[37]. Using linkage analysis and whole genome sequencing, the Multiple System Atrophy Research Collaboration team in Japan identified mutations in the COQ2 gene in members of two multiplex families with autopsy-proven MSA[8]. These mutations include a homozygous mutation (M78V-V343A/M78V-V343A) and compound heterozygous mutations (R337X/V343A). Moreover, a common variant (V343A) and multiple rare variants in the COQ2 gene were found to be associated with sporadic MSA. The frequency of the V343A allele is significantly higher in MSA patients than in controls (4.8% vs 1.6%-2.2%). The V343A variant has been found exclusively in Japanese individuals. Thus, this variant represents a susceptibility factor rather than a causative factor for MSA.
Each variant of COQ2 was functionally impaired in yeast complementation assays. Intracellular CoQ10 levels and COQ2 enzyme activities in lymphoblast cell lines established from MSA patients with the two variant alleles were substantially lower than those in controls. Intracellular levels of CoQ10 in the brain tissue of individuals with the homozygous mutation (M78V-V343A) were much lower than in controls. A previous study revealed that the activity of mitochondrial complex I was significantly lower in muscle mitochondria from patients with MSA than in mitochondria from age-matched controls[38]. Because COQ2 mutations are associated with an increased risk of MSA, oral CoQ10 supplementation may be beneficial for patients with MSA, similar to findings in other primary CoQ10 deficiencies.
Statins are the most effective medications currently in use for reducing low-density lipoprotein cholesterol levels. Statins competitively inhibit HMG-CoA reductase, thereby blocking the synthesis of mevalonate, a critical intermediate in the cholesterol synthesis pathway (Figure 1). Although statins have revolutionized clinical cardiology and are generally safe, statin therapy has been associated with a variety of muscle complaints from myalgia to life-threatening rhabdomyolysis[39-41]. The mechanism of statin-related myopathy is unknown but may involve mitochondrial dysfunction resulting from intramuscular CoQ10 deficiency which, in turn, may be due to statin interference with CoQ10 biosynthesis in the same mevalonate pathway.
Statins have been found to reduce circulating CoQ10 levels in humans but low-dose statin treatment does not appear to reduce intramuscular CoQ10 levels. Studies using muscle biopsy materials from patients with statin-related myopathy have yielded conflicting results, with one study suggesting that morphological changes are consistent with mitochondrial dysfunction, while another found that the muscle CoQ10 level is mildly decreased but there was no biochemical or histochemical evidence of mitochondrial myopathy[42,43]. CoQ10 supplementation can increase circulating CoQ10 levels but it is not clear whether this relieves muscle complaints.
Collectively, no definite evidence has implicated CoQ10 deficiency as the cause of statin-related myopathy. However, case reports have described patients with mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes whose symptoms were temporally related to statin therapy[44-46], suggesting that statins may provoke mitochondrial diseases in susceptible individuals. The same may be true for individuals susceptible to MSA as well as to other primary and secondary CoQ10 deficiencies.
CoQ10 deficiencies are clinically and genetically heterogeneous. Although they are rare, their recognition is important because clinical improvement after CoQ10 supplementation has been repeatedly documented in many patients. The discovery of a link between a CoQ10 synthesizing enzyme and MSA provides new insights into the pathogenesis of MSA and suggests the potential benefit of CoQ10 supplementation. Further studies may lead to effective therapies for MSA and other CoQ10 deficiencies.
P- Reviewers: Orlacchio A, Zhou W S- Editor: Song XX L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20:591-598. [PubMed] |
| 2. | Aberg F, Appelkvist EL, Dallner G, Ernster L. Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys. 1992;295:230-234. [PubMed] |
| 3. | Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579-584. [PubMed] |
| 4. | Pignatti C, Cocchi M, Weiss H. Coenzyme Q10 levels in rat heart of different age. Biochem Exp Biol. 1980;16:39-42. [PubMed] |
| 5. | Littarru GP, Tiano L. Clinical aspects of coenzyme Q10: an update. Nutrition. 2010;26:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Mugoni V, Postel R, Catanzaro V, De Luca E, Turco E, Digilio G, Silengo L, Murphy MP, Medana C, Stainier DY. Ubiad1 is an antioxidant enzyme that regulates eNOS activity by CoQ10 synthesis. Cell. 2013;152:504-518. [PubMed] |
| 7. | Quinzii CM, DiMauro S, Hirano M. Human coenzyme Q10 deficiency. Neurochem Res. 2007;32:723-727. [PubMed] |
| 8. | Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 9. | Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171-199. [PubMed] |
| 10. | Rötig A, Mollet J, Rio M, Munnich A. Infantile and pediatric quinone deficiency diseases. Mitochondrion. 2007;7 Suppl:S112-S121. [PubMed] |
| 11. | Quinzii CM, Hirano M. Primary and secondary CoQ(10) deficiencies in humans. Biofactors. 2011;37:361-365. [PubMed] |
| 12. | Mollet J, Giurgea I, Schlemmer D, Dallner G, Chretien D, Delahodde A, Bacq D, de Lonlay P, Munnich A, Rötig A. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;117:765-772. [PubMed] |
| 13. | López LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, Dimauro S, Hirano M. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125-1129. [PubMed] |
| 14. | Quinzii C, Naini A, Salviati L, Trevisson E, Navas P, Dimauro S, Hirano M. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet. 2006;78:345-349. [PubMed] |
| 15. | Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773-2780. [PubMed] |
| 16. | Scalais E, Chafai R, Van Coster R, Bindl L, Nuttin C, Panagiotaraki C, Seneca S, Lissens W, Ribes A, Geers C. Early myoclonic epilepsy, hypertrophic cardiomyopathy and subsequently a nephrotic syndrome in a patient with CoQ10 deficiency caused by mutations in para-hydroxybenzoate-polyprenyl transferase (COQ2). Eur J Paediatr Neurol. 2013;17:625-630. [PubMed] |
| 17. | Jakobs BS, van den Heuvel LP, Smeets RJ, de Vries MC, Hien S, Schaible T, Smeitink JA, Wevers RA, Wortmann SB, Rodenburg RJ. A novel mutation in COQ2 leading to fatal infantile multisystem disease. J Neurol Sci. 2013;326:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Salviati L, Trevisson E, Rodriguez Hernandez MA, Casarin A, Pertegato V, Doimo M, Cassina M, Agosto C, Desbats MA, Sartori G. Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J Med Genet. 2012;49:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, Xie LX, Salviati L, Hurd TW, Vega-Warner V. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121:2013-2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 20. | Lagier-Tourenne C, Tazir M, López LC, Quinzii CM, Assoum M, Drouot N, Busso C, Makri S, Ali-Pacha L, Benhassine T. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Mollet J, Delahodde A, Serre V, Chretien D, Schlemmer D, Lombes A, Boddaert N, Desguerre I, de Lonlay P, de Baulny HO. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Gerards M, van den Bosch B, Calis C, Schoonderwoerd K, van Engelen K, Tijssen M, de Coo R, van der Kooi A, Smeets H. Nonsense mutations in CABC1/ADCK3 cause progressive cerebellar ataxia and atrophy. Mitochondrion. 2010;10:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Horvath R, Czermin B, Gulati S, Demuth S, Houge G, Pyle A, Dineiger C, Blakely EL, Hassani A, Foley C. Adult-onset cerebellar ataxia due to mutations in CABC1/ADCK3. J Neurol Neurosurg Psychiatry. 2012;83:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Duncan AJ, Bitner-Glindzicz M, Meunier B, Costello H, Hargreaves IP, López LC, Hirano M, Quinzii CM, Sadowski MI, Hardy J. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Emmanuele V, López LC, Berardo A, Naini A, Tadesse S, Wen B, D’Agostino E, Solomon M, DiMauro S, Quinzii C. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch Neurol. 2012;69:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 26. | Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med. 2008;358:2849-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 27. | Bonakdar RA, Guarneri E. Coenzyme Q10. Am Fam Physician. 2005;72:1065-1070. [PubMed] |
| 28. | Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7 Suppl:S78-S88. [PubMed] |
| 29. | Hidaka T, Fujii K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors. 2008;32:199-208. [PubMed] |
| 30. | Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc Natl Acad Sci USA. 1998;95:8892-8897. [PubMed] |
| 31. | Ferrante KL, Shefner J, Zhang H, Betensky R, O’Brien M, Yu H, Fantasia M, Taft J, Beal MF, Traynor B. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65:1834-1836. [PubMed] |
| 32. | Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2050] [Cited by in RCA: 2341] [Article Influence: 137.7] [Reference Citation Analysis (0)] |
| 33. | Wenning GK, Stefanova N. Recent developments in multiple system atrophy. J Neurol. 2009;256:1791-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Flabeau O, Meissner WG, Tison F. Multiple system atrophy: current and future approaches to management. Ther Adv Neurol Disord. 2010;3:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125:1070-1083. [PubMed] |
| 36. | Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Köllensperger M, Goebel G, Pfeiffer KP, Barone P. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12:264-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 380] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 37. | Stemberger S, Scholz SW, Singleton AB, Wenning GK. Genetic players in multiple system atrophy: unfolding the nature of the beast. Neurobiol Aging. 2011;32:1924.e5-1924.14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Blin O, Desnuelle C, Rascol O, Borg M, Peyro Saint Paul H, Azulay JP, Billé F, Figarella D, Coulom F, Pellissier JF. Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson’s disease and multiple system atrophy. J Neurol Sci. 1994;125:95-101. [PubMed] |
| 39. | Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150:858-868. [PubMed] |
| 40. | Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231-2237. [PubMed] |
| 42. | Lamperti C, Naini AB, Lucchini V, Prelle A, Bresolin N, Moggio M, Sciacco M, Kaufmann P, DiMauro S. Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol. 2005;62:1709-1712. [PubMed] |
| 43. | Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JD, Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581-585. [PubMed] |
| 44. | Chariot P, Abadia R, Agnus D, Danan C, Charpentier C, Gherardi RK. Simvastatin-induced rhabdomyolysis followed by a MELAS syndrome. Am J Med. 1993;94:109-110. [PubMed] |
| 45. | Thomas JE, Lee N, Thompson PD. Statins provoking MELAS syndrome. A case report. Eur Neurol. 2007;57:232-235. [PubMed] |
| 46. | Tay SK, Dimauro S, Pang AY, Lai PS, Yap HK. Myotoxicity of lipid-lowering agents in a teenager with MELAS mutation. Pediatr Neurol. 2008;39:426-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |