Peer-review started: November 1, 2015
First decision: November 30, 2015
Revised: December 19, 2015
Accepted: January 27, 2016
Article in press: January 29, 2016
Published online: May 2, 2016
Processing time: 180 Days and 17.5 Hours
Dermal mucinosis is often associated with collagen diseases such as rheumatoid arthritis, lupus erythematosus, and dermatomyositis, in addition to autoimmune thyroiditis. We report eight cases of dermal mucin deposition secondary to typical dermatomyositis with cutaneous lesions known as heliotrope rash and Gottron’s papules. Striking mucin deposition was observed in both the papillary dermis and reticular dermis of all biopsy specimens. Immunohistochemical analysis showed that CD34+ dermal dendritic cells (DDCs) in the perilesional area in combination with vimentin+ cells within the mucinous lesion might be important in giving rise to abnormal deposition of dermal mucin. On the other hand, numbers of factor XIIIa+ DDCs and tryptase+ mast cells were reduced within and surrounding the mucin deposition, as compared with those in the dermis of normal controls. A pathogenic mechanism of dermal mucin deposition is proposed.
Core tip: Immunohistochemical analysis of skin biopsy specimens with dermatomyositis showed the involvement of CD34+ dermal dendritic cells, α-smooth muscle actin+ myofibroblasts and possibly mast cells, as well as vimentin+ fibroblasts for abnormally dermal mucin production. Further pathophysiological studies are required to more precisely clarify secondary cutaneous mucin deposition by CD34+ dermal dendritic cells. CD34+ dermal dendritic cells and mast cells might be important in giving rise to deposition of dermal mucin in dermatomyositis.
- Citation: Yokoyama E, Nakamura Y, Okita T, Nagai N, Muto M. CD34+ dermal dendritic cells and mucin deposition in dermatomyositis. World J Dermatol 2016; 5(2): 65-71
- URL: https://www.wjgnet.com/2218-6190/full/v5/i2/65.htm
- DOI: https://dx.doi.org/10.5314/wjd.v5.i2.65
Dermal mucin deposition has been seen in several skin diseases, including granuloma annulare, autoimmune thyroiditis, and collagen diseases (dermatomyositis, scleroderma, and lupus erythematosus). These observations led us to imagine dermal mucinosis as a heterogeneous group of pathologies showing a common factor of dermal mucin deposition.
We have previously reported a rare case of self-healing papular mucinosis (SHPM) in a patient with rheumatoid arthritis[1]. We suggested that CD34+ or Factor XIIIa+ (FXIIIa+) dermal dendritic cells (DDCs) and tryptase+ mast cells (MCs) in the perilesional area in combination with vimentin+ cells in the mucinous lesion might have been involved in the dermal deposition of mucin. DDCs and MCs would then presumably play a key role in the development of mucinosis.
The present study tentatively defined DDCs as all the cells in connective tissue morphologically showing a dendritic shape. These fibroblast-like cells in the connective tissue have been classified into the following groups[2-8]: (1) true fibroblasts; (2) myofibroblasts; (3) CD34+ DDCs; (4) FXIIIa+ DDCs; and (5) others. Fibroblasts are regarded as those cells positive only for vimentin, and myofibroblasts as those positive for both vimentin and α-smooth muscle actin (α-SMA)[2,8]. DDCs are divided into CD34+ and FXIIIa+ DDCs[2-7]. Other fibroblast-like cells (i.e., not categories 1-4) are not specified further, and are collected as other fibroblast-like cells.
The aim of the present study was to elucidate the involvement of DDCs and MCs in the dermal mucin deposition seen in dermatomyositis.
Participants comprised 8 patients who had been clinicopathologically diagnosed with dermatomyositis. This study followed eight cases of dermatomyositis showing clear mucin deposition in biopsied skin tissues taken at the time of the first medical examination. Fifteen volunteers who underwent removal of nevus cell nevi (including normal skin) were used as site-matched controls. Mean (± standard deviation) ages in the patient and control groups were 53 ± 26 years (range, 34-86 years) and 22 ± 20 years (range, 12-64 years), respectively. Male-to-female ratios were 3:5 in the patient group and 6:9 in controls.
Skin biopsy specimens from the 8 patients with dermatomyositis were obtained from the thigh in 3 cases, from the chest in 3 cases, and from the dorsum of the hand in 2 cases. Normal skin consisting of the remaining unaffected portion of surgically removed nevus cell nevus was derived from various corresponding control sites. Informed consent was obtained from all subjects prior to participation in the present study.
Archival paraffin embedded tissues from dermatomyositis and normal skin were utilized for this study. In each case, formalin-fixed, paraffin-embedded, 4-μm-thick sections were stained by the periodic acid-Schiff (PAS) technique, with Alcian blue (pH 2.5). Fibroblast-like cells were immunohistochemically recognized using antibodies for vimentin, CD34, FXIIIa, α-SMA, and desmin. MCs were identified by immunohistochemical staining for tryptase.
Sections from all cases were stained with an avidin-biotin peroxidase technique, using an ENVISION kit (Dako, Carpinteria, CA). Antibodies used in the present study are shown in Table 1. Cells were stained with mouse monoclonal and rabbit polyclonal antibodies. Sections were deparaffinized in xylene and rehydrated in a graded alcohol series. Endogenous peroxidase activity was removed by immersion in methanol with 3% hydrogen peroxide for 10 min. Non-specific binding was blocked by incubation for 5 min at room temperature with non-immune goat serum. Primary antibodies were then applied to sections and incubated for 45 min at room temperature. Secondary rabbit anti-mouse immunoglobulin (Ig) was applied for 45 min at room temperature. Finally, specimens were developed with 3,3’-diamino benzidine solution and 1% hydrogen peroxide, then counterstained with Mayer’s hematoxylin.
| Antibody | Type | Source | Dilution |
| Vimentin | Mouse | Dako | 1:40 |
| CD34 | Mouse | Nichirei | 1:1 |
| Factor XIIIa | Rabbit | Biogenesis | 1:50 |
| α-smooth muscle actin | Mouse | Dako | 1:50 |
| Desmin | Mouse | Dako | 1:1 |
| Tryptase | Mouse | Dako | 1:50 |
DDC and MC counts were assessed as the number of positive cells per 10 high-power fields (× 400) on each skin specimen by a single observer. Statistical significance was analyzed using Student’s t-test. Student’s t-test was applied for comparisons of mean numbers of positive cells between dermatomyositis and normal skin samples. Values of P < 0.05 were considered significant.
The profiles of eight patients with dermatomyositis are shown in Table 2. No patients had yet received any treatments (including steroids) for dermatomyositis at the time of biopsy. The interval between observation of the first skin symptom to first medical examination ranged from 1 to 6 mo (1 mo, n = 2; 5 mo, n = 2; 6 mo, n = 1; 3 mo, n = 1; 2 mo, n = 1; unknown, n = 1). Three cases (cases 2, 3 and 6) had muscle weakness, two cases (cases 3 and 7) had arthralgia, and one case (case 1) developed respiratory failure. Only one case (case 8) showed lung cancer; this patient died after 1 year. None of the other seven patients had internal malignancy.
| No | Age | Sex/period | Clinical findings | Sight of skin biopsy | Pathological findings |
| 1 | 86 | Male | Rash erythema of whole body respiratory failure | Right tight | Vacuolar change, mucin in papillary and reticular dermis |
| One month | |||||
| 2 | 59 | Female | Lilac rash of eyelids, erythema rash of limbs, dorsal hands of Gottron’s papules arthralgia | Right tight | Vacuolar change, subepidermal blister, mucine in papillary dermis |
| One month | |||||
| 3 | 53 | Female | Dorsal hands of Gottron’s papules, scary erythema of face, chest, limbs, arthralgia, muscle weakness | Light tight | Vacuolar change, periadnexal infiltration |
| Two months | mucin in papillary and reticular dermis | ||||
| 4 | 63 | Female | Lilac rash of eyelids, dorsal hands of Gottron’s papules, scary erythema of face, neck, chest | Chest | Vacuolar change, periadnexal infiltration mucin in papillary and reticular dermis |
| Five months | |||||
| 5 | 46 | Male | Dale reddish erythema of face, edematous erythema of neck | Limbs | Vacuolar change, melanine incontinen mucin in papillary and reticular dermis |
| Unidentified | |||||
| 6 | 34 | Female | Dorsal hands of Gottron’s papules rash erythema of eyelid, nasal grooves muscle weakness | Chest | Hyperkeratosis, vacuolar change, mucin in papillary dermis |
| Five months | |||||
| 7 | 46 | Female | Dorsal hands of Gottron’s papules, scary erythema of knee and hip, arthralgia | Light dorsal hand | Hyperkeratosis, vacuolar change, mucin in papillary and reticular dermis |
| Three months | |||||
| 8 | 57 | Male1 | Edematous erythema of face, neck, chest and shoulder | Chest | Vacuolar change, mucin in papillary and reticular dermis |
| Six months |
In terms of histopathology, mucin deposition was in the papillary dermis in two cases, and in the papillary and reticular dermis in six cases. All cases showed vacuolar change and one had subepidermal blistering (case 2). Two cases (cases 3 and 4) had periadnexal infiltration.
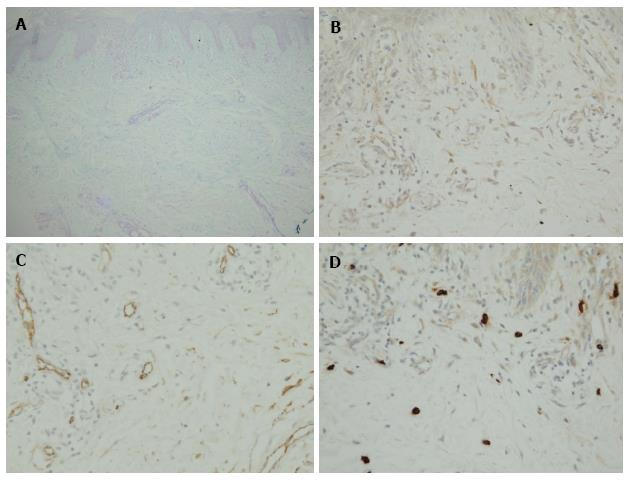
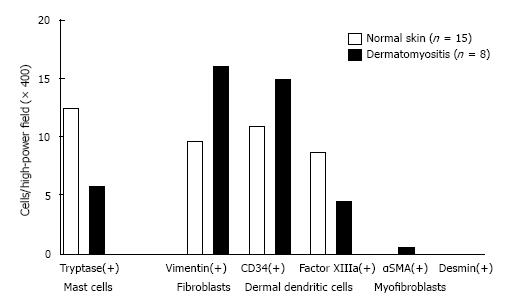
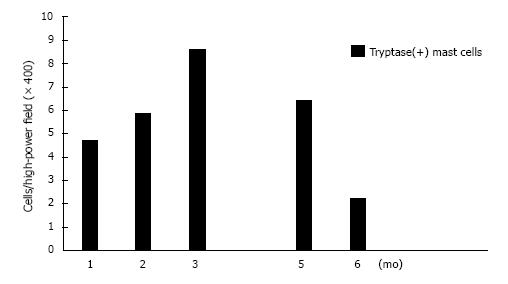
Our findings are shown in Figures 1 and 2 and Table 3. PAS-negative and Alcian blue-positive mucin deposition was identified in all 8 skin samples from dermatomyositis patients. Histologically, mucin deposition demonstrable with Alcian blue staining was distributed diffusely but not focally in the papillary dermis, and to a lesser extent between collagen bundles of the reticular dermis with or without sparse lymphocytic infiltrates. Vimentin+ fibroblasts (16.01 ± 3.23 in dermatomyositis vs 9.65 ± 1.37 in controls; P = 0.052), CD34+ DDCs (14.94 ± 3.35 in dermatomyositis vs 10.93 ± 1.13 in controls; P = 0.18), and α-SMA+ myofibroblasts (0.48 ± 0.45 in dermatomyositis vs 0 in controls; P = 0.14) tended to be moderately increased in numbers, whereas numbers of FXIIIa+ DDCs were decreased in dermatomyositis skin, compared to normal skin (4.40 ± 1.62 in dermatomyositis vs 8.71 ± 2.17 in control; P = 0.17), although no significant differences were evident between diseased and control groups. In contrast, significant differences between the two groups were only seen for numbers of tryptase-positive MCs (5.79 ± 0.81 in dermatomyositis vs 12.32 ± 1.00 in controls; P = 0.00027). MCs were diffusely scattered without forming cell clusters. DDCs and MCs were counted separately in areas of periadnexal matrix and interstitial portions of the dermis. However, no significant differences were seen between groups. Furthermore, histopathological examination revealed no increase in vascularization within the dermis among the 8 patients with dermatomyositis. We examined the tryptase(+) MC count according to progress before performing a biopsy after the onset of exanthema (Figure 3). For 3 mo, a tendency to increase was seen, followed by a gradual decrease, and, for 1 mo, it is with a low value most in 6 mo. Unfortunately, the extremely limited number of cases adversely impacted the statistical power of our comparisons.
| Normal skin (n = 15) mean ± SE cells/high-power field (× 400) | Dermatomyositis (n = 8) mean ± SE cells/high-power field (× 400) | P value | |
| Mast cells tryptase | 12.320 ± 0.997 | 5.788 ± 0.805 | 0.000270 |
| Dendritic cells | |||
| Vimentin | 9.654 ± 1.374 | 16.0125 ± 3.257 | 0.0524 |
| CD34 | 10.933 ± 1.131 | 14.937 ± 3.257 | 0.177 |
| Factor XIIIa | 8.708 ± 2.172 | 4.40 ± 1.619 | 0.166 |
| α-SMA | 0 | 0.488 ± 0.447 | 0.141 |
| Desmin | 0 | 0 |
The present immunohistochemical analysis of dermatomyositis showed a moderate increase in CD34+ DDCs and vimentin+ cells lacking CD34 or FXIIIa related to mucin deposition in the dermis. We have previously reported a rare case of self-healing papular mucinosis (SHPM) in a patient with rheumatoid arthritis[1]. In that lesion, well-circumscribed mucin deposition was demonstrated in the papillary dermis with Alcian blue staining. The overlying epidermis showed the formation of an epidermal collarette. On the other hand, in the case of dermatomyositis, diffuse mucin deposition was demonstrated with Alcian blue staining in the papillary dermis and between collagen bundles of the reticular dermis with or without sparse lymphocytic inflammatory infiltrates. Mucin deposition was particularly prominent in the papillary dermis. At the time of clinical diagnosis of dermatomyositis and immunohistochemical study, seven of the patients with dermatomyositis had no internal malignancies such as gastric or breast cancer, while one 57-year-old male patient showed lung cancer. There was also no evidence to support thyroid abnormalities among any of the eight patients.
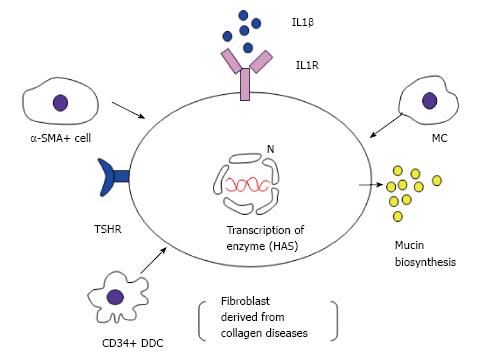
Interstitial mucin deposition is a well-known occurrence in dermatomyositis[9]. However, the pathogenic mechanisms responsible for dermal mucin deposition remain unclear (Figure 4). Mucin is normally produced in small amounts by dermal fibroblasts, and chemically consists of acidic glycosaminoglycans. Rapoport et al[10] hypothesized that the overproduction of mucin results from autoantibodies against thyroid-stimulating hormone receptor stimulating dermal fibroblasts to produce deposition of mucin, mainly as hyaluronic acid. However, our cases showed no evidence of thyroid dysfunction. Another hypothesis is that interleukin (IL) 1β can induce glycosaminoglycan synthesis by fibroblasts via the prostaglandin E2 pathway through cyclooxygenase-2, an enzyme responsible for prostaglandin E2 synthesis[11]. IL1β can be produced by many cells, including fibroblasts, myofibroblasts, and MCs[12]. In our study, a small number of α-SMA+ DDCs were observed in dermatomyositis, whereas none were present among normal controls. The possibility that α-SMA+ myofibroblasts could produce mucin thus remains plausible. A third hypothesis is that Tominaga et al[13] observed an increased hyaluronan content in lesional skin compared to non-lesional skin in a patient with reticular erythematous mucinosis and proposed that the cells responsible for the deposition of hyaluronan in lesional skin were FXIIIa+/hyaluronan synthase 2+ DDCs rather than dermal fibroblasts. However, we found no evidence for an increase in FXIIIa + DDCs in the patient group with dermatomyositis (Figure 2).
Finally, Pugashetti et al[14] noted that local tissue hypoxia in response to chronic venous insufficiency could potentially increase the biosynthetic activity of fibroblasts and thus dermal mucin deposition. Our data offered no clinicopathological evidence for venous insufficiency. Since the numbers of vimentin+ spindle-shaped cells were increased in our cases, mesenchymally derived vimentin+/CD34- fibroblasts, histologically indistinguishable from CD34+ and FXIIIa+ DDCs, appeared likely to represent the source of mucin deposition.
What are the CD34+ cells? In the present study, numbers of CD34+ DDCs tended to be increased, although no significant difference was evident between the patient and normal control groups at the 5% level. Immunohistochemically, CD34 is a human progenitor cell antigen expressed not only on vascular endothelial cells but also on a population of dendritic fusiform cells around cutaneous appendages and in the interstitium, principally in the deep dermis[2,6]. In contrast, FXIIIa immunoreactivity is noted on populations of dendritic cells in the upper portion of the dermis and around blood vessels and cutaneous appendages. Taken together with our data regarding collagen disease involving dermatomyositis and rheumatoid arthritis[1], CD34+ DDCs seem to play important supportive functions in the production of mucin within diseased skin.
Much remains to be learned about CD34-expressing cells in the skin regarding the functional relationships of dermal mucin production between CD34+ DDCs and other cells (FXIIIa+ DDCs, α-SMA+ myofibroblasts, vimentin+ fibroblasts, and tryptase+ MCs). Although we did not identify the DDCs in dermatomyositis more precisely, use of specific antibodies for DDCs such as blood dendritic cell antigens [BDCA-1 for myeloid DCs, BDCA-2 for plasmacytoid DCs (PDCs)] might be valuable for subtyping DDCs, as Shrestha et al[15] recently reported. Using juvenile patients with dermatomyositis, they suggested that increased numbers of mature PDCs with CD34 markers as well as MCs are the major producers of interferon (IFN)α, in which IFNα itself can conversely modulate DCs subsets. The major infiltrating DDCs around mucin-deposited skin lesions in dermatomyositis might plausibly represent PDCs, subsequently influencing the subsequent effector functions of T cells.
MCs positive for tryptase were frequently seen in the perilesional area of predominantly the papillary dermis, although a significant reduction in total numbers of tryptase+ MCs were seen compared to numbers in normal controls. With respect to quantification of MCs, our data did not show any significant increase in the number of MCs at sites of dermatomyositis, as compared with the skin of controls. However, the present study also found greater numbers of MCs in the papillary dermis than in the reticular dermis for both control subjects and patients with dermatomyositis having mucin deposition. Abd El-Aal et al[16] reported that increased numbers of MCs could stimulate dermal fibroblasts to produce mucin in lichen myxedema. Martins et al[17] speculated mucin production from fibroblasts derived from patients with cutaneous mucinosis through the interaction of elevated serum levels of IgE between MCs bearing FcεRIα. We cannot discard this possibility, because we did not examine serum IgE levels for our 8 patients with dermatomyositis.
According to Smith et al[18], colloidal iron stain-positive mucin is present in 97% of skin biopsy samples from dermatomyositis cases and is a characteristic finding on views of dermatomyositis examining the pathological organization, but is not seen in all cases. Mucin deposition can represent an important sign of dermatomyositis, and its association with convalescence is unknown. This study examined the presence of MCs and DDCs in clear cases of mucin deposition with dermatomyositis.
The dermatomyositis and normal skin groups in this study showed similar results, with only MCs counts showing a significant difference. MCs tended to be decreased in our 8 dermatomyositis cases, who had not received treatment as of the time of biopsy. MCs may thus decrease at some stage during the progress of dermatomyositis. We showed this in the tryptase(+)MC count according to progress before performing biopsy after exanthem appeared. A tendency toward an increase was seen for three months after presentation, followed by a decrease.
Immunohistochemical analysis of skin biopsy specimens with dermatomyositis showed the involvement of CD34+ DDCs, α-SMA+ myofibroblasts and possibly MCs, as well as vimentin+ fibroblasts for abnormally dermal mucin production. Further pathophysiological studies are required to more precisely clarify secondary cutaneous mucin deposition by CD34+ DDCs.
P- Reviewer: Cuevas-Covarrubias SA, Hu SCS, Kaliyadan F, Vasconcellos C S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Yokoyama E, Muto M. Adult variant of self-healing papular mucinosis in a patient with rheumatoid arthritis: predominant proliferation of dermal dendritic cells expressing CD34 or factor XIIIa in association with dermal deposition of mucin. J Dermatol. 2006;33:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | McNutt NS, Reed JA. Tumors of the fibrous tissue. Pathology of the skin. 2nd ed. New York: McGraw-Hill 2000; 1160-1161. |
| 3. | Headington JT. The dermal dendrocyte. Chicago: Yearbook Medical Publishers 1986; 159-179. |
| 4. | Headington JT, Cerio R. Dendritic cells and the dermis: 1990. Am J Dermatopathol. 1990;12:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Narvaez D, Kanitakis J, Faure M, Claudy A. Immunohistochemical study of CD34-positive dendritic cells of human dermis. Am J Dermatopathol. 1996;18:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Nickoloff BJ. The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi’s sarcoma. Arch Dermatol. 1991;127:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 162] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Cerio R, Griffiths CE, Cooper KD, Nickoloff BJ, Headington JT. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol. 1989;121:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 255] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Eyden B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol. 2001;25:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis. A histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 76] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Rapoport B, Alsabeh R, Aftergood D, McLachlan SM. Elephantiasic pretibial myxedema: insight into and a hypothesis regarding the pathogenesis of the extrathyroidal manifestations of Graves’ disease. Thyroid. 2000;10:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Schmitz T, Leroy MJ, Dallot E, Breuiller-Fouche M, Ferre F, Cabrol D. Interleukin-1beta induces glycosaminoglycan synthesis via the prostaglandin E2 pathway in cultured human cervical fibroblasts. Mol Hum Reprod. 2003;9:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Mia MM, Boersema M, Bank RA. Interleukin-1β attenuates myofibroblast formation and extracellular matrix production in dermal and lung fibroblasts exposed to transforming growth factor-β1. PLoS One. 2014;9:e91559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Tominaga A, Tajima S, Ishibashi A, Kimata K. Reticular erythematous mucinosis syndrome with an infiltration of factor XIIIa+ and hyaluronan synthase 2+ dermal dendrocytes. Br J Dermatol. 2001;145:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Pugashetti R, Zedek DC, Seiverling EV, Rajendran P, Berger T. Dermal mucinosis as a sign of venous insufficiency. J Cutan Pathol. 2010;37:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Shrestha S, Wershil B, Sarwark JF, Niewold TB, Philipp T, Pachman LM. Lesional and nonlesional skin from patients with untreated juvenile dermatomyositis displays increased numbers of mast cells and mature plasmacytoid dendritic cells. Arthritis Rheum. 2010;62:2813-2822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Abd El-Aal H, Salem SZ, Salem A. Lichen myxedematosus: histochemical study. Dermatologica. 1981;162:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Martins C, Nascimento AP, Monte-Alto-Costa A, Alves Mde F, Carneiro SC, Porto LC. Quantification of mast cells and blood vessels in the skin of patients with cutaneous mucinosis. Am J Dermatopathol. 2010;32:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Smith ES, Hallman JR, DeLuca AM, Goldenberg G, Jorizzo JL, Sangueza OP. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. 2009;31:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |