Published online Aug 10, 2017. doi: 10.5306/wjco.v8.i4.360
Peer-review started: April 17, 2017
First decision: May 22, 2017
Revised: June 11, 2017
Accepted: June 30, 2017
Article in press: July 3, 2017
Published online: August 10, 2017
Processing time: 113 Days and 15.2 Hours
Driver mutations in patients with non-small cell lung cancer (NSCLC) can lead to distinct behaviors and patterns of metastasis. Mutations in the proto-oncogene B-raf (BRAF) occur in approximately 3% of NSCLC cases. In the literature, reports of patients with lung adenocarcinomas metastasizing to the duodenum are rare, and most of the only 21 cases reported were from before the advent of next-generation sequencing. We present here a case involving a 57-year-old female who had a lytic lesion in her lesser trochanter. Biopsy showed metastatic adenocarcinoma of lung origin. Chest X-ray showed a large left upper lobe mass. Next-generation sequencing analysis confirmed the presence of BRAF V600Q mutation. The patient presented with persistent anemia and melena. Esophagogastroduodenoscopy confirmed the presence of duodenal metastasis. She also had suspected paraneoplastic leukemoid reaction. To our knowledge, this is only the second well-documented case of gastrointestinal metastasis from BRAF-mutated lung cancer.
Core tip: We report a rare and interesting case of BRAF-mutated lung adenocarcinoma with metastases to the bone and duodenum, and extreme leukocytosis. We found next-generation sequencing to be helpful in prognostication and determination of some of the unique clinical behaviors of lung adenocarcinoma. This is only the second case of BRAF-mutated lung adenocarcinoma with well documented metastases to the gastrointestinal tract. The addition of this case to the literature should prompt interest in studying the propensity of BRAF-mutated malignancies to metastasize to the gastrointestinal tract.
- Citation: Qasrawi A, Tolentino A, Abu Ghanimeh M, Abughanimeh O, Albadarin S. BRAF V600Q-mutated lung adenocarcinoma with duodenal metastasis and extreme leukocytosis. World J Clin Oncol 2017; 8(4): 360-365
- URL: https://www.wjgnet.com/2218-4333/full/v8/i4/360.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i4.360
Non-small cell lung cancer (NSCLC) has been traditionally classified and treated as a single disease. However, recent research has helped us to better understand the molecular pathogenesis of lung cancers in general. For example, mutations in the epidermal growth factor receptor (EGFR) and rearrangements of the anaplastic lymphoma kinase (ALK) gene were discovered in 2004 and 2007, respectively[1]. These mutations, often called “driver mutations”, have been shown to drive NSCLC tumorigenesis and are now being exploited as a targeted strategy for treatment-the application of which consisting mostly of tyrosine kinase inhibitors. Tumors with different driver mutations have been shown to have different clinical backgrounds, pathological features and prognoses[2]. In addition, different driver mutations can lead to distinct patterns of metastatic spread[3]. Generally, however, small bowel metastases from lung cancer is very uncommon, and duodenal metastases are particularly rare[4,5]. It is unknown if certain driver mutations can lead to an increased predisposition to gastrointestinal spread in patients with lung cancer.
In this report, we present a case of a metastatic lung adenocarcinoma with a V600Q mutation in the proto-oncogene B-raf (BRAF) and which had an atypical course of duodenal metastasis and extreme leukocytosis. Because this represents such a rare case, we also provide a review of the literature regarding BRAF-mutated lung cancers and of previous reports of duodenal metastasis originating from lung cancer. Finally, we also provide reasoned hypotheses as to the causes of the accompanying leukocytosis.
Our patient was a 57-year-old female with a known history of metastatic lung adenocarcinoma. Her history dated back to December 2015, when she developed left hip pain. It was initially treated conservatively and imaging examination was not performed. However, over the ensuing 4 mo, the pain worsened and became gnawing and constant. She was afebrile. Results from laboratory work-up revealed leukocytosis (19.3 × 109/L; reference range: 4.3-11 × 109/L) with 84% neutrophils, microcytic anemia (7.1 g/dL; reference range: 12-15.5 g/dL) with mean corpuscular volume (MCV) of 71 fL, and thrombocytosis (595 × 109/L; reference range: 150-450 × 109/L). At 4 mo prior, her hemoglobin had been 13.0 g/dL (reference range: 12-15.5 g/dL).
A computed tomography (CT) scan of the left hip was obtained, and showed marked irregularity of the lesser trochanter with cortical bone destruction. A soft tissue mass was also seen in the region of the cortex. A plain chest film revealed a large left lung mass. Iron studies revealed a ferritin level of 86 ng/mL, iron of < 10 μg/dL (reference range: 50-160 μg/dL) and total-iron binding capacity of 353 μg/dL (reference range: 270-380 μg/dL). Other causes of anemia were ruled out. A peripheral smear showed microcytic hypochromic anemia and granulocytosis without left-shift.
The patient was transfused with a unit of packed red blood cells (PRBCs) and taken to the operating room. She underwent intralesional curettage, partial excision of the lesser trochanter, and open arthotomy of the left hip with extraction of the mass. Pathological examination of the extracted bone and soft-tissue mass revealed poorly differentiated metastatic adenocarcinoma cells. Immunohistochemical staining revealed strong reactivity for cytokeratin 7 (CK7) and thyroid transcription factor-1 (TTF-1) and negative reactivity for cytokeratin 20 (CK20) and GATA binding protein 3 (GATA3); these findings are most consistent with lung origin. CT scans revealed a large left upper lobe mass, measuring 8.5 cm × 6.7 cm × 10.7 cm, with extensive local invasion. In addition, there was a left adrenal mass indicative of metastatic disease.
Genetic testing of the tumor was carried out using Caris Molecular Intelligence® (Caris Life Sciences, Irving, TX, United States). The next-generation sequencing (NGS) analysis revealed exon 15 BRAF V600Q and exon 7 TP53 G215V mutations. No mutations or rearrangements were found in the genes for Kirsten ras viral oncogene (KRAS), neuroblastoma ras viral oncogene (NRAS), anaplastic lymphoma receptor tyrosine kinase (ALK), tyrosine-protein kinase Met (cMET), EGFR, ROS1, retinoblastoma-1 (RB1), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), or ret proto-oncogene (RET).
The patient received palliative radiotherapy to the left femur. Her anemia was considered likely multifactorial, given the active malignancy with possible iron deficiency. Ferrous sulfate supplementation was initiated (oral; 325 mg regular-release twice daily), but only minimal improvement in her anemia was observed. Of note, her leucocyte count remained elevated after the surgery (18.9-56.4 × 109/L). She had no signs of infection or inflammation.
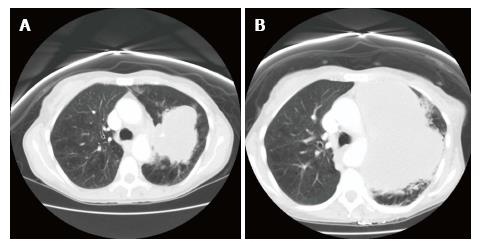
After the radiotherapy, two cycles of carboplatin and pemetrexed were administered. However, shortly after the second cycle, the patient presented to the emergency room with increasing shortness of breath and weakness. She also reported intermittent melanotic stool for the past few days. Physical exam revealed pallor and almost no air entry into the left part of the chest on auscultation. She was afebrile. Laboratory investigations showed a leucocyte count of 80.2 × 109/L with 92% neutrophils, 6% monocytes and 2% lymphocytes, hemoglobin of 6.0 g/dL, and platelet count of 519 × 109/L. Guaiac fecal occult blood test was positive. CT scan showed extensive growth of the upper lobe mass (to 14.5 cm × 10.0 cm × 17.4 cm) with progressive mediastinal invasion (Figure 1).
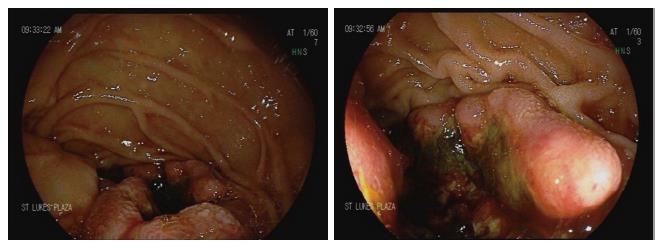
The patient was admitted to the hospital and transfused with 1 U of PRBCs. Esophagogastroduodenoscopy was performed, and an ulcerated bleeding 1-cm mass with malignant appearance was found in the second part of the duodenum (Figure 2). The scope could not traverse the lesion, and the exam could not be finished. Cold forceps biopsies were taken. On pathological exam, poorly differentiated adenocarcinoma was determined. The morphological and immunohistochemical characteristics of the tumor were similar to the findings on the original bone biopsy, being consistent with lung origin.
The patient’s leukocytosis worsened (up to 102 × 109/L, with 92% neutrophils, 3% monocytes, 2% myelocytes, 1% metamyelocytes, 1% promyelocytes and 1% lymphocytes). She did not have fever or other signs of infection. She did not receive any granulocyte-stimulating agent with her chemotherapy and did not receive steroids. A peripheral smear showed absolute neutrophilia with coarse toxic granulation and Döhle bodies in numerous neutrophils and with occasional metamyelocytes and myelocytes. In addition, rare nucleated red blood cells were observed. Peripheral flow cytometry did not show any increase in blast count. Mutational analysis showed no mutation in the genes for Janus kinase-2 (JAK-2), calreticulin (CALR) and colony-stimulating factor 3 receptor (CSF3R). In addition, reverse-transcriptase polymerase chain reaction assay of peripheral blood gave negative results for BCR-ABL b2a2, b3a2, and e1a2 fusion gene transcripts. This finding lessened the likelihood of chronic myeloid leukemia as well as of chronic myeloproliferative disorders. Given the patient’s very poor prognosis, bone marrow examination was not performed.
Considering the patient’s rapid course of progression and development of resistance to front-line chemotherapy, she was started on the off-label combination of dabrafenib with trametinib, which has Federal Drug Administration approval for use in BRAF-mutated melanoma. Unfortunately, her clinical condition deteriorated quickly and she died around 2 wk after her presentation.
The BRAF gene on chromosome 7 (7q34) is a proto-oncogene that encodes the serine/threonine specific protein kinase family member BRAF[6]. The BRAF protein participates in the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway, which is also known as the Ras-Raf-mitogen-activated protein kinase kinase (MEK)-ERK pathway[1]. It is a chain of proteins that functions in the signaling from cell surface to the nucleus[1]. Activation of this pathway leads to synthesis of transcription factors that are important in cell cycle regulation[7]. Mutations in MAPK/ERK can lead to uncontrolled growth and neoplastic transformation. BRAF mutations were first described in 2002 and occur in varying frequencies in melanoma, colorectal carcinomas, and lung, thyroid and other types of malignancies[8].
BRAF is mutated in approximately 3% of patients with NSCLC (mainly of adenocarcinoma type)[9]. The most commonly observed mutation in BRAF is the valine (V) to glutamic acid (E) substitution at codon 600 (BRAF V600E) on exon 15[10]. BRAF V600E accounts for about 50% of the BRAF mutations in NSCLC cases[11]. A large meta-analysis found that the BRAF V600E mutation was more frequent in women and was closely related a history of never-smoking[9]. In addition, one study showed that V600E-mutated tumors had an aggressive histotype and were significantly associated with shorter disease-free and overall survival rates[12]. Two other studies showed that V600E-mutated tumors responded less favorably to platinum-based chemotherapy, although the finding did not reach statistical significance[13,14]. In contrast, other studies have shown that overall survival was not statistically different between patients with wild-type BRAF and those with V600E or non-V600E BRAF mutations[10,11,15].
Our patient had metastasis to the duodenum. The immunohistochemical pattern of her bone and duodenal biopsies was suggestive of adenocarcinoma originating in the lung. In general, positive staining for TTF-1 and CK7, in addition to negative CK20 staining (i.e., TTF1+/CK7+/CK20- pattern) strongly supports a lung origin, as opposed to a gastrointestinal origin[16]. Metastases to the small bowel from lung cancer are very rare and usually asymptomatic[4]. According to a literature review by Hillenbrand et al[4] published in 2005, clinically-manifested small bowel metastasis was documented in 58 reports between 1961 and 2003. The most common symptoms were perforation and/or obstruction, or less commonly, bleeding.
Duodenal metastasis from lung cancer is exceedingly rare. AlSaeed et al[5] reported a case of duodenal metastasis from lung adenocarcinoma and cited another 11 previous reports. In addition, we found 9 more cases of lung cancer with duodenal metastasis[17-23]. Out of the 21 total cases reported, 9 showed adenocarcinoma histology, 7 showed squamous cell histology, 2 showed large cell histology, 2 showed small cell histology, and 1 was deemed unspecified NSCLC. Symptoms of gastrointestinal bleeding and/or iron deficiency occurred in 13 cases. Other reported clinical features include obstructive jaundice, abdominal pain, obstruction, and perforation. None of the reported cases had accompanying molecular analyses data for driver mutations.
We have previously reported a case of BRAF V600E-mutated lung adenocarcinoma, which had an aggressive clinical course and gastric metastases[24]. Herein, we present another case with a similar aggressive course and duodenal metastasis. Previous reports and studies have not indicated the association between certain genetic mutations in lung cancer and the predilection to gastrointestinal metastasis. In addition, gastric or intestinal metastases are rare and difficult to diagnose on imaging, and most of the reported cases of gastrointestinal metastases from lung cancer occurred before the discovery of driver mutations. Therefore, it cannot be proven that the BRAF mutation contributed to the gastric or duodenal metastasis in the previously reported cases.
We suggest that in cases of lung cancer with BRAF V600E mutations, an aggressive behavior must be expected. In addition, gastrointestinal tract involvement must be kept in mind. Clinical research on a larger scale (rather than relying on the rare case reports) will be imperative to understand the incidence of gastrointestinal involvement. In addition to mutated BRAF, our patient had a missense mutation in codon 245 of exon 7 of the TP53 gene (G245V). This mutation occurred in the DNA-binding site of the protein and was previously reported in lung cancer[25]. A previous, large meta-analysis found that TP53 mutations conferred worse clinical outcomes in patients with NSCLC, especially in those with adenocarcinoma histology[26]. It is possible that the TP53 mutation in our patient contributed to the aggressive clinical course and resistance to chemotherapy.
Another interesting feature of our case was the extreme neutrophilia. Infections and myeloproliferative disorders were unlikely in the absence of fever or signs of infection, JAK-2 mutations, or BCR-ABL rearrangements. Bone marrow infiltration from adenocarcinoma cells was a possibility, given the presence of rare nucleated red blood cells in the peripheral smear. Unfortunately, the patient’s bone marrow was not examined. It is also possible that her poor outcome was related to a paraneoplastic leukemoid reaction. Interestingly, neutrophilic leukemoid reaction was reported in a patient with BRAF V600E-mutated metastatic melanoma[27]. In another report, a case of squamous cell carcinoma with peritoneal carcinomatosis and an eosinophilic leukemoid reaction showed coexistence of the BRAF V600E and oncogenic KRAS G12A mutations[28]. Up-regulation of granulocyte colony-stimulating factor (G-CSF) through RAS/RAF/MEK pathway activation can lead to a paraneoplastic leukemoid reaction[29].
Finally, dabrafenib and trametinib are small molecule inhibitors of BRAF and MEK1/MEK2, respectively. Their oral administration combination is approved for treatment of BRAF V600E-mutated melanoma and is currently being investigated for lung cancer harboring the mutation[30]. In that trial, the overall response rate was 63%, with the median duration of response being 9 mo. We started our patient on dabrafenib and trametinib. Unfortunately, she died of her disease before any response was observed.
In conclusion, certain molecular mutations in NSCLC might lead to unique clinical behaviors. We have described a case of lung adenocarcinoma which had an atypical and aggressive clinical course, with duodenal metastasis and extreme leukocytosis. We have performed molecular analysis using NGS, which showed the mutations of exon 15 BRAF V600Q and exon 7 TP53 G245V. To the best of our knowledge, this is only the second reported case of well-documented BRAF-mutated lung adenocarcinoma with metastases to the gastrointestinal tract. Indeed, the continued use of modern molecular methods, such as NGS, will allow us to explore possible correlations between certain mutations and clinical behaviors.
A 57-year-old female with metastatic lung adenocarcinoma mutation in the proto-oncogene B-raf (BRAF) gene with presentation of fatigue, increasing shortness of breath and melena.
Pallor and almost no air entry into the left part of the chest on auscultation.
Peptic ulcer disease, esophagitis, gastritis, duodenitis, vascular lesions or tumors.
Anemia, extreme leukocytosis, and positive hemoccult stool test.
Computed tomography chest scan showing rapid progression of the cancer. Esophagogastroduodenoscopy with duodenal mass demonstrating a metastatic deposit of lung origin.
The morphological and immunohistochemical characteristics of the tumor were similar to the findings on the original biopsy, being consistent with lung origin.
Dabrafenib and trametinib were started, but the patient died before any response could be measured.
This is only the second well-documented case of gastrointestinal metastasis from BRAF-mutated lung cancer.
The BRAF gene is a proto-oncogene that encodes the serine/threonine specific protein kinase family member BRAF. The BRAF protein participates in the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway.
BRAF-mutated lung adenocarcinoma can be aggressive. Further studies are needed to explore possible correlations between BRAF mutations and clinical behaviors. Furthermore, treatment with dabrafenib and trametinib has promising results.
The object is interesting and the manuscript clearly reported. This is the second well-documented case of gastrointestinal metastasis from BRAF-mutated lung cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Peters GJ, Tontini GE, Velayos B S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | de Langen AJ, Smit EF. Therapeutic approach to treating patients with BRAF-mutant lung cancer: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev. 2010;29:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, Oton AB, Bunn PA Jr, Barón AE, Franklin WA, Aisner DL, Varella-Garcia M, Camidge DR. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118:4502-4511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Hillenbrand A, Sträter J, Henne-Bruns D. Frequency, symptoms and outcome of intestinal metastases of bronchopulmonary cancer. Case report and review of the literature. Int Semin Surg Oncol. 2005;2:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | AlSaeed EF, Tunio MA, AlSayari K, AlDandan S, Riaz K. Duodenal metastasis from lung adenocarcinoma: A rare cause of melena. Int J Surg Case Rep. 2015;13:91-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Hussain MR, Baig M, Mohamoud HS, Ulhaq Z, Hoessli DC, Khogeer GS, Al-Sayed RR, Al-Aama JY. BRAF gene: From human cancers to developmental syndromes. Saudi J Biol Sci. 2015;22:359-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 949] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 8. | Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7459] [Cited by in RCA: 7626] [Article Influence: 331.6] [Reference Citation Analysis (0)] |
| 9. | Chen D, Zhang LQ, Huang JF, Liu K, Chuai ZR, Yang Z, Wang YX, Shi DC, Liu Q, Huang Q. BRAF mutations in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e101354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Villaruz LC, Socinski MA, Abberbock S, Berry LD, Johnson BE, Kwiatkowski DJ, Iafrate AJ, Varella-Garcia M, Franklin WA, Camidge DR. Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer. 2015;121:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Tissot C, Couraud S, Tanguy R, Bringuier PP, Girard N, Souquet PJ. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer. 2016;91:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, Viola P, Pullara C, Mucilli F, Buttitta F. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29:3574-3579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 13. | Ding X, Zhang Z, Jiang T, Li X, Zhao C, Su B, Zhou C. Clinicopathologic characteristics and outcomes of Chinese patients with non-small-cell lung cancer and BRAF mutation. Cancer Med. 2017;6:555-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, Yeap BY, Sholl LM, Johnson BE, Jänne PA. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19:4532-4540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Kinno T, Tsuta K, Shiraishi K, Mizukami T, Suzuki M, Yoshida A, Suzuki K, Asamura H, Furuta K, Kohno T. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol. 2014;25:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Su YC, Hsu YC, Chai CY. Role of TTF-1, CK20, and CK7 immunohistochemistry for diagnosis of primary and secondary lung adenocarcinoma. Kaohsiung J Med Sci. 2006;22:14-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Jeba J, Backianathan S, Ishitha G, Singh A. Oral and gastrointestinal symptomatic metastases as initial presentation of lung cancer. BMJ Case Rep. 2016;2016:bcr2016217539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Iwamuro M, Uetsuka H, Makihata K, Yamamoto K. Metastatic tumors in the duodenum: A report of two cases. J Cancer Res Ther. 2015;11:648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Linsen PV, Linsen VM, Buunk G, Arnold DE, Aerts JG. Iron deficiency anemia as initial presentation of a non-small cell lung carcinoma: A case report. Respir Med Case Rep. 2015;16:109-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Lee KA, Lee SK, Seo DW, Kim MH. Duodenal metastasis from lung cancer presenting as obstructive jaundice. Gastrointest Endosc. 2001;54:228. [PubMed] |
| 21. | Nakamura H, Mizokami Y, Iwaki Y, Shiraishi T, Ohtsubo T, Miura S, Narasaka T, Matsuoka T. Lung cancer with metastases to the stomach and duodenum: report of three cases. Digest Endosc. 2003;15:210-215. |
| 22. | Raijman I. Duodenal metastases from lung cancer. Endoscopy. 1994;26:752-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Goh BK, Teo MC, Chng SP, Tan HW, Koong HN. Upper gastrointestinal bleed secondary to duodenal metastasis: a rare complication of primary lung cancer. J Gastroenterol Hepatol. 2006;21:486-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Qasrawi A, Abu Ghanimeh M, Albadarin S, Yousef O. Gastric Metastases from Lung Adenocarcinoma Causing Gastrointestinal Bleeding. ACG Case Rep J. 2017;4:e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Feng H, Wang X, Zhang Z, Tang C, Ye H, Jones L, Lou F, Zhang D, Jiang S, Sun H. Identification of Genetic Mutations in Human Lung Cancer by Targeted Sequencing. Cancer Inform. 2015;14:83-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Gu J, Zhou Y, Huang L, Ou W, Wu J, Li S, Xu J, Feng J, Liu B. TP53 mutation is associated with a poor clinical outcome for non-small cell lung cancer: Evidence from a meta-analysis. Mol Clin Oncol. 2016;5:705-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Gouveia E, Sousa M, Passos MJ, Moreira A. Paraneoplastic leukemoid reaction in a patient with BRAF V600E-mutated metastatic malignant melanoma. BMJ Case Rep. 2015;2015:bcr2014208645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Li B, Lu JC, He D, Wang J, Zhou H, Shen L, Zhang C, Duan C. Rapid onset lung squamous cell carcinoma with prominent peritoneal carcinomatosis and an eosinophilic leukemoid reaction, with coexistence of the BRAF V600E and oncogenic KRAS G12A mutations: A case report. Oncol Lett. 2014;8:589-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | McCoach CE, Rogers JG, Dwyre DM, Jonas BA. Paraneoplastic Leukemoid Reaction as a Marker of Tumor Progression in Non-Small Cell Lung Cancer. Cancer Treat Commun. 2015;4:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Planchard D, Besse B, Groen HJ, Souquet PJ, Quoix E, Baik CS, Barlesi F, Kim TM, Mazieres J, Novello S. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 646] [Article Influence: 71.8] [Reference Citation Analysis (0)] |