Published online Nov 27, 2016. doi: 10.5313/wja.v5.i3.44
Peer-review started: July 21 2016
First decision: September 5, 2016
Revised: October 13, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: November 27, 2016
Processing time: 121 Days and 3.3 Hours
Arrhythmogenic right ventricular dysplasia (ARVD) is an inherited heart muscle disease. Myocyte apoptosis and fibro-fatty scar tissue predisposes patients to malignant ventricular arrhythmias. Patients may present to variety of surgical procedures with diagnosed ARVD. Surgical insult, catecholamine surge and physiological disturbance can be hazardous on the vulnerable myocardium and may result in life-threatening ventricular tachycardia or sudden cardiac death in the perioperative period. Anaesthetists have particular role in perioperative management of this patient population, meticulous perioperative planning, close haemodynamic monitoring and maintenance of physiological stability throughout helps to avoid devastating perioperative loss.
Core tip: Arrhythmogenic right ventricular dysplasia (ARVD) is an inherited disease of the cardiac muscle, characterised by progressive myocyte death and scarring of the myocardium associated with ventricular tachycardia and sudden cardiac death. Electrical instability is exacerbated by physiological changes induced by surgical insult and may lead to unexpected perioperative death. Careful anaesthetic management can minimise stress response and reduce the incidence of malignant ventricular arrhythmias in the perioperative period. In this article we discuss the available literature with the aim to provide some guidance for the clinical anaesthetist encountering patient with ARVD.
- Citation: Blaskovics I, Valchanov K. Anaesthesia for patients with arrhythmogenic right ventricular dysplasia. World J Anesthesiol 2016; 5(3): 44-53
- URL: https://www.wjgnet.com/2218-6182/full/v5/i3/44.htm
- DOI: https://dx.doi.org/10.5313/wja.v5.i3.44
Arrhythmogenic right ventricular dysplasia (ARVD) is inherited cardiomyopathy characterised by progressive death of the myocytes most commonly in the right ventricle. Fibro-fatty replacement of the myocardium forms scar tissue causes malignant ventricular arrhythmias, sudden cardiac death (SDC), or cardiac failure. In the late stages it affects both left and right ventricles. Early diagnosis could offer control over progress of the disease and prevention of malignant arrhythmias and cardiac arrest. Once arrhythmia control is achieved AVRD could become a stable condition[1,2].
Patients with ARVD undergo variety of surgical procedures in their life span. This is why the condition is of a particular interest for anaesthetists and peri-operative physicians. Physiological changes and medication in the perioperative period may have proarrhytmogenic effect. Surgical insult activates stress response with catecholamine surge while adverse effects of anaesthetic agents induce significant cardiovascular instability.
AVRD have autosomal dominant inheritance pattern with an estimated prevalence rate of 1:5000 that may be underestimated. Forensic autopsy following unexpected perioperative death in young individuals proved ARVD in eighteen out of fifty patients[3]. It is common enough for most anaesthetists to encounter a patient with ARVD at some point in their career either: (1) In the subclinical stage, leading to sudden perioperative death in seemingly healthy patients undergoing low-risk surgical intervention. In such cases post mortem examination could confirm the diagnosis of ARVD; or (2) in patients with established diagnosis of ARVD presenting for elective or emergency surgical procedure. Disease may be in the early or advanced stage at the time of presentation, symptoms may be well or poorly controlled.
Literature on the management of ARVD in the anaesthetic practice is based on a small number of individual case reports describing anaesthetic technique used in patients with subclinical or diagnosed ARVD. Straightforward link between anaesthetic agents and perioperative mortality is difficulty to establish. Previously uneventful general or regional anaesthesia does not exclude ARVD. It could be that perioperative death is associated with physiological instability, rather than administration individual anaesthetic agent. Majority of the recommendations on perioperative management of these patients are based on case reports when many things have gone wrong, and it is difficult to untangle whether a particular medication was a culprit. Not many anaesthetists have had frequent exposure to ARVD patients to be able to scientifically approach the management. In this review we will discuss the literature on presentation and perioperative management of these patients, and add some of the experience of our centre where there is an unusually high concentration of ends stage cardiac failure ARVD patients.
ARVD in an heritable cardiac muscle disease, most commonly follows autosomal dominant inheritance with variable penetrance in families and wide spectrum of disease severity[4]. AVRD does not present at birth. Symptoms develop in adolescence, and diagnosis is commonly made in the second and third decade of life. ARVD affects young men three times more frequently than women and an important cause of SCD in young adults and athletes[5-7].
ARVD incidence remains unclear, prevalence is estimated between 1 in 2000-5000 showing high variability between geographical regions. Positive family history presents in up to 50% of the cases. High incidence was observed in Vento region of Italy where forensic autopsy proved ARVD behind SCD in 20% of the cases[8,9]. Autosomal recessive forms are less common, Naxos disease is associated with palmoplantar keratosis and severe cardiac features[10].
AVRD develops due to desmosomal and non-desmosomal gene mutation. Gene mutation alters protein synthesis. Desmosomes (also called macula densa) are the structural and functional units of the heart muscle cell, connecting cells in the intercalate discs and mediating intracellular signal conduction. Disrupted desmosomal structure cannot withstand physical activity and shear forces and induces cardiomyocyte death. Apoptotic cells are replaced with fibro-fatty tissue causing increased excitability and structural abnormality. Initially focal process becomes generalised leaving extensive scar tissue with scattered residual myocytes within the thin ventricular wall. Gene mutation alters the structure of the following five desmosomal proteins: Plakoglobin, Desmoplakin, Plakophillin, Desmoglein and Desmocollin.
Non-desmosomal gene mutations are also found to be linked to AVRD. Ryanodine receptor-2 mutation causes ryanodine receptor dysfunction and alter intracellular calcium release from the sarcoplasmatic reticulum. Transforming growth factor-B3 gene mutation may have a role in myocardial fibrosis whilst TMEM43 gene mutation may cause dysregulation in adipogenic pathway and induces fibro-fatty replacement of the cardiomyocytes[2,11,12].
AVRD is difficult to diagnose when asymptomatic. First presentation is cardiac arrest in up to 50% of the clinical cases. Post mortem examination confirms fibro-fatty histology. Screening of family members is important to identify and risk stratify genetically affected relatives to prevent SDC.
Diagnostic test specific for ARVD has not been invented. In 1994, the International Task Force proposed the first diagnostic criteria system and combined multiple diagnostic information (structural, histological, arrhythmic, echocardiographic, genetic and familiar features). This system highly specific but failed to identify early, asymptomatic and familiar cases. International Task Force Criteria has been modified in 2010. Diagnosis was based on the combination of major and minor criteria[13].
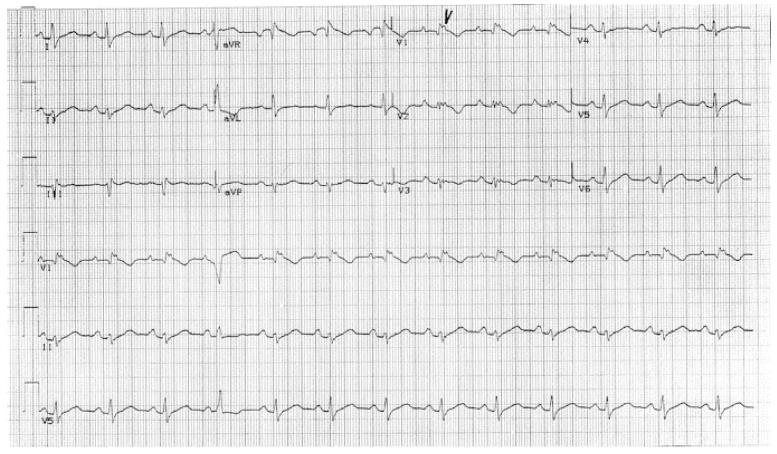
Electrocardiogram (ECG) abnormalities observed include T-wave inversion in anterior precordial leads, left axis deviation, wide QRS complexes and epsilon waves. Ventricular tachy-arrhythmias may present in a wide spectrum on resting ECG from premature ventricular extrasystoles, non-sustained ventricular tachycardia and monomorphic ventricular tachyarrhythmia with left bundle branch block.
Echocardiography may detect regional or global dysfunction, predominantly affecting the RV. RV wall thinning and dilation with tricuspid regurgitation are the features of advanced right heart failure.
Cardiac magnetic resonance imaging (MRI) is a non-specific diagnostic modality, however allows reliable quantification of the right ventricle volume however may fail to detect the ventricular adipose tissue and wall thinning accurately.
Right ventricular angiogram is considered a reliable invasive method for evaluating regional right ventricular wall motional abnormality. Cardiac MRI and 3D Echocardiography are non-invasive and preferred over right ventricular angiography for assessing morphology and functional abnormalities of the right ventricle[14].
Ventricular biopsy may confirm the suspected diagnosis of ARVD however negative biopsy should be interpreted with caution. Myocardial sample may be unreliable as AVRD often does not involve the subendocardium whilst the myocardium exhibits patchy involvement. Negative biopsy does not exclude the diagnosis of ARVD[15]. Involvement of a specialist pathologist experienced in this condition is important.
Immunohistochemical analysis: Reference diagnostic test highly sensitive and specific to ARVD would provide accurate early diagnosis in asymptomatic cases. Immunohistochemical analysis of plakoglobin in the intercalate discs found reduced signal level in patients with ARVD and may be of value in diagnosing ARVD[16,17] (Figure 1).
Current data suggest that asymptomatic patients with established ARVD do not benefit from prophylactic therapeutic interventions.
Once the clinical diagnosis of ARVD is established, treatment modalities target three main goals.
Clinical evidence suggests the strenuous physical exercise accelerates myocyte apoptosis and increase the risk of SDC in competitive athletes. Physical exercise may also be responsible for “hot disease phase” when myocyte atrophy accelerates. This phase is characterised by increased excitability[18,19]. Restriction from strenuous exercise and competitive sports is thought to be slow down myocardial cell death.
Ventricular arrhythmias manifest in a wide spectrum. Sporadic premature ventricular extrasystole may progress to non-sustained or sustained ventricular tachycardia and VF. VF leads to sudden cardiac arrest. Long term arrhythmia control may be achieved with implantable cardioverter defibrillator (ICD) alone or in combination with antiarrhythmic drugs.
The effect of β-blockers or Amiodarone in treatment of sustained VT has been proven. Sotalol provides the most effective arrhythmia control. Amiodarone is usually preserved for advance heart failure. In combination with B-blocker its efficacy is comparable to Sotalol[20,21].
ICD is considered for primary prevention in high risk cases and proved to be successful when cardiac dysfunction occurs. However ICD for AVRD treatment will require long term follow up and may be complicated with adverse effects associated with procedures. When cardiac failure is a problem the ICD can be also used as a biventricular re-synchronisation therapy too[22].
Catheter ablation: Developing fibro-fatty scar tissue with scattered myocytes increases the risk of re-entrant tachycardia. Catheter ablation is reserved for patients with recurrent ventricular arrhythmia not responsive to pharmacological treatment as it only offers short-term success, long-term efficacy remained debatable[23].
Symptomatic heart failure is rare and occurs predominantly in advanced stage. Cardiac failure should be treated in conjunction with conventional heart failure guidelines, most commonly with angiotensin-converting enzyme inhibitors, diuretics, digoxin and anticoagulants. In the rare occasions, when heart failure is unresponsive to medical management, heart transplantation may be necessary.
Given a prevalence rate of 1:5000, most anaesthetists will encounter a patient with ARVD during their practice. SDC during general anaesthesia is unresponsive to cardiopulmonary resuscitation. Disease awareness, understanding pathophysiology and hazardous conditions help improve safety in these patients. Perioperative management is divided in two groups: For patients with already diagnosed ARVD, and for patients who present with malignant arrhythmias and SDC.
Preoperative evaluation of patients with AVRD should include collecting medical history, documenting physical findings and obtaining specialized diagnostic tests. All elective patients must be consulted with a cardiologist with experience with the disease. History of provoked arrhythmias and safe medication is essential.
Subclinical stage of ARVD is symptomless or characterised by non-specific clinical symptoms such as palpitation, chest pain and syncope. Linking these features to a rare cardiac muscle disease may be difficult. Previous uneventful general or regional anaesthesia does not exclude ARVD[24]. ECG is not used for routine assessment in young and healthy patients.
Patient with confirmed diagnosis of ARVD should undergo regular follow up to evaluate efficacy of arrhythmia control and cardiac function. Review of case notes and discussion with a cardiologist would enable the anaesthetist to gain information about the course of the disease. Physical examination carries limited significance in early stages, signs and symptoms of systemic or pulmonary congestion become apparent in advanced stage. Twenty-four hour holter-monitor is a reliable non-invasive method to discover sustained and non-sustained ventricular tachycardia. Echocardiography is available in most institutions to offer a quick insight to cardiac function. Preoperative optimisation carries high impact on perioperative mortality. Multidisciplinary team approach including cardiologist, anaesthetist and operating surgeon facilitate safe perioperative planning. Elective procedure should be postponed until ventricular arrhythmia controlled and heart failure is optimised. Regular anti-arrhytmic drugs should be continued until surgery. Patient with ICD should undergo device check to assess optimal function and number of appropriate ICD discharges. Electrical safety may warrant inactivation of the device in the immediate preoperative period[25].
Maintaining haemodynamic state near to the normal physiological conditions is likely to offer best arrhythmia protection. Close haemodynamic monitoring is essential for early recognition of physiological instability and assessment cardiovascular response to intervention. Routine monitoring with ECG, oxygen saturation probe, non-invasive blood pressure cuff and capnography may be supplemented with invasive arterial blood pressure cannula even in case of minor surgical procedures. Invasive arterial pressure monitoring will allow for blood pressure monitoring even in the face of arrhythmia, when NIBP could be unreliable. Major interventions warrant more invasive monitoring. Central venous pressure provides information about right heart function and filling status. The role of pulmonary artery catheters may be controversial outside specialised cardiothoracic institutions due to induction of malignant ventricular arrhythmia and ventricular perforation during placement. However, intraoperative transoesophageal echocardiography offers superior diagnostic and avoids risks of arrhythmia. Less invasive forms of continuous cardiac output monitoring allow assessment of contractility, ventricular filling.
Malignant ventricular arrhythmia and SCD could occur at induction of general anaesthesia, during the surgical procedure or in the immediate postoperative stage. Therefore, appropriate post-operative monitoring and care locations must be chosen according to institutional organisation.
Understanding the pharmacokinetics and adverse effects of the anaesthetic agents: Hemodynamic variation during anaesthesia is related to specific effects of the anaesthetic agents on the sympathetic nervous system. Patient with extensive sympathetic blockade are prone to develop reduced systemic vascular resistance, reduced cardiac output and severe arterial hypotension.
Midazolam causes little direct myocardial depression and was reported to be safe for sedation in conjunction with opioid analgesics or for induction of general anaesthesia in combination with a different induction agent[26]. However in two case reports administration of Midazolam was associated with perioperative death[27,28]. Propofol is known to induce significant arterial hypotension and myocardial suppression. Judicious dosing or slow infusion for induction may help to overcome these unfavourable pharmacological properties making it one of the commonly used induction agent. Maintenance of general anaesthesia using Propofol gains increasing popularity. Case reports describe Propofol as a safe agent for induction[27] and maintenance of general anaesthesia[26]. Ketamine has a stable haemodynamic profile however anticholinergic effect can result in sinus tachycardia.
Opioid analgesia should be selected carefully. Perioperative death in patients with diagnosed ARVD was reported after administration of Sufentanyl[28]. Fentanyl is considered to be safe[28,29]. High dose of Fentanyl was used in a cardiothoracic patient population[26].
Volatile anaesthetic agents should be administered cautiously to patients with ARVD as these are known to induce dose-dependent myocardial depression, tachycardia and arterial hypertension. Isoflurane was associated with intraoperative SCD but paradoxically, uneventful anaesthesia was also described[27-31].
Muscle relaxants have several unfavourable side effects on the circulation. Careful selection helps to avoid haemodynamic instability. Perioperative death was observed in a patient receiving Suxamethonium and Atracurium[32]. This had been explained by histamine release followed haemodynamic instability. Cisatracurium has minimal effect on the cardiovascular system and was associated with positive patient outcome[31]. Pancuronium is likely to induce sinus tachycardia due to its vagolytic effect and thought to be contraindicated in patients with ARVD. However Pancuronium was the muscle relaxant of choice in successful case series of intraoperative management of patients with advance cardiac failure ARVD patients undergoing orthotopic cardiac transplantation. There were no deleterious effects reported[26,33].
Anticipating and promptly responding physiological disturbance. These are the principles of balanced anaesthesia for all operations. However, there are no more important in any other group of patients other than ARVD.
Hypertension increase afterload on the diseased ventricle. Hypertension may be treated with deepening the plane of anaesthesia. Intravenous Nicardipine has been described as a safe choice. Although there is lack of experience, it is possible that other systemic blood pressure lowering agents are safe too.
Hypotension could diminish coronary perfusion and oxygen delivery. Although direct vasoactive drugs can provoke arrhythmias, these may need to be used to maintain haemodynamic parameters. Vasopressor infusions have been previously described. Dopamine is known to increase the incidence arrhythmias whilst used to treat arterial hypotension[34], however safe administration in patients with ARVD was reported in the literature[26].
Tachycardia increases the incidence of premature ventricular complexes and ventricular extrasystole predisposing to malignant arrhythmias. During general anaesthesia tachycardia may be explained by for several factors. Pain control, adequate depth of anaesthesia and muscle relaxation should be maintained at all time. Agents with positive dromotropic and batmotropic effects have to be avoided where possible. Controlling electrolyte levels of potassium and magnesium can offer some protection. Persistent tachycardia may be treated with short acting intravenous β-blocker (Esmolol). When premature ventricular extrasystole increases in number, Amiodarone infusion should be commenced.
Excessive intravenous fluid administration is hazardous in case of ventricular dysfunction as it can distend the heart. Hypovolaemia secondary to dehydration or large fluid shift during complex abdominal and thoracic procedures should be treated intravenous crystalloid. Anticipating and promptly responding to haemorrhage helps to avoid decompensated blood. Compensatory tachycardia to maintain cardiac out and oxygen delivery predisposes to malignant tachyarrhythmia.
Controlled ventilation maintains normal arterial carbon-dioxide partial pressure (pCO2). Tachycardia may be the first manifestation of increased pCO2. Muscle relaxation in major surgical cases could offer also reduction of oxygen demand due to reduced striated muscle oxygen demand.
Hypothermia increases the risk of shivering and increased oxygen consumption and predispose to tachyarrhythmia.
Calcium, magnesium and potassium play important role in regulation of cardiac function and vessel tone. Deranged plasma level can precipitate haemodynamic instability. Metabolic acidosis has deleterious effect on cardiac contractility and reduces sensitivity to endogenous and exogenous catecholamines.
Postoperative care plays an important role, malignant arrhythmia in the postoperative phase must be anticipated and predisposing factors prevented or promptly eliminated. ARVD may present exclusion criteria for day surgery even following low risk surgical interventions. Patient with ARVD should be cared for in a clinical area where close monitoring can be maintained.
Maintaining physiological parameters near to the normal values decreases the incidence of malignant arrhythmias and SDC.
Adequate arterial perfusion pressure is essential to maintain coronary perfusion pressure, myocardial oxygen supply and end-organ perfusion. Perioperative clinicians should have low threshold to treat bradycardia and tachycardia. Close monitoring of fluid balance and early recognition of significant aim to maintain haemodynamic stability.
Supplemental oxygen may help to avoid arrhythmias induced by hypoxia, however, importance of adequate carbon-dioxide removal must not be overlooked.
Adequate pain control reduces anxiety, maintains autonomic stability and prevents further catecholamine surge. Multimodal approach with non-opioid analgesic drugs may be appropriate choice after minor surgical interventions. Opioids analgesics have significant side effects on the cardiovascular and respiratory systems and their use is reserved to treat severe postoperative pain after. Regional and neuroaxial blockade may be useful after major surgical interventions.
Satisfactory treatment of postoperative nausea and vomiting eliminates vagotonic effect. Electrolyte balance maintains electrical stability of the diseased myocardium.
When malignant supraventricular or ventricular arrhythmia occurs, pharmacological treatment or electrical cardioversion should not be delayed. Prompt consideration of reversible causes and electrolyte abnormalities may help to restore cardiovascular stability. Early involvement of experienced cardiologist should be thought. Management of cardiovascular collapse follows Adult Life Support (ALS) guidelines (Table 1).
| Preoperative management | |
| History | Symptomatic arrhythmia, cardiac drug history, cardiology follow-up |
| Physical findings | Signs of systemic and pulmonary congestion |
| Specific diagnostic tests | ECG, Holter monitor, Echocardiography |
| Preoperative optimisation | Multidisciplinary approach involving cardiologist, surgeons, anaesthetists and intensivists |
| Intraoperative management | |
| Monitoring | Minor surgical intervention: ECG, non-invasive blood pressure monitoring, SpO2, capnography Major surgical interventions: Central venous pressure, pulmonary artery catheter and cardiac output monitoring, temperature monitoring, intraoperative transoesophageal echocardiography |
| Haemodynamic stability | Maintaining adequate depth of anaesthesia and analgesia Adequate arterial perfusion pressure Avoiding tachycardia, bradycardia and arrhythmias Avoiding blood loss, hypovolaemia and fluid overload Maintaining adequate gas exchange Temperature control Electrolyte balance Acid-base balance |
| Choice of anaesthetic agents and vasoactive drugs | Understanding pharmacokinetics and side effects of the individual anaesthetic agents and anticipating cardiovascular effects |
| Postoperative management | Cardiovascular stability: Avoiding hypo- and hypertension, tachycardia, bradycardia and arrhythmias Prompt recognition of blood loss and hypovolaemia Normoxia and normocapnia Temperature control Adequate pain control Early treatment of postoperative nausea and vomiting Electrolyte and acid-base balance |
In pregnancy all maternal organ system undergoes physiological changes in order to adapt to increased metabolic demand of the foetus and prepare the maternal body for delivery. Cardiovascular changes occur throughout pregnancy: Systemic vascular resistance decreases initially due to progesterone effect and low resistance placental vascular bed later on. Cardiac output may increase up to 50% at term due to left ventricular hypertrophy. Intravascular volume expansion induces physiological anaemia. Healthy gravidas tolerate adaptation well, women with structural heart disease may experience worsening symptoms while pregnant.
Limited available data describes pregnancy safe and well-tolerated in women with mild to moderate form of ARVD, however all women with ARVD in child-bearing age should receive counselling before pregnancy.
In an ideal world all pregnant women with ARVD should be assessed by multidisciplinary team including specialist cardiologist, obstetrician and anaesthetist as soon as pregnancy discovered. Early assessment enables the medical team to plan essential investigation, maintain effective control of malignant arrhythmias and establish a robust plan for delivery and the postpartum period.
Anaesthetists are likely to encounter the following issues whilst managing pregnant women with AVRD: (1) discontinuation of pharmacological control of malignant arrhythmias: Omission of regular antiarrhytmic drugs have been reported with minimal effect[35]; (2) amiodarone is associated with severe adverse effect on the foetus therefor it needs to be used only to treat life-treating arrhythmias. β-blockers may be associated with intra-uterine growth retardation however Bisoprolol is considered to be safe[36]; (3) alteration of heart failure medications due to their teratogenic effect: While Warfarin and ACE-inhibitors may be associated with faulty foetal organogenesis, most diuretics, digoxin and low molecule weight heparin can be used without having an adverse effect on the foetus[36,37]; and (4) worsening cardiac function and symptomatic ventricular arrhythmia: Despite of continuous rhythm control, women may experience increased incidence of palpitation. Evaluation of cardiac function with echocardiograph and holter monitor is described to be beneficial.
ARVD is not a contraindication of vaginal delivery, however significant deterioration of pre-existing heart failure might indicate surgical delivery. Planned delivery may provide a better control of perinatal events.
In labour cardiac output increases 85% above the non-pregnant level. This is due to increased venous return from the placental vessels with each uterine contraction. Furthermore, anxiety and pain results in sympathetic stimulation and increase heartrate. Low dose spinal or effective epidural anaesthesia achieves good pain relief and blunts autonomic response but also warrants higher level of haemodynamic monitoring to avoid cardiovascular instability.
Successful surgical delivery of healthy new born has been described both under regional and general anaesthesia[35,36,38]. Suitable technique is determined by maternal choice and the degree of heart failure. There is no evidence to supports one technique over the other. The ultimate goal is to maintain cardiovascular stability. Haemodynamic instability is more common with spinal anaesthesia, epidural anaesthesia allows better control over instability and more time for adaptation.
Most commonly used oxytocin has deleterious side effects such as hypotension and tachycardia, dilution and slow administration may overcome. It is important to judge whether use of oxytocin is needed for the individual ARVD patient at all, rather than follow the routine protocol for all patients. Involvement of experienced obstetrician in these cases is important.
Increased incidence of malignant arrhythmias has been reported in the third trimester and the postpartum period. High level of monitoring is recommended in the early postpartum phase. Lactation raises concern about electrolyte derangement however there is no report of ventricular tachyarrhythmia in women who opted for breast feeding. Evaluation of cardiac function 3 mo after delivery provides good guide regarding the need for modifying pharmacological arrhythmia control.
Local anaesthetic drugs exert effect by blocking voltage sensitive sodium channels and inhibit propagation of action potential via the nerve fibres.
Regional blocks may provide anaesthesia as a sole technique or used as an adjunct to general anaesthesia to facilitate adequate analgesia and blunt stress response to pain.
Cardiovascular changes are the most important effects of central neural block. These responses are due to arterial vasodilation and decreased contractility due to sympathetic denervation. Reflex tachycardia aims to maintain cardiac output however significant decrease in systemic vascular resistance due to sympathectomy reduces venous return and paradoxically induces bradycardia or asystole.
Anaesthetists should anticipate these changes when planning central neuraxial blockade for a patient with ARVD. Remembering of non-specific symptoms such as nausea, vomiting and syncope may be helpful to avoid cardiovascular collapse.
Epidural anaesthesia provides better controlled onset of the blockade with less marked effect on cardiovascular system. However, it also uses large doses of local anaesthetics, which can cause arrhythmias after their systemic absorption.
Local anaesthetics exhibit dose dependent sodium channel blockage on the cardiac muscle, inducing prolongation of the PR interval and widening the QRS complexes. Direct cardiac toxicity depends on the potency. Bupivacaine is the most commonly used local anaesthetic agent however its higher plasma level is associated with severe adverse effect on the cardiovascular system.
There is no sufficient data to support any local anaesthetic agent being superior to other, Bupivacaine was associated with perioperative death in patients with ARVD and used successfully during surgical delivery[29,38]. In fact, it may support the concept that perioperative death is associated with deleterious haemodynamic instability rather than the choice of the local anaesthetic agent.
Ephedrine is frequently administered concomitantly with local anaesthetic drugs with the aim to prevent rapid absorption to the circulation and avoid toxic plasma level. It also increases the duration of the blockade. Subcutaneous and extravascular administration is associated with minimal cardiovascular sequels. Intravascular use could induce hypertension, tachycardia and increased myocardial oxygen consumption predisposing patients with ARVD to malignant ventricular arrhythmias and SDC.
Laparoscopic surgery an important diagnostic and therapeutic tool and gaining increased popularity over the past two decades. Growing surgical experience and better understanding of the physiological effect of pneumoperitoneum lead to that more and more major surgical interventions are being performed laparoscopically.
Laparoscopy offers lower perioperative morbidity, better wound healing, fewer wound infection and better pain control allowing early mobilisation and shorter hospital stay.
Anaesthetists should be aware of the physiological changes induced by laparoscopic surgery whilst preparing a patient with diagnosed ARVD for surgery: Mechanical effect of pneumoperitoneum has significant cardiovascular consequences: Reduced venous return due to compression of the inferior vena cava, increased myocardial afterload and bradycardia secondary to increased systemic vascular resistance. Compensatory tachycardia precipitates malignant arrhythmias. Carbon-dioxide insufflation may induce respiratory acidosis. Perforation of the solid organs or great vessels precipitates decompensated blood loss.
Laparoscopy does not have absolute contraindications, however patients with diagnosed ARVD or advanced heart disease need to be assessed on an individual basis. Operative planning should involve surgeon, anaesthetist and specialist cardiologist. Experienced surgeon can reduce intraoperative complication and the duration of the surgical intervention. Anaesthetist should anticipate and respond to cardiovascular changes. Invasive haemodynamic monitoring may be necessary for minor procedures. Postoperative nausea and vomiting are common, prevention with combined antiemetic drugs is recommended. Persisting haemodynamic concerns should prompt early discussion with the operating surgeon and conversion to open procedure should be thought.
Undiagnosed ARVD often presents as unexpected perioperative death in young and fit patient undergoing minor surgical intervention. Cardiovascular collapse is commonly unresponsive to cardiopulmonary resuscitation.
It such cases all anaesthetists ask for help, and it is the culture of perioperative case. Once cardiovascular collapse occurs, management should follow the ALS guidelines. Defibrillation is almost always needed. Administration of Amiodarone bolus and infusion should follow ALS guidelines. Other reversible causes should be considered, intravascular volume, electrolytes should be replaced.
Patent airway and controlled ventilation aim to maintain adequate arterial oxygen and carbon-dioxide pressure and avoid arrhythmia induced by hypoxia and hypercarbia.
AVRD is a rare form of cardiomyopathy, subclinical stage may be asymptomatic or characterised by non-specific symptoms such as palpitation or chest pain. Awareness amongst anaesthetists may help to raise the suspicion. When malignant tachycardia occurs is healthy individual in the perioperative period, differential diagnosis should include ARVD. Early recognition and prompt treatment of these arrhythmias may help to avoid cardiovascular collapse.
Patients with established diagnosis of ARVD should be managed by a multidisciplinary team. Robust perioperative plan should include preoperative workup and optimisation involving a cardiologist. Surgical intervention must be performed by an experienced consultant surgeon with the least haemostatic disturbance. Experienced consultant anaesthetist in charge for the anaesthetic management should understand the pathophysiology of ARVD and physiological anomalies predisposing to adverse events. Cardiovascular stability should be maintained throughout. Malignant arrhythmias and cardiovascular collapse can occur at any time during the perioperative care therefor emergency vasoactive and anti-arrhythmia drugs and defibrillator should be available. Postoperative recovery is the best to take place in a clinical area with high level of monitoring. Stable haemodynamic parameters, adequate analgesia and temperature control, fluid and electrolyte balance aim to avoid arrhythmias and facilitate early recovery, mobilisation and hospital discharge. Once adverse event occurs at any point during perioperative care, experience cardiologist can offer valuable input.
Most anaesthetists would encounter patients with subclinical or diagnosed ARVD. Available literature on management of ARVD in the anaesthetic practice is not sufficient to establish evidence-based recommendations. Clinical data remains conflicting about the safety of the individual pharmacological agents. Careful perioperative assessment and solid management plan focusing on the maintenance of physiological stability may help to avoid perioperative loss and provide the highest level of safety in this patient population.
Manuscript source: Invited manuscript
Specialty type: Anesthesiology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amornyotin S, Shorrab AA S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823-3832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 2. | Thiene G, Basso C. Arrhythmogenic right ventricular cardiomyopathy: An update. Cardiovasc Pathol. 2001;10:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Tabib A, Loire R, Miras A, Thivolet-Bejui F, Timour Q, Bui-Xuan B, Malicier D. Unsuspected cardiac lesions associated with sudden unexpected perioperative death. Eur J Anaesthesiol. 2000;17:230-235. [PubMed] [Cited in This Article: ] |
| 4. | Corrado D, Fontaine G, Marcus FI, McKenna WJ, Nava A, Thiene G, Wichter T. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: need for an international registry. Study Group on Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy of the Working Groups on Myocardial and Pericardial Disease and Arrhythmias of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the World Heart Federation. Circulation. 2000;101:E101-E106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Borjesson M, Pelliccia A. Incidence and aetiology of sudden cardiac death in young athletes: an international perspective. Br J Sports Med. 2009;43:644-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 945] [Cited by in F6Publishing: 791] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 7. | Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med. 1998;339:364-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 636] [Cited by in F6Publishing: 540] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Matsumori A, Furukawa Y, Hasegawa K, Sato Y, Nakagawa H, Morikawa Y, Miura K, Ohno Y, Tamakoshi A, Inaba Y. Epidemiologic and clinical characteristics of cardiomyopathies in Japan: results from nationwide surveys. Circ J. 2002;66:323-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Peters S, Trümmel M, Meyners W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int J Cardiol. 2004;97:499-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Protonotarios N, Tsatsopoulou A, Patsourakos P, Alexopoulos D, Gezerlis P, Simitsis S, Scampardonis G. Cardiac abnormalities in familial palmoplantar keratosis. Br Heart J. 1986;56:321-326. [PubMed] [Cited in This Article: ] |
| 11. | Murray B. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): a review of molecular and clinical literature. J Genet Couns. 2012;21:494-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Kapplinger JD, Landstrom AP, Salisbury BA, Callis TE, Pollevick GD, Tester DJ, Cox MG, Bhuiyan Z, Bikker H, Wiesfeld AC. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia-associated mutations from background genetic noise. J Am Coll Cardiol. 2011;57:2317-2327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 13. | Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533-1541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1527] [Cited by in F6Publishing: 1378] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 14. | Tavano A, Maurel B, Gaubert JY, Varoquaux A, Cassagneau P, Vidal V, Bartoli JM, Moulin G, Jacquier A. MR imaging of arrhythmogenic right ventricular dysplasia: What the radiologist needs to know. Diagn Interv Imaging. 2015;96:449-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Kiès P, Bootsma M, Bax J, Schalij MJ, van der Wall EE. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: screening, diagnosis, and treatment. Heart Rhythm. 2006;3:225-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Munkholm J, Christensen AH, Svendsen JH, Andersen CB. Usefulness of immunostaining for plakoglobin as a diagnostic marker of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2012;109:272-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 18. | Sen-Chowdhry S, Morgan RD, Chambers JC, McKenna WJ. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med. 2010;61:233-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Saguner AM, Brunckhorst C, Duru F. Arrhythmogenic ventricular cardiomyopathy: A paradigm shift from right to biventricular disease. World J Cardiol. 2014;6:154-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 20. | Wichter T, Paul TM, Eckardt L, Gerdes P, Kirchhof P, Böcker D, Breithardt G. Arrhythmogenic right ventricular cardiomyopathy. Antiarrhythmic drugs, catheter ablation, or ICD? Herz. 2005;30:91-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Ermakov S, Scheinman M. Arrhythmogenic Right Ventricular Cardiomyopathy - Antiarrhythmic Therapy. Arrhythm Electrophysiol Rev. 2015;4:86-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Mugnai G, Tomei R, Dugo C, Tomasi L, Morani G, Vassanelli C. Implantable cardioverter-defibrillators in patients with arrhythmogenic right ventricular cardiomyopathy: the course of electronic parameters, clinical features, and complications during long-term follow-up. J Interv Card Electrophysiol. 2014;41:23-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Dalal D, Jain R, Tandri H, Dong J, Eid SM, Prakasa K, Tichnell C, James C, Abraham T, Russell SD. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007;50:432-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Staikou C, Chondrogiannis K, Mani A. Perioperative management of hereditary arrhythmogenic syndromes. Br J Anaesth. 2012;108:730-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Alexoudis AK, Spyridonidou AG, Vogiatzaki TD, Iatrou CA. Anaesthetic implications of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Anaesthesia. 2009;64:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Valchanov K, Goddard M, Ghosh S. Anesthesia for heart transplantation in patients with arrhythmogenic right ventricular dysplasia. J Cardiothorac Vasc Anesth. 2014;28:355-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Bastien O, Guérin JM, Artru F, Lehot JJ. Arrhythmogenic right ventricular dysplasia: an underestimated cause of perioperative death? J Cardiothorac Vasc Anesth. 2002;16:357-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Bonnet F, Samain E, Bocquet R, Le Corre F, Marty J. Perioperative management of severe head injury in a patient with arrhythmogenic right ventricular dysplasia. Anesthesiology. 2001;95:255-256. [PubMed] [Cited in This Article: ] |
| 29. | Houfani B, Meyer P, Merckx J, Roure P, Padovani JP, Fontaine G, Carli P. Postoperative sudden death in two adolescents with myelomeningocele and unrecognized arrhythmogenic right ventricular dysplasia. Anesthesiology. 2001;95:257-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Martínez Torrente F, Orts Castro A, García-Montoto F, Pérez-Cerdá F. Patient with right ventricular arrhythmogenic dysplasia, ascites and ulcerative colitis: anesthetic management during major abdominal surgery. Rev Esp Anestesiol Reanim. 2005;52:631-633. [PubMed] [Cited in This Article: ] |
| 31. | Morita K, Takeuchi M, Oe K, Iwasaki T, Taga N, Hirakawa M, Sano S. Perioperative management of Fontan operation for two patients with arrhythmogenic right ventricular dysplasia. J Anesth. 2002;16:169-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Toh KW, Nadesan K, Sie MY, Vijeyasingam R, Tan PS. Postoperative death in a patient with unrecognized arrhythmogenic right ventricular dysplasia syndrome. Anesth Analg. 2004;99:350-352, table of contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Massen H, Cosnay JP, Bouzaher M, Mercat C. Arrhythmogenic right ventricular dysplasia revealed during anesthesia. Ann Fr Anesth Reanim. 1986;5:436-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1194] [Cited by in F6Publishing: 1091] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 35. | Hodes AR, Tichnell C, Te Riele AS, Murray B, Groeneweg JA, Sawant AC, Russell SD, van Spaendonck-Zwarts KY, van den Berg MP, Wilde AA. Pregnancy course and outcomes in women with arrhythmogenic right ventricular cardiomyopathy. Heart. 2016;102:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Krul SP, van der Smagt JJ, van den Berg MP, Sollie KM, Pieper PG, van Spaendonck-Zwarts KY. Systematic review of pregnancy in women with inherited cardiomyopathies. Eur J Heart Fail. 2011;13:584-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Burt C, Durbridge J. Management of cardiac disease in pregnancy. BJA Education. 2009;44-47. [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Bauce B, Daliento L, Frigo G, Russo G, Nava A. Pregnancy in women with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Obstet Gynecol Reprod Biol. 2006;127:186-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |