Published online Dec 10, 2017. doi: 10.5306/wjco.v8.i6.429
Peer-review started: May 23, 2017
First decision: June 14, 2017
Revised: September 6, 2017
Accepted: October 30, 2017
Article in press: October 30, 2017
Published online: December 10, 2017
Processing time: 198 Days and 7.4 Hours
Non-alcoholic fatty liver disease (NAFLD) associated hepatocellular carcinoma (HCC) incidence is increasing worldwide, paralleling the obesity epidemic. Although most cases are associated with cirrhosis, HCC can occur without cirrhosis in NAFLD. Diabetes and obesity are associated risk factors for HCC in patients. Given the sheer magnitude of the underlying risk factors (diabetes, obesity, non-cirrhotic NAFLD) screening for HCC in the non-cirrhotic population is not recommended. Optimal screening strategies in NAFLD cirrhosis are not completely elucidated with Ultrasound having significant limitations in detection of liver lesions in the presence of obesity and steatosis. Consequently NAFLD-HCC is more often diagnosed at a later stage with larger tumors and reduced opportunities for curative treatments as opposed to HCC in other causes of cirrhosis. When HCC is found at a curative stage treatments including liver transplantation, resection and loco-regional therapies are associated with good results similar to that seen in HCV-HCC. Future strategies under study include the use of chemopreventive and antioxidant agents to reduce development of cirrhosis and non-alcoholic steatohepatitis (NASH). Strategies to reverse NASH via weight loss, control of associated conditions like diabetes are key strategies in reducing the increasing incidence of NASH-HCC. Novel therapeutic agents for NASH are in trials and if successful in achieving reversal of NASH will be an important strategy in reducing NAFLD-HCC.
Core tip: Non-alcoholic fatty liver disease (NAFLD) related hepatocellular carcinoma (HCC) is rapidly increasing worldwide. HCC in NAFLD is often detected at a more advanced stage than in hepatitis C virus (HCV). Challenges include earlier recognition of cirrhosis in NAFLD to allow earlier screening for liver cancer. NAFLD also has a higher proportion of HCC occurring in the absence of cirrhosis. Given the sheer number of patients with non-cirrhotic NAFLD, screening for HCC in this population is not practical. Instead prevention and treatment of non-alcoholic steatohepatitis to prevent cirrhosis should be an important strategy. When NAFLD-HCC is found at a curative stage, results with liver transplant, resection and loco-regional therapy are similar to that seen in HCV-HCC.
- Citation: Said A, Ghufran A. Epidemic of non-alcoholic fatty liver disease and hepatocellular carcinoma. World J Clin Oncol 2017; 8(6): 429-436
- URL: https://www.wjgnet.com/2218-4333/full/v8/i6/429.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i6.429
Non-alcoholic fatty liver disease (NAFLD) has become the leading chronic liver disorder in the developed world, with a worldwide prevalence ranging from 6% to 35%[1]. The incidence of NAFLD is continuing to rise worldwide, paralleling the epidemic of metabolic syndrome worldwide. NAFLD is caused by an insulin-resistant state, occurring in the presence of diabetes, obesity, and metabolic syndrome[2].
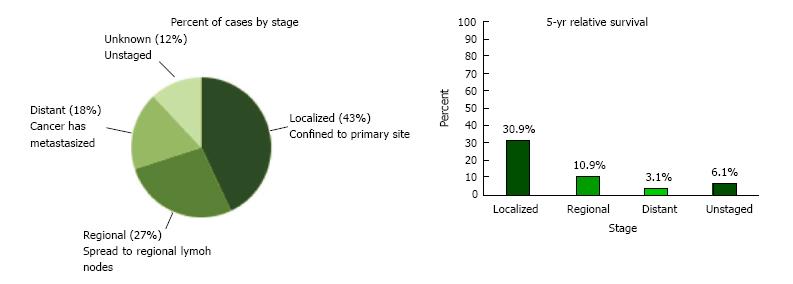
The incidence of hepatocellular carcinoma (HCC) in the United States and worldwide continues to rise, with an age-adjusted incidence rising from 1.5 to 6.7 per 100000 individuals in the past 30 years[3,4]. The most recent Surveillance, Epidemiology and End Results (SEER) registry data show that the rates for new liver and intrahepatic bile duct cancer cases have been rising on average 3.0% each year over between 2004 and 2013 (Figure 1)[5]. Most cases of HCC in the United States occur over age 50, with over 70% of cases occurring in men[4] and the ethnics groups with the highest incidence rates in the United States are Asian/Pacific Islanders and Hispanics (Table 1)[6].
| Outcome | Age (yr) | All races | None-hispanic | Hispanic | |||||||
| White | Black | API | |||||||||
| Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | Rate | 95%CI | ||
| HCC | Overall | 5.9 | (5.8-5.9) | 4.2 | (4.2-4.3) | 7.5 | (7.3-7.8) | 11.7 | (11.3-12.0) | 9.5 | (9.3-9.8) |
| Incidence | 35-49 | 2.2 | (2.1-2.3) | 1.4 | (1.3-1.5) | 2.5 | (2.2-2.8) | 4.7 | (4.3-5.2) | 3.2 | (2.9-3.4) |
| SEER 18 | 50-64 | 16.5 | (16.2-16.8) | 12.2 | (11.9-12.6) | 26.9 | (25.8-28.1) | 23.5 | (22.4-24.7) | 24.3 | (23.3-25.3) |
| > 65 | 22.3 | (21.9-22.7) | 16.0 | (15.5-16.4) | 22.4 | (20.9-23.9) | 54.7 | (52.4-57.0) | 40.5 | (38.7-42.4) | |
| Liver cancer | Overall | 4.3 | (4.3-4.3) | 3.6 | (3.5-3.6) | 6.4 | (6.3-6.6) | 8.2 | (7.9-8.4) | 7.0 | (6.9-7.2) |
| Mortality | 35-49 | 1.2 | (1.2-1.2) | 0.9 | (0.8-0.9) | 2.0 | (1.9-2.2) | 2.8 | (2.6-3.1) | 1.4 | (1.3-1.5) |
| US | 50-64 | 9.7 | (9.5-9.8) | 7.7 | (7.6-7.8) | 18.6 | (18.2-19.1) | 13.0 | (12.4-13.6) | 13.5 | (13.0-13.9) |
| > 65 | 20.1 | (19.9-20.3) | 17.2 | (17.0-17.5) | 24.5 | (23.7-25.3) | 43.2 | (41.6-44.8) | 36.7 | (35.6-37.8) | |
In the United States, SEER registries 4929 cases of HCC between 2004-2009 were examined[7]. 14.1% of HCC were due to NAFLD and between 2004-2009 NAFLD-HCC showed a 9% annual increase. However, the rise in incidence now may be plateauing in the United States[8].
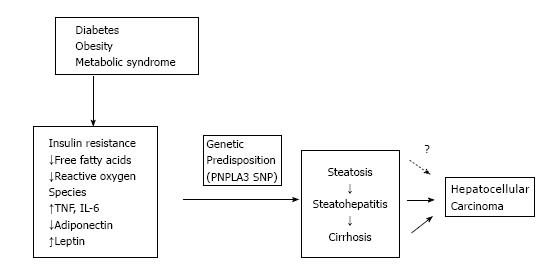
Cirrhosis is the most common underlying cause of HCC, with 80%-90% of patients diagnosed with HCC having underlying cirrhosis[9]. There is new data emerging to suggest that NAFLD may be an independent risk factor for HCC, even in the absence of cirrhosis[10-12] (Figure 2).
Diabetes and obesity are known independent risk factors for the development of HCC. There appears to be a common pathway via insulin resistance and its subsequent inflammatory cascade in the development of NASH and HCC. HCC is increased in patients with diabetes[13,14] as well as obesity[15]. In a prospective United States study of more than 900000 adults, overweight and obesity were associated with excess cancer mortality with an Odds ratio of 4.52 for liver cancer mortality in men and an Odds ratio of 1.68 in women. In a case control study of HCC, diabetes was associated with a 2-3 fold increased risk of HCC regardless of presence of other risk factors[8].
Genetic polymorphisms (I148M) in the gene encoding patatin-like phospholipase domain-containing protein 3 (PNPLA3) is a known risk factor for histologic steatosis as well as NASH, fibrosis and cirrhosis[16]. It has now also been shown to be an independent risk factor for development of HCC with a meta-analysis showing that PNPLA3 rs738409 SNP is associated with an Odds ratio of 1.40 for HCC in cirrhosis including NAFLD[17]. The common genetic mutations of hemochromatosis (C282Y and H63D) have been implicated in risk of developing NAFLD and HCC as well. In a recent meta-analysis a significantly increased risk of NAFLD and HCC was discovered. H63D polymorphism was associated with increased risk of developing non-cirrhotic HCC in the African population[18]. Putatively this is due to increased iron overload leading to hepatic inflammation, fibrosis and carcinogenesis.
Insulin resistance also leads to release of free fatty acids (FFA) and other reactive oxygen species that cause oxidative stress and inflammation. Trans-4-hydroxy-2-nonenal, a product of lipid peroxidation has been shown to cause mutations of the p53 tumor suppressor gene that is associated with more than half of human cancers including HCC[11]. The inflammation caused by oxidative stress leads to an increased release of inflammatory and inhibitory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and nuclear factor kappa B (NF-κB)[19]. Presence of these chemical mediators leads to hepatocyte death, compensatory proliferation, and ultimately carcinogenesis.
Leptin is a pro-inflammatory adipokine that is elevated in patients with NAFLD[20]. Leptin is associated with increasing expression of pro-inflammatory cytokines like TNF-α and IL-6[21], and activation of Janus Kinase (JAK)[22]. It can cause tumor growth and has been associated with HCC recurrence after treatment[23]. Adiponectin is an anti-inflammatory adipokine that has been shown to inhibit angiogenesis via modulation of apoptosis in an animal model. Adiponectin deficiency in obese states has been linked to carcinogenesis[24]. It is specific to adipose tissue and is decreased in insulin-resistant states, and thus may potentially play a role in development of HCC.
NAFLD associated HCC occurs in patients with cirrhosis at variably reported rates. A single center cohort study reported HCC at an annual incidence of 2.6%[25] compared to a 4% incidence in those with hepatitis C related cirrhosis. Other studies have reported overall incidence of HCC in NAFLD Cirrhosis from 2.4% at 7 years to 12.8% over 3 years[26].
Given the rising incidence of NAFLD, and the advances in curative options for Hepatitis C infection, NAFLD is expected to become the leading cause of HCC in developed nations[10]. Known risk factors for HCC in cirrhotic NAFLD include male gender, age over 70, and underlying diabetes and hypertension[27].
There is increasing evidence to suggest that NAFLD contributes to non-cirrhotic HCC as well[10]. Also, while the presence of hepatic steatohepatitis is an established risk factor for development of cirrhosis and HCC[28], there are case reports of HCC complicating underlying NAFLD in the absence of hepatitis or advanced fibrosis, making associations between steatosis, steatohepatitis, cirrhosis, and HCC complex[10].
While several studies have reported HCC in non-cirrhotic NAFLD the risk seems to be lower (0% to 3% over 20 years)[26]. Recent studies however have shown that non-cirrhotic HCC may be more common in NAFLD compared to other chronic liver diseases. Studies from multiple countries including United States, Japan and France of NAFLD-associated HCC have shown that a significant proportion of HCC occurs in the absence of cirrhosis in NAFLD. In a histologic analysis of resected NAFL-HCC from France the majority of patients did not have cirrhosis and 65% had stage 0-2 fibrosis[29]. In the study from Japan of 87 HCC patients only 51% had histologic cirrhosis and 28% had stage 1-2 fibrosis only[27]. In a United States veterans Study of 1500 HCC cases, 8% of the HCC cohort had NAFLD associated HCC and only 65% of the NAFLD-HCC cohort had cirrhosis (NAFLD had > 5 fold risk of HCC without cirrhosis compared to HCV related cirrhosis[30].
Several large-scale epidemiological studies have shown that there is a higher incidence of HCC in patients with obesity and diabetes, as well as poorer outcomes[10]. The relative risk of liver cancer was 117% for overweight subjects and 189% for the obese[31], while risk of mortality in men with a BMI > 35 kg/m2 can be as high as 4.5 times that in men with a normal BMI[15]. Similarly, presence of diabetes alone increases the risk of development of HCC three-fold[8]. Other independent risk factors for HCC in NAFLD include polymorphisms in the PNPLA3 gene (I148M)[32]. In the most recent met-analysis, HFE mutation (C282Y and H63D) was also shown to be associated with HCC risk in NAFLD populations including for the H63D mutation in non-cirrhotic African populations[18].
In the US SEER registries 4929 cases of HCC between 2004-2009 were examined[7]. Fourteen point one percent were due to NAFLD. Patients with NAFLD-HCC were older, had more advanced tumor stage at presentation and had shorter survival time and were less likely to receive a liver transplant.
These findings are seen in non-United States centers as well. In a large retrospective cohort study from Germany, of 1119 patients with HCC, those with NASH-HCC were older, had higher metabolic complications but better liver function at presentation[33]. Resection was performed in only 17.8% and transplant in 4.4% of these patient and overall survival for NAFLD-HCC was lower than that for HCV-HCC.
An Italian multicenter observational study of NAFLD-HCC vs HCV-HCC was performed[34]. Compared to HCV-HCC, NAFLD-HCC was more again likely associated with larger tumors, more infiltrative tumors and was more likely to be detected outside surveillance. Survival was significantly shorter in NAFLD-HCC (25.5 mo vs HCV-HCC (33.7 mo) regardless of tumor stage. However analysis of patients with HCC in Milan criteria sent for curative treatments showed similar survival in NASH-HCC and HCV-HCC (38.6 mo vs 41 mo).
In a study from 2 centers in the United Kingdom, 275 HCV related HCC patients were compared with 212 NAFLD related HCC patients. Patients with NAFLD-HCC had lower rates of cirrhosis and were significantly older and had larger tumors than those with HCV-HCC. Those with NAFLD-HCC were less likely to receive curative therapy than HCV-HCC including liver transplant (21/212 of NAFLD-HCC) vs 80/275 of HCV-HCC. Despite this overall survival from diagnosis was similar for NAFLD-HCC (56% at 1year and 23% at 3 years) and HCV-HCC (58% at 1 year and 21% at 3 years)[35].
The reasons for these tumor stage differences are multi-factorial. The current AASLD Guidelines recommend that patients with cirrhosis undergo regular surveillance for HCC with ultrasound every 6 mo[36]. While this may suffice in most patients with cirrhosis, patients with NASH are often obese, thus limiting the diagnostic ability of ultrasound[37]. The ITALICA study group showed that HCC was significantly less likely to be diagnosed during surveillance in patients with cryptogenic cirrhosis compared to HCV patients, translating into a greater prevalence of advanced HCC stage and poor survival[38]. MRI, therefore, may offer a better enhanced surveillance for HCC but comes at a higher cost and reduced accessibility.
Underdiagnosis of cirrhosis is a common problem as it leads to lack of screening and potentially increased stage of HCC when diagnosed resulting in worse outcomes. In a large cohort of HCC patients (1201) in the VA, 24.6% had undiagnosed cirrhosis prior to HCC diagnosis and patients with NAFLD had higher odds of having undiagnosed cirrhosis (OR = 4.77)[39].
Similar to other causes of HCC, NAFLD associated HCC occurs in the context of liver disease and liver function and portal hypertension are integral in multimodality assessment and treatment of HCC.
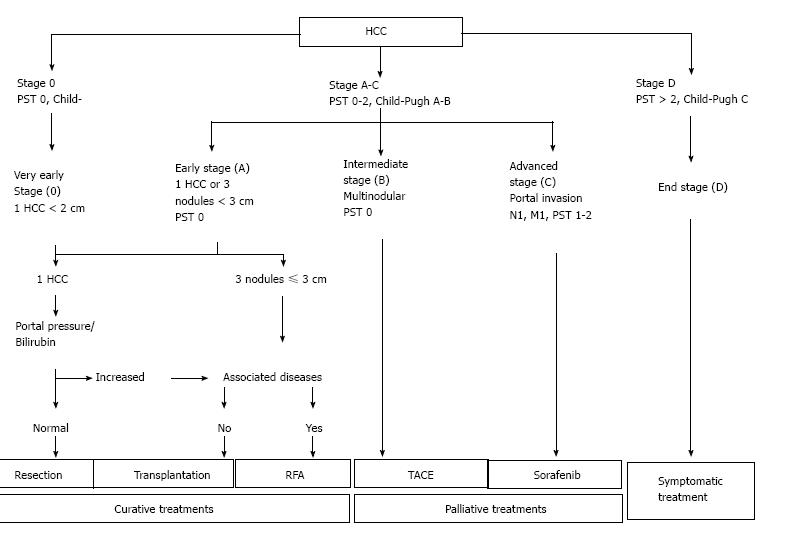
In addition to actual tumor stage, the severity of liver impairment also affects the available options for management of HCC. Thus, the most commonly used schema for management of HCC is Barcelona Clinic Liver Cancer (BCLC)[36,40] which incorporates both stage of the tumor as well as disease severity of underlying liver impairment (Figure 3)[36].
As per current SEER data, 42.9% of patients with HCC are diagnosed at the local stage, and the 5-year survival for localized liver and intrahepatic bile duct cancer is 30.9%[5]. Curative therapies include resection for early stage HCC with thermal ablative therapies also showing comparable results for single small tumors. Liver transplantation is a good option for patients with Milan (T2) criteria tumors who have complications of cirrhosis and portal hypertension[41].
Patients with larger tumors can be candidates for intra-arterial therapy including trans-arterial chemoembolization (TACE) and Y-90 radioembolization that are utilized with goals of tumor control and not always for potentially curative ends. Thus finding tumors that are small and limited to the liver offers patients the widest array of potential treatments and hope of cure[42].
With the growing epidemic of NAFLD, NAFLD associated HCC is the second most common indication for liver transplant (LT) for HCC in the United States after HCV since 2006, increasing 4 fold since 2002[43]. In this study, Wong et al[43] analyzed 10061 patients with HCC who underwent LT for HCC between 2002-2012. Since MELD system was implemented in 2002, HCC related liver transplants increased significantly from 3.3% (n = 143) of all LT in 2002 to 23.3% (n = 1336) in 2012. NASH related HCC also increased significantly from 8.3% of all HCC related LT in 2002 to 13.5% in 2012 and were the second leading reason for HCC related LT behind HCV.
In a study from 2 United Kingdom liver transplant centers between 2000 and 2014, 487 patients with HCC associated with NAFLD or HCV presented to the transplant centers. 275 had HCC secondary to HCV and 212 secondary to NAFLD[35]. Patients with NAFLD were significantly older than HCV patients at time of HCC diagnosis (69.6 years vs 58.6 years). Absence of cirrhosis was more common in NAFLD patients (13%) vs HCV patients (1%). The non-cirrhotic patients were more likely to be older, have DM and had larger tumor size (likely due to non-surveillance). NAFLD patients had significantly larger tumors at presentation and were less likely to receive liver transplant than HCV-HCC patients and were more likely to receive TACE. Overall survival was however similar between NAFLD and HCV HCC at 3 years from diagnosis (21% and 23%).
Outcomes for LT for NAFLD-HCC were compared in a US study with LT for HCC-HCV and HCC-ALD. Over a 50 mo median follow up there was no difference in tumor free survival and overall survival (curative treatments vs HCV, ALD)[44].
In an Italian study that compared resection in HCC-associated with metabolic syndrome with HCC-HCV outcomes were similar in 96 HCC-Metabolic Syndrome and 96 HCC-HCV patients after resection. All patients had Child A cirrhosis and operative mortality was 2.1% (similar between the 2 groups). Morbidity and liver failure rates were also similar and were impacted by cirrhosis need for major hepatectomy and MELD score but not by histologic steatohepatitis. Five year overall survival was better in HCC-Metabolic Syndrome vs HCC-HCV (65.6% vs 61.4%, P = 0.03)[45].
Reddy et al[44] reported 3-year survival after resection was better in NASH vs HCV-ALD patients (60.9% vs 36.2%) including on multivariable analysis. Therefore curative treatments like resection are an acceptable treatment for NASH patients.
Liver-directed therapy with percutaneous ablation is a frontline therapeutic option in patients with HCC in its early stage. These options include ablation by chemical agents (acetic acid or ethanol) or by heat (radiofrequency, microwaves, laser, or cryotherapy). The efficacy of these therapies is followed by contrast-enhanced CT scan, with lack of contrast uptake suggestive of adequate response[36]. NASH-HCC outcomes with ablation are reported to be as efficacious as HCV-HCC or ALD-HCC[44].
TACE and Sorafenib are available non-curative options for HCC[36,40]. TACE may sometimes be employed to downstage a tumor prior to use of transplantation as a curative option.
Given the current limitation in treatment options, there is currently an interest in targeting the known molecular pathways as treatment options for HCC. NAFLD and obesity associated HCC has been linked to oxidative stress, hyperinsulinemia, and chronic inflammation. Addressing metabolic syndrome can be a preventative measure against development of HCC. These options include, but are not limited to, exercise, weight loss, and optimal control of diabetes, and hypertension, if present. In fact, one study showed a lower relative risk of developing HCC in vigorously active subjects compared to those with a sedentary lifestyle[46]. This reduction in risk was found to be independent of BMI.
In preliminary studies, dietary antioxidants like, vitamin C and E, selenium and coenzyme Q, vitamin D supplementation, and a Mediterranean diet have been shown to prevent hepatic carcinogenesis[47]. This is of particular interest given the known role of antioxidants in limiting and even reverting fibrosis in patients with NASH[48]. Similarly, the use of metformin, known to reduce insulin resistant and subsequent steatohepatitis, has been shown to be associated with reduced incidence of HCC in diabetic patients[47].
In animal models anti-inflammatory and anti-oxidant compounds like green tea, BCAA and acyclic retinoids have shown promise in preventing HCC. BCAA supplementation in cirrhosis is associated with improvements in insulin resistance and inhibition of IGF-1 and IGF-2 expression in the db/db obese mouse liver and reduced expression of liver cancers[49]. In Human study long term supplementation with BCAA reduced HCC in obese patients with cirrhosis[50]. Green tea extracts have been associated with beneficial effects on weight loss, insulin resistance and inflammatory cytokines in animal models[51]. In animal models beneficial effects on carcinogenesis have been reported as well by modulation of tyrosine kinase and Pi13/AKT pathways[52] and in reducing hepatic tumors in DEN treated db/db/mice. With green tea extracts no human data have been published showing efficacy. Acyclic Retinoids derivates of vitamin A exert their effect through nuclear receptors including RXRalpha, which is found in abundant supply in human liver. Supplementation of retinoids has shown beneficial effects in maintaining hepatocyte homeostasis in hepatocyte carcinogenesis[53]. In a long term human study acyclic retinoids reduced the chance of HCC recurrence and death by 40%[54]. Given the significant association of diabetes with HCC, metformin has been studied in cohort and case-control studies and a meta-analysis showed a significantly reduced risk of HCC with metformin use in diabetics[55].
In summary, there is indisputable evidence showing the increased risk of HCC in patients with NAFLD regardless of the presence of advanced fibrosis and steatohepatitis. Patients with HCC and NAFLD are increasing more rapidly than any other indication for liver transplantation. Patients with NAFLD are candidates for curative and non-curative therapies with encouraging results.
Diagnosing HCC in advanced stages of tumor or liver disease can render curative options futile and call for development of alternate guidelines for enhanced HCC surveillance in patients with metabolic syndrome. The current challenges include developing optimal surveillance options in targeted populations. There is also a huge potential in development of therapies targeting NASH and molecular pathways as preventive options for HCC in patients with cirrhosis in general and NAFLD in particular.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Agrawal S, Patial V, Servillo G, Zhong JA S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 2. | Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1326] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 4. | White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812-820.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 331] [Article Influence: 41.4] [Reference Citation Analysis (1)] |
| 5. | Available from: https://seer.cancer.gov/. |
| 6. | Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 355] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 7. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 618] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 8. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 9. | Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 536] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 11. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1014] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 12. | Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Adami HO, Chow WH, Nyrén O, Berne C, Linet MS, Ekbom A, Wolk A, McLaughlin JK, Fraumeni JF Jr. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 256] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Raff EJ, Kakati D, Bloomer JR, Shoreibah M, Rasheed K, Singal AK. Diabetes Mellitus Predicts Occurrence of Cirrhosis and Hepatocellular Cancer in Alcoholic Liver and Non-alcoholic Fatty Liver Diseases. J Clin Transl Hepatol. 2015;3:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5283] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 16. | Speliotes EK, Butler JL, Palmer CD, Voight BF; GIANT Consortium; MIGen Consortium; NASH CRN, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 17. | Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, Waljee AK. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 18. | Ye Q, Qian BX, Yin WL, Wang FM, Han T. Association between the HFE C282Y, H63D Polymorphisms and the Risks of Non-Alcoholic Fatty Liver Disease, Liver Cirrhosis and Hepatocellular Carcinoma: An Updated Systematic Review and Meta-Analysis of 5,758 Cases and 14,741 Controls. PLoS One. 2016;11:e0163423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 20. | Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497-2507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 387] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 23. | Watanabe N, Takai K, Imai K, Shimizu M, Naiki T, Nagaki M, Moriwaki H. Increased levels of serum leptin are a risk factor for the recurrence of stage I/II hepatocellular carcinoma after curative treatment. J Clin Biochem Nutr. 2011;49:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 474] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 25. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 965] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 26. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 575] [Article Influence: 44.2] [Reference Citation Analysis (2)] |
| 27. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-433; quiz e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [PubMed] |
| 29. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 30. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-131.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 492] [Article Influence: 54.7] [Reference Citation Analysis (1)] |
| 31. | Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 332] [Cited by in RCA: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 32. | Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, Hara T, Okajima A, Umemura A, Nishikawa T. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: Association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol Res. 2017;47:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Weinmann A, Alt Y, Koch S, Nelles C, Düber C, Lang H, Otto G, Zimmermann T, Marquardt JU, Galle PR. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 465] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 35. | Than NN, Ghazanfar A, Hodson J, Tehami N, Coldham C, Mergental H, Manas D, Shah T, Newsome PN, Reeves H. Comparing clinical presentations, treatments and outcomes of hepatocellular carcinoma due to hepatitis C and non-alcoholic fatty liver disease. QJM. 2017;110:73-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 37. | Uppot RN, Sahani DV, Hahn PF, Gervais D, Mueller PR. Impact of obesity on medical imaging and image-guided intervention. AJR Am J Roentgenol. 2007;188:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Giannini EG, Marabotto E, Savarino V, Trevisani F, di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M, Borzio F, Caturelli E, Chiaramonte M; Italian Liver Cancer (ITALICA) Group. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 2009;7:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Walker M, El-Serag HB, Sada Y, Mittal S, Ying J, Duan Z, Richardson P, Davila JA, Kanwal F. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther. 2016;43:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 41. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5309] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 42. | Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 43. | Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 586] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 44. | Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 45. | Viganò L, Conci S, Cescon M, Fava C, Capelli P, D’Errico A, Torzilli G, Di Tommaso L, Giuliante F, Vecchio FM. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter matched analysis with HCV-related HCC. J Hepatol. 2015;63:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Piguet AC, Saran U, Simillion C, Keller I, Terracciano L, Reeves HL, Dufour JF. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J Hepatol. 2015;62:1296-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 48. | Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50:171-180. [PubMed] |
| 49. | Iwasa J, Shimizu M, Shiraki M, Shirakami Y, Sakai H, Terakura Y, Takai K, Tsurumi H, Tanaka T, Moriwaki H. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci. 2010;101:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, Kumada H, Ohashi Y; Long-Term Survival Study (LOTUS) Group. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677-1683. [PubMed] |
| 52. | Shimizu M, Shirakami Y, Moriwaki H. Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, EGCG. Int J Mol Sci. 2008;9:1034-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Yasuda I, Shiratori Y, Adachi S, Obora A, Takemura M, Okuno M, Shidoji Y, Seishima M, Muto Y, Moriwaki H. Acyclic retinoid induces partial differentiation, down-regulates telomerase reverse transcriptase mRNA expression and telomerase activity, and induces apoptosis in human hepatoma-derived cell lines. J Hepatol. 2002;36:660-671. [PubMed] |
| 54. | Okita K, Izumi N, Ikeda K, Osaki Y, Numata K, Ikeda M, Kokudo N, Imanaka K, Nishiguchi S, Kondo S. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J Gastroenterol. 2015;50:667-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |