Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.266
Peer-review started: December 5, 2016
First decision: March 28, 2017
Revised: April 12, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: June 10, 2017
To verify whether recurrence-free survival (RFS) surrogates overall survival (OS) in phase III trials for resectable colorectal liver metastases (CRLM).
MEDLINE, EMBASE, and Scopus databases were consulted. Eligible studies were phase III trials testing any type of systemic therapy (neoadjuvant, adjuvant or perioperative) added to surgery in patients with resectable CRLM. A linear regression model based on hazard ratios (HR) of OS and RFS was performed.
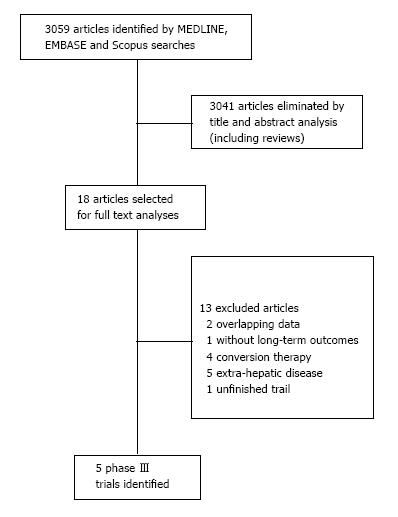
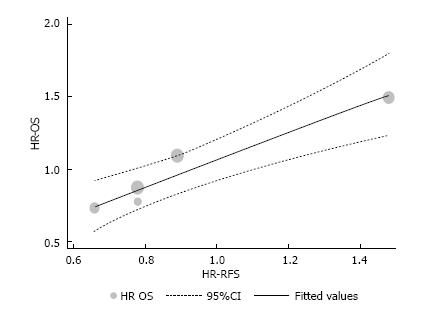
Of 3059 studies, 5 phase III trials (1162 patients) were included for analyses. A linear regression weighted by each trial was used to estimate the association between each HR and RFS. The originated formula was: OS HR = (0.93 × RFS HR) + 0.14; with RFS 95%CI (0.48-1.38), with P = 0.007.
This association suggests that RFS could work as a putative surrogate endpoint of OS in this population, avoiding bigger, longer and more resource-consuming trials. The OS could be assumed based on RFS and our model could be useful to better estimate sample size calculations of phase III trials of CRLM aiming for OS.
Core tip: This study addresses a systematic review of curative-intent treatment of colorectal liver metastasis looking for oncologic outcomes. We describe the association between overall survival (OS) and recurrence free survival in the setting of resectable colorectal liver metastases (CRLM). It suggests that recurrence free survival could work as a putative surrogate of OS in this population, avoiding bigger, longer and more resource-consuming trials. We do believe that our model can be useful to better estimate sample size calculations of superiority phase III trials of CRLM aiming for OS.
- Citation: Araujo RLC, Herman P, Riechelmann RP. Recurrence-free survival as a putative surrogate for overall survival in phase III trials of curative-intent treatment of colorectal liver metastases: Systematic review. World J Clin Oncol 2017; 8(3): 266-272
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/266.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.266
Randomized clinical trials (RCT) represent a high level of evidence and a mainstream analysis of oncologic outcomes. However, they involve a time-consuming methodology with inherent high costs. Moreover, trials using overall survival (OS) as their primary endpoint in patients with slow progressive malignancies, such as colorectal cancer, must have longer follow-up for events to arise and thus properly evaluate potential differences in OS. In turn, long-term follow up increases cost associated with image and laboratory tests, salaries of research coordinators, pharmacists and research nurses, investigators fees, medications, etc. Therefore, such trials claim for fundings that are not always provided by governmental agencies or by pharmaceutical companies. For example, Emanuel et al[1] reviewed the cost of conducting clinical trials and demonstrated that monitoring and treating 20 patients in a 12-mo randomized placebo-controlled trial of a new chemotherapeutic agent cost more than United States $ 6900 per enrolled subject in an industry-sponsored trial.
In order to reduce the cost and time to conduct RCT, investigators have looked at surrogate endpoints of OS, such as progression free survival (PFS) and recurrence free survival (RFS), as measures of clinical benefit in cancer trials. Gains in RFS associating chemotherapy to surgery vs surgery alone for initially resectable colorectal liver metastases (CRLM) have been demonstrated by phase III trials[2-4]. While surgery plus chemotherapy has not been associated with improvements in OS in phase III trials, it has been suggested by a meta-analysis of published data[5]. However, it is unknown whether RFS can substitute, and if so to which extent, OS in RCT of CRLM. In this regard we hypothesized that if gains in RFS predicted gains in OS, trials of new drugs in the setting of CRLM could use RFS as a surrogate endpoint, and thus expedite drug development. The objectives of this systematic review were to evaluate RCT of curative-intent treatment to resectable CRLM and to verify whether RFS surrogates OS in phase III trials for this population.
Type of studies: All published RCTs with curative-intent treatment for initially resectable CRLM were evaluated; curative-intent therapies were surgery alone vs associated systemic cancer-directed therapy. Two considerations were made to assume curative-intent treatment: Patients were not treated for conversion therapy because they were already resectable at the time of study enrollment and removal of all macroscopic disease (no residual disease). No language restriction was applied. Studies with extra-hepatic disease were generally excluded, but when extra-hepatic disease was present in no more than 5% they were accepted. Studies using regional chemotherapy or presenting initially unresectable disease were also excluded. For situations in which two studies from the same institution were identified, the most recent or the most informative study was selected unless different periods were evaluated or the data of overlapping patients could be subtracted.
Type of interventions: Only treatments with curative-intent treatment for initially resectable hepatic lesions were evaluated. However, any standardized description of resectable disease was used to define this group of patients, as they were defined according to clinic-radiological evaluation of each tumor board of their respective authors’ institutions. Any additional systemic treatment was considered as the following: Adjuvant chemotherapy (surgery followed by systemic therapy), neoadjuvant chemotherapy (preoperative chemotherapy followed by surgery), perioperative chemotherapy (preoperative chemotherapy followed by surgery and postoperative chemotherapy), and targeted agents at any perioperative period. This study did not discriminate between type of liver resection or surgical techniques because all of them were procedures with curative-intent. The study also did not aim to compare types of systemic therapies or times of its administration.
Type of outcome measure: The primary end point of the study was to describe the association between OS and RFS in the setting of resectable CRLM. Calculation of OS was based on survivorship status (deceased or alive) at the last follow-up visit as reported by RCT. Calculation of RFS was based on the first detected recurrence or the last follow-up visit without recurrence. Start time was counted as defined by each study. Imaging tests were mostly performed at 3-mo intervals until disease recurrence, as defined by RCT. The terminology chosen was RFS since all patients did not present any residual macroscopic disease after curative-intent treatment. We counted reappearance of distance and/or liver-only disease as recurrence.
Search: The MEDLINE, EMBASE, and Scopus databases were searched using the mesh terms (‘‘colorectal liver metastases” or “colorectal liver metastasis”) and (surgery or surgical or chemotherapy or “drug therapy” or “Antineoplastic Agents”) and (Clinical Trial or Comparative Study or Randomized Controlled Trial). They were filtered from January 1990 to February 2015 and only for studies in humans.
Data collection process: Relevant data were extracted independently from all the studies by two reviewers (Raphael LC Araujo and Rachel P Riechelmann) and included study features, population characteristics, and data needed for quality assessment. For the purpose of this study, only OS and RFS were extracted according to the description provided by the authors.
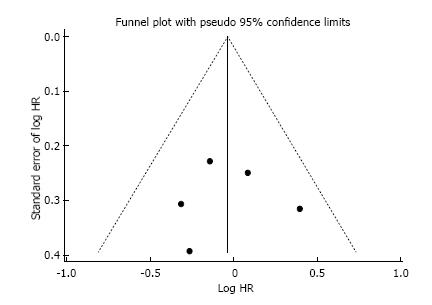
Quality assessment: The RCTs were evaluated by individual components based on the Cochrane Risk-of-Bias Tool (version 5.1.0). The qualitative evaluation was performed and discriminated for each RCT. This study was performed according to the recommendations of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement. We used Begg’s funnel plot as an analytic tool to detect publication bias[6,7].
Linear regression was performed to examine the association between HR for both outcomes. Demographics were demonstrated as percentages as appropriate. Survival probabilities were estimated by cited hazard ratios (HR) accordingly to each published study. The graph of linear regression was based on linear prediction of OS HR according to RFS HR, along with a 95%CI based on the mean. For all tests, statistical significance was defined by a two-sided P value lower than 0.05. All analyses were performed by STATA 13 statistical software (StataCorp, College Station, TX, United States).
We identified five RCT addressing curative-intent treatment with surgery alone or with systemic therapy for initially resectable CRLM. They were selected among 3059 articles. The flowchart of selection process is summarized in a flow diagram in Figure 1.
This systematic review was made properly to the PRI-SMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-Analysis). Additionally, the Cochrane Risk-of-Bias Tool was used to qualitative evaluation of RCTs, and it is described in Table 1. As frequently seen in surgical trials, the difficulty concealing the allocation of patients and blinding in the randomization between chemotherapy and surgery first could not be granted. However, it was not considered as a drawback neither affecting outcomes directly nor compromising the primary endpoint of our review. No publication bias was demonstrated using Begg’s funnel plot as depicted in Figure 2.
| Criteria | Langer | Portier | Nordlinger | Ychou | Primrose |
| Random sequence generation | Unclear | Low | Low | Low | Low |
| Allocation concealments | Low | Low | Low | Low | Low |
| Blinding of participants and personnel1 | Low | Low | Unclear | Low | Low |
| Blinding of outcome assessment1,2 | Low | Low | Low | Low | Low |
| Incomplete outcome data | Unclear | Low | Low | Unclear | Low |
| Selective reporting | Low | Low | Low | Low | Low |
| Other bias | Unclear | Low | Low | Low | Low |
Only five phase III trials were accepted for this review, and all of them were looking for initially resectable CRLM[2-4,8,9]. Comparative distributions of accessible baseline characteristics in the studies are depicted in Table 2. Three of them compared surgery alone vs surgery plus chemotherapy, and two RCT compared surgery plus chemotherapy on both arms, with one of them testing the addition of cetuximab, a monoclonal antibody against epidermal growth factors receptor (EGFR). Four studies had RFS as their primary endpoint. Only Langer et al[2] pursued OS as primary endpoint, but they failed to show a significant difference with postoperative chemotherapy. A total of 1162 patients (per protocol) were included in this pooled analysis. Most of them were male, the median age ranged from 60 to 64 years old, most presented colon as their primary site and with a single hepatic lesion. Comparisons of original planned and analyzed design of RCT are demonstrated in Table 3.
| Characteristics | Langer n = 107 (%) | Portier n = 171 (%) | Nordlinger n = 364 (%) | Ychou n = 306 (%) | Primrose1n = 257 (%) | |||||
| Surg n = 55 | S + C n = 52 | Surg n = 85 | S + C n = 86 | Surg n = 182 | S + C n = 182 | S + 5-FU n = 153 | S + FOLFIRI n = 153 | S + C n = 128 | S + C + Cetuximab n = 129 | |
| Median age | 60 | 63.5 | 63 | 63 | 62 | 64 | 61 | 63 | 64 | 63 |
| Gender (male) | 65.4 | 65.4 | 62.4 | 53.5 | 63 | 70 | 65.4 | 58.8 | 63 | 71 |
| Primary site (rectum) | 30.9 | 26.9 | 40 | 40.7 | 37 | 46 | 26.1 | 28.8 | - | - |
| DFI ≤ 12 mo | 38.2 | 34.6 | 74.1 | 74.4 | 24 | 27 | 62.3 | 61.4 | - | - |
| Node-positive primary | 45.4 | 50 | 50.6 | 44.3 | 57 | 55 | - | - | - | - |
| No. of lesions > 1 | 32.7 | 36.5 | 30.1 | 31.4 | 52 | 51 | 35.9 | 36 | - | - |
| Largest met ≥ 5 cm | - | - | - | - | - | - | - | - | - | - |
| Chemotherapy | 5-FU | 5-FU | FOLFOX | 5 –FU | FOLFIRI | 5-FU + OX or 5-FU + Cap or FOLFIRI | 5-FU + OX or 5-FU + Cap or FOLFIRI or + Cetuximab | |||
| Studies | Initial design | No. of patients | Chemotherapy | % | Median FU | RFS | OS | ||||||||||
| by author | Primary endpoint | Planned HR | Type of analyses | Plan-ned | Acc-rued | ITT enrolled | PP (weight) | Regimen | Std Arm | Exp Arm | Resected | Std Arm | Exp Arm | Std Arm | Exp Arm | Std Arm | Exp Arm |
| Langer | OS | NR | PP | NR | 129 | 129 | 107 (9) | Adj | 0 | 5-FU × 6 | 100% | NR | NR | 20 | 39 | 43 median | 53 median |
| Portier | RFS | 20% abs dif 2 yr1 | ITT | 200 | 173 | 171 | 171 (15) | Adj | 0 | 5-FU × 6 | 100% | 87.4 | 87.4 | 17.6 | 24.4 | 46.4 median | 62.1 median |
| Nord-linger | RFS | 0.714 | Both | NR | 364 | 364 | 342 (29) | Periop | 0 | FOLFOX × 12 | 93% | 8.7 yr | 8.7 yr | 20 | 12.5 | 54.3 | 61.3 |
| Ychou | RFS | NR | PP | 420 | 321 | 321 | 306 (26) | Adj | 1 5FU | FOLFIRI × 6 | 100% | 42.4 | 41.7 | 21.6 | 24.7 | 72% at 3-yr | 73% at 3-yr |
| Pri-mrose | RFS | 0.68 | ITT | 268 | 272 | 257 | 236 (20) | Periop | FOL-FOX | Cetux + FOLFOX (70%) | 85% (Chemo) | 21.1 | 19.8 | 14.1 | 20.5 | 39.1 | NR |
| 82% (Cetux) | |||||||||||||||||
A linear regression was used to fit a predict model for OS based on RFS and using HR values. The assumption of linearity was based on this formula: OS HR = (0.93 × RFS HR) + 0.14; with RFS 95%CI (0.48-1.38); standard error of 0.14, and P = 0.007. HR for RFS and OS, the originals and those assumed by the formula above are described in Table 4 and depicted in Figure 3 (the intention-to-treat analysis from Nordlinger et al[3] was used).
| Studies (by author) | n total (weight %) | RFS | OS | Assumption OS HR | ||||
| HR | 95%CI | HR | 95%CI | |||||
| Langer | 107 (9) | 0.78 | 0.46 | 1.31 | 0.77 | 0.42 | 1.4 | 0.87 |
| Portier | 171 (15) | 0.66 | 0.45 | 0.96 | 0.73 | 0.48 | 1.1 | 0.75 |
| Nordlinger1 | 342 (29) | 0.78 | 0.61 | 0.99 | 0.87 | 0.66 | 1.14 | 0.87 |
| Ychou | 306 (26) | 0.89 | 0.66 | 1.19 | 1.09 | 0.72 | 1.64 | 0.97 |
| Primrose | 236 (20) | 1.48 | 1.04 | 2.12 | 1.49 | 0.86 | 2.6 | 1.52 |
This systematic review and pooled analyses of published RCT of resectable CRLM demonstrates that RFS can be considered a surrogate endpoint for OS in this setting. We found a linear association between RFS gains, as measured by HR, and OS increments. This finding has numerous implications for future trial designs of new cancer-directed therapies added to curative-intent hepatic resection of CRLM.
The practice of evidence-based medicine (EBM) has been in vogue in the last 30 years. Sacket et al[10] categorized levels of evidence according to quality of study designs, ranging from expert opinion (level V - the lowest level) to RCT (level I - highest level). While RCT represent the best way to deliver evidence-based medicine, over the last decades, their costs have skyrocketed, what may limit national fundings and consequently, demand for-profit sponsorship[11]. This is particular relevant for clinical cancer research[12]. The cost of RCT, including cancer trials, can be split in fixed (trial administration, hospital facilities, personnel training, equipment, infrastructure, etc.), variable (randomization, recruitment cost, patient track and follow-up) and indirect costs (hospital overhead, public relations and networking, and legal consultancy)[11]. In this context, expensive new cancer drugs can cost to society in two different ways: Firstly, it costs directly to payers; and secondly, their high prices preclude new trials to compare their effectiveness against effective but cheaper alternatives[13]. Looking for oncologic outcomes in resectable CRLM, RCT can be even more expensive since OS is usually a required primary endpoint. For example, it is clear that slow-progressive tumors demand longer follow-up (more than 5 years to reach median OS, e.g.,) in phase III trials than those just looking for RFS. Therefore, there are several pros and cons of utilizing OS as a primary endpoint for a cancer RCT. The first issue with the measurement of OS is that the curative-intent treatment works as just first-line therapy; when disease progresses, the patient can still undergo further lines of systematic therapy or R0 surgery, what contaminates and dilutes eventual OS gains from first line. This phenomenon can sometimes be overcome by planning trials with large samples aimed to look for small statistical OS benefits. This is turn, increases the cost of conducting RCT in oncology. The argument in favor of using only OS as the primary endpoint in cancer RCT is that survival is a hard endpoint, not subjected to measurement biases. On the other hand, those in favor of surrogate endpoints for OS, such as PFS and RFS, highlight benefits in terms of faster trial results, less cost and perceived clinical benefit by patients and physicians. We argue that while both OS and surrogates endpoints can be used depending on the scenario, surrogates endpoints, if mathematically demonstrated, are useful tools to expedite clinical research and avoid unrealistically large and expensive trials, and also to early identify and stop enrollment into futile trials.
PFS has been demonstrated to be a surrogate endpoint for OS in treatments for metastatic[14,15] as well as for early stage colorectal cancer. In the adjuvant, i.e., curative setting, Sargent et al[16] pooled individual data of 18 RCT (20898 patients) for early stage colorectal cancer, showing that gains in disease free survival predicted for gains in OS. Our study resembles the results of the Sargent et al[16] because we selected studies with a population more inclined to be cured since patients presented potentially resectable CRLM and underwent curative-intent treatment. In RFS, likewise OS, recurrence is a hard endpoint that is not subject to measurement bias, although it is dependent on the intervals of radiological evaluation.
In this review four studies were powered to RFS but not OS[3,4,8,9]. Although Langer et al[2] investigated OS as the primary endpoint, it failed to show any benefits of adjuvant chemotherapy compared to surgery alone. Nordlinger et al[17] reported the long-term outcomes with median follow-up of 8.5 years, which also did not find differences in OS. We recently published a systematic review and meta-analysis concerning also surgery alone vs surgery plus chemotherapy, and we found a relative increasing of 23% in OS at 5 years[5]. However, it was only possible using published data from both randomized and non-randomized trials. One may think that all these negative trials for OS suggest that larger trials would still be necessary to detect small differences in OS. We argue that this is unrealistic and that surrogates endpoints such as RFS should replace OS in RCT of CRLM.
Our study has some limitations. Despite our extensive search, only five studies were suitable to our analysis and it was conducted based on published instead of individual data. As most patients presented low volume disease, our model likely reflect CRLM patients with better prognosis and might not be generalizable to settings of bulky or conversion CRLM. Moreover, as expected, part of those patients will recur but they will still be candidate to rescue treatments (chemotherapy with or without surgery or radiofrequency ablation)[18,19]. For theses reasons, we do not consider our formula useful for individual estimative of OS in clinical practice. The patterns of recurrence are heterogeneous and our correlations could not address such questions since they were not addressed in the original trials. The limitations of our study are those inherent of systematic reviews. And because of that, we think predictive value of RFS demonstrated by our model should be externally tested in future studies. However, we attempted to search as wide as possible, and moreover, we did not detect publication bias. Another limitation to our study and to all others in the field of CRLM is that colorectal cancer is a heterogeneos disease, with patients presenting variable outcomes even when following similar treatments.
Recently, colorectal cancer has been molecularly classified as four distinct prognostic subgroups: CMS1 (microsatellite instability and immune activation features, better prognosis), CMS2 (epithelial, with marked WNT and MYC signaling activation), CMS3 (metabolic dysregulation) and CMS4 (mesenchymal features, worse outcomes)[20]. It is clear that while perioperative benefits patients with resectable CRLM, many relapse and are not cured. Hence is crucial to properly identify the patients who are more likely to be cured or not by hepatectomy. Once this is done, the surrogacy of RFS on OS will have to be revaluated according to treatments tailored to each of these molecular subgroups.
Based on RCT, it seems that chemotherapy should always be offered as additional treatment to curative-intention liver resections, increasing RFS, and likely OS[5]. However, given the lack of evidence on OS gain by RCT, we foresee that surgical trials of systemic treatment for CRLM may prefer OS as their main endpoint. We think such approach should be revisited since larger sample than those already used would be necessary. Based on this systematic review and pooled analysis, we suggest RFS as surrogate of OS for phase III trials comprising patients with resectable CRLM could be used.
In summary, this study demonstrates a linear prediction of OS based on RFS of RCT of patients with resectable CRLM who were managed by curative-intent surgery and systemic therapy. This association suggests that RFS could work as a putative surrogate of OS in this population, avoiding bigger, longer and more resource-consuming trials. Our model can be useful to better estimate sample size calculations of superiority phase III trials of CRLM aiming for OS. However, future RCT should test this model to externally validate its efficiency.
The statistical methods of this study were reviewed by Marcos Alves Lima, biostatistician from Epidemiology and Biostatistics Center, Institute of Learning and Research, at Barretos Cancer Hospital.
Gains in recurrence-free survival (RFS) for resectable colorectal liver metastases (CRLM) have been demonstrated by phase III trials, but have not been associated with improvements in overall survival (OS). This systematic review verified whether RFS surrogates OS in phase III trials for resectable CRLM.
Although OS is considered the most appropriate outcome sought in oncology clinical trials, its use is not always feasible in trials of curative-intent treatment of CRLM. Most studies have evaluated RFS as their primary endpoint and none of them had demonstrated benefit in OS, except for a meta-analysis of published randomized trials. The study hypothesized a linear correlation between RFS and OS for this population after a systematic review of literature.
This study addresses an alternative option for analyses of oncologic outcomes in patients who have undergone a curative-intent treatment for CRLM. The linearity identified suggests a corresponding comportment between RFS and OS. The authors’ model could be useful to calculate the sample size for new trials in this field.
The use of modern chemotherapies and surgical resection for CRLM has made the comportment of this disease change into a more indolent profile, with many patients achieving long-term survival. Therefore clinical trials looking for OS as their primary endpoint are associated with long follow up times and high cost. The present study proposes a paradigm change in oncology clinical research because it sought to investigate another outcome which associated with patient benefit, RFS, that may be used in future research to avoid resource-consuming trials.
The paper is well written, properly designed, and comprehensive.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Facciorusso A, Palacios-Eito A, Peters GJ, Sukocheva OA, Vinh-Hung V, Wang GY S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Emanuel EJ, Schnipper LE, Kamin DY, Levinson J, Lichter AS. The costs of conducting clinical research. J Clin Oncol. 2003;21:4145-4150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Langer B, Bleiberg H, Labianca R, Shepard L, Nitti D, Marsoni S, Tu D, Sargeant A, Fields A. Fluorouracil (FU) plus l-leucovorin (l-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): results of the ENG (EORTC/NCIC CTG/GIVIO) randomized trial. Proc Am Soc Clin Oncol. 2002;21:Abstract 592. [Cited in This Article: ] |
| 3. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1478] [Cited by in F6Publishing: 1383] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 4. | Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 396] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Araujo RL, Gönen M, Herman P. Chemotherapy for patients with colorectal liver metastases who underwent curative resection improves long-term outcomes: systematic review and meta-analysis. Ann Surg Oncol. 2015;22:3070-3078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Mavridis D, Salanti G. Exploring and accounting for publication bias in mental health: a brief overview of methods. Evid Based Ment Health. 2014;17:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Based Ment Health. 2014;17:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, Hornbuckle J, Peterson M, Rees M, Iveson T. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15:601-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 325] [Cited by in F6Publishing: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 9. | Ychou M, Hohenberger W, Thezenas S, Navarro M, Maurel J, Bokemeyer C, Shacham-Shmueli E, Rivera F, Kwok-Keung Choi C, Santoro A. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964-1970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72. [PubMed] [Cited in This Article: ] |
| 11. | Shore BJ, Nasreddine AY, Kocher MS. Overcoming the funding challenge: the cost of randomized controlled trials in the next decade. J Bone Joint Surg Am. 2012;94 Suppl 1:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Fireman BH, Fehrenbacher L, Gruskin EP, Ray GT. Cost of care for patients in cancer clinical trials. J Natl Cancer Inst. 2000;92:136-142. [PubMed] [Cited in This Article: ] |
| 13. | Mailankody S, Prasad V. Comparative effectiveness questions in oncology. N Engl J Med. 2014;370:1478-1481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25:4562-4568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Giessen C, Laubender RP, Ankerst DP, Stintzing S, Modest DP, Mansmann U, Heinemann V. Progression-free survival as a surrogate endpoint for median overall survival in metastatic colorectal cancer: literature-based analysis from 50 randomized first-line trials. Clin Cancer Res. 2013;19:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Sargent DJ, Patiyil S, Yothers G, Haller DG, Gray R, Benedetti J, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569-4574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 18. | Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, Abascal A, Majno P, Engerran L. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225:51-60; discussion 60-62. [PubMed] [Cited in This Article: ] |
| 19. | Wicherts DA, de Haas RJ, Salloum C, Andreani P, Pascal G, Sotirov D, Adam R, Castaing D, Azoulay D. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg. 2013;100:808-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3303] [Cited by in F6Publishing: 3040] [Article Influence: 337.8] [Reference Citation Analysis (0)] |