Published online Mar 27, 2014. doi: 10.5313/wja.v3.i1.134
Revised: December 10, 2013
Accepted: December 17, 2013
Published online: March 27, 2014
Processing time: 129 Days and 4.4 Hours
Dexmedetomidine is indicated as a sedative agent in intensive care units (ICUs). While several clinical trials and two meta-analyses have compared this agent with propofol or midazolam, the results were variable depending on the specific end-point (e.g., duration of mechanical ventilation, ICU mortality, maintaining a target depth of sedation, incidence of delirium episodes, length of hospital stay). Hence, the effectiveness of this new agent vs the comparators seems to be controversial. Trial sequential analysis (TSA) is a statistical technique that can estimate the optimal, cumulative number of patients that would be needed to generate a conclusive result. We therefore applied a TSA model to the most recent meta-analysis evaluating dexmedetomidine. A total of 10 randomized controlled trials were included in our analysis. According to our results, the comparison of dexmedetomidine vs propofol showed no proof of incremental effectiveness for the end-points of length of ICUs stay and incidence of delirium episodes. In contrast, futility (i.e., proof of no incremental effectiveness) was demonstrated for the end-point of mechanical ventilation. Hence, the results for the comparison of dexmedetomidine vs propofol were inconclusive for the first two end-points; on the other hand, conclusiveness was reached for the third end-point. We conclude that the place of dexmedetomidine in therapy of critically ill patients is very uncertain and further controlled trials are still needed.
Core tip: Dexmedetomidine, a sedative agent for critically ill patients, has been studied in several randomized trials and in two meta-analyses. The clinical results were conflicting because of the diversity of the end-points and the small size of most studies. Since trial sequential analysis can improve the interpretation of controversial meta-analyses, we applied this technique to dexmedetomidine. According to our results, the comparison of dexmedetomidine vs propofol showed no proof of incremental effectiveness (for length of intensive care unit (ICU) stay and incidence of delirium) or of no incremental effectiveness (for duration of mechanical ventilation). Hence, the therapeutic role of dexmedetomidine in ICU is still uncertain.
-
Citation: Fadda V, Maratea D, Trippoli S, Messori A. Dexmedetomidine
vs propofol in intensive care unit patients. World J Anesthesiol 2014; 3(1): 134-136 - URL: https://www.wjgnet.com/2218-6182/full/v3/i1/134.htm
- DOI: https://dx.doi.org/10.5313/wja.v3.i1.134
Dexmedetomidine is increasingly being used as a sedative agent in intensive care units (ICUs)[1,2]. Several clinical trials have compared this relatively new agent with propofol or midazolam[2], based on the end-point of maintaining a target depth of sedation (i.e., score of 0 to -3 according to the Richmond Agitation Sedation Scale). Most of these trials have shown non-inferiority[3] or no difference[4,5] for dexmedetomidine vs the comparator. Sedative agents are often associated with clinically relevant adverse events (e.g., prolonged mechanical ventilation, prolonged ICU stay and high incidence of neurocognitive adverse events like delirium). Dexmedetomidine is supposed to lower the incidence of these events[3] but the effectiveness of this new agent vs the comparators is still uncertain.
Two meta-analyses[1,6] have evaluated the effectiveness of dexmedetomidine as a sedative agent in ICUs. The most recent one was conducted by Xia et al[1] and included 10 randomized controlled trials that compared dexmedetomidine with propofol according to a variety of end-points (namely: length of ICU stay, ICU mortality, duration of mechanical ventilation and incidence of delirium episodes). The pooled results showed no difference between the two treatment strategies in duration of mechanical ventilation (5 trials, 895 patients) and ICU mortality (5 trials, 267 patients). On the other hand, dexmedetomidine showed a significantly lower incidence of delirium (3 trials, 658 patients) and shorter length of ICU stay (5 trials, 655 patients) than propofol.
Trial sequential analysis (TSA)[7] is a relatively new technique that can be applied to the clinical material included in a meta-analysis. The main advantage of TSA lies in its ability to re-interpret a non-significant meta-analysis and, in particular, to differentiate its results between inconclusiveness (i.e., no proof of difference) and demonstrated non-inferiority/futility (i.e., proof of no difference). Another advantage is that TSA estimates the “optimal information size” for the comparison under examination and is therefore able to indicate how many patients would be required to generate a conclusive result[8-12]. As regards its limitations, on the one hand TSA shares virtually all limitations already known for meta-analysis; on the other hand, one specific limitation of TSA is represented by the need to declare a pre-specified margin for the incremental clinical benefit (i.e., the threshold separating a clinically irrelevant benefit from clinically relevant one); this margin is essentially the same as that commonly employed for sample size estimation or non-inferiority statistics.
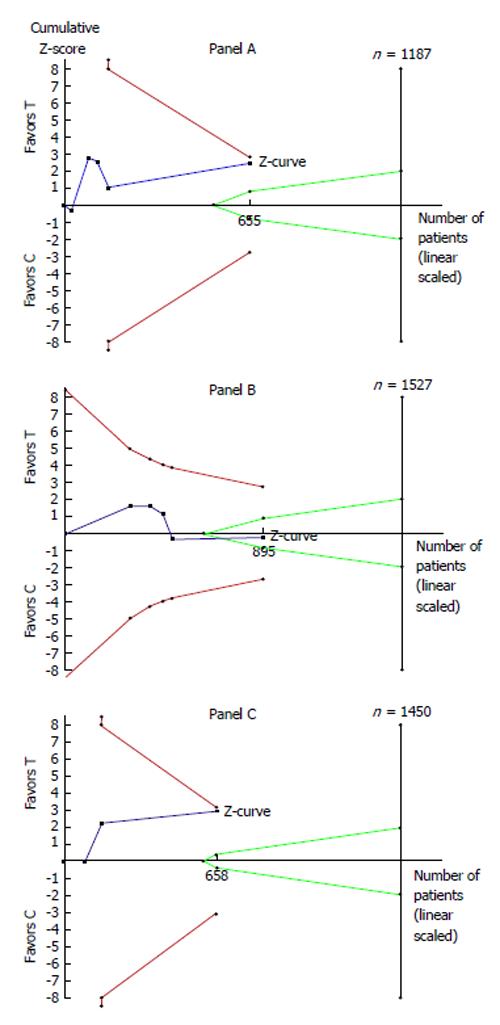
To test to which degree the results of the above mentioned meta-analysis were conclusive and to determine the optimal information size for this therapeutic problem, we carried out a TSA to re-analyze the data of Xia et al[1]. Our analysis examined the following three end-points: length of ICU stay, duration of mechanical ventilation and incidence of delirium episodes. Our assumptions included two-sided testing, type 1 error = 5%, power = 80%. The assumption of no difference (or margin) was defined as a difference of ≤ 1 d for the end-point of length of ICU stay, a difference of ≤ 6 h for the end-point of duration of mechanical ventilation, and a relative risk reduction of ≤ 40% for the incidence of delirium episodes. As usual, the output of the analysis was represented by the Z-curve graph; in this graph, the boundaries for superiority, inferiority and futility were determined according to the O’Brien-Fleming alpha-spending function. All calculations were carried out using specific statistical software (TSA, User Manual for TSA, Copenhagen Trial Unit 2011, software downloadable at http://www.ctu.dk/tsa).
Figure 1 summarizes the results of our TSA. Overall, our findings indicate that the comparison of dexmedetomidine vs propofol is inconclusive (i.e., no proof of incremental effectiveness) for the two end-points of length of ICU stay (Panel A) and incidence of delirium episodes (Panel C). On the other hand, our results demonstrate futility (i.e., proof of no incremental effectiveness) for the end-point of mechanical ventilation (Panel B). As shown in Figure 1, the last point of the Z-curve remained within the area of inconclusiveness (since the curve did not cross any boundaries) in Panels A and C; in contrast, in Panel B, the Z-curve crossed the boundary of futility and therefore reached a conclusive but negative result. More importantly, in the two panels showing inconclusiveness (i.e., Panels A and C), the number of patients enrolled in the available trials was much lower than the optimal information size as determined by the TSA model.
We conclude that further data are still needed to assess the place of dexmedetomidine in therapy of critically ill patients.
P- Reviewers: Camara-Lemarroy CR, Hariharan S, Velisek J, Kim DK S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Xia ZQ, Chen SQ, Yao X, Xie CB, Wen SH, Liu KX. Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: a meta-analysis of randomized clinical trials. J Surg Res. 2013;185:833-843. [PubMed] |
| 2. | Adams R, Brown GT, Davidson M, Fisher E, Mathisen J, Thomson G, Webster NR. Efficacy of dexmedetomidine compared with midazolam for sedation in adult intensive care patients: a systematic review. Br J Anaesth. 2013;111:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 634] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 4. | Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1117] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 5. | Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576-584. [PubMed] |
| 6. | Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 848] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 8. | Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. 2009;9:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 755] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 9. | Messori A, Fadda V, Maratea D, Trippoli S. Same-day discharge after percutaneous coronary intervention: trial sequential analysis of outcomes. J Am Coll Cardiol. 2014;63:376-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Messori A, Fadda V, Maratea D. Parenteral vs oral iron in patients with inflammatory bowel disease: quantifying information size by trial sequential analysis. J Crohns Colitis. 2013;7:e495-e496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Maratea D, Fadda V, Trippoli S, Messori A. Effectiveness of drug-eluting balloons: quantifying the information size from clinical trials. Int J Cardiol. 2013;168:4435-4437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Messori A, Fadda V, Maratea D, Trippoli S. Intra-aortic balloon pump in high-risk percutaneous coronary interventions without cardiogenic shock: trial sequential analysis of outcomes. Int J Cardiol. 2013;168:4534-4536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |