Published online Apr 10, 2017. doi: 10.5306/wjco.v8.i2.158
Peer-review started: October 14, 2016
First decision: November 14, 2016
Revised: December 14, 2016
Accepted: January 2, 2017
Article in press: January 4, 2017
Published online: April 10, 2017
To evaluate the treatment effects of recombinant human interleukin-12 (rhIL-12) on radiotherapy complications, such as severe myelosuppression or pancytopenia, the decline or imbalance of immune function, etc.
The patients received high-dose and short-course precise radiotherapy, such as Cyber knife and image-guided radiotherapy (IGRT), which can cause myelosuppression or pancytopenia and immune function decline within a short time. One-hundred subjects were enrolled in the study, and 50 were randomized to a treatment group which used rhIL-12 and 50 were randomized to a control group which used symptomatic and supportive therapy after radiotherapy. The 50 subjects in the treatment group were further divided into five subgroups and intervened with rhIL-12 at a dose of 50, 100, 150, 200 or 250 ng/kg respectively. The dose-effect relationship was observed.
RhIL-12 significantly attenuated the decrease of peripheral blood cells in the treatment group, and immune function was improved after treatment. Due to the different radiation doses, there was a fluctuation within 12 h after treatment but mostly showing an increasing trend. As to the clinical manifestations, 2 patients in the 250 ng/kg subgroup showed low fever after administration, 1 patient in the 200 ng/kg subgroup and 2 patients in the 250 ng/kg subgroup showed mild impairment of liver function during the observation period.
RhIL-12 has effective therapeutic and protective effects on complications following radiotherapy, such as the decline of blood cells, myelosuppression and the decline or imbalance of immune function, which indicated good prospects for development and application.
Core tip: Recombinant human interleukin-12 (rhIL-12) is a new kind of biological agent secreted by Chinese hamster ovary cells. Study has shown that it has the advantage of promoting recovery of hematopoietic function, regulating the body’s immunity and inhibiting angiogenesis growth. At present, the research of rhIL-12 stays in the foundational realm and in animal experimentation. In our study, however, there were 100 patients with large or numerous tumors (more than two) and who received precision radiotherapy (Cyber knife or image-guided radiotherapy). The results showed that rhIL-12 can prevent radiation damage, improve hematopoietic function, regulate immunity, reduce the side effect of radiotherapy and improve the quality of life of patients.
- Citation: Guo N, Wang WQ, Gong XJ, Gao L, Yang LR, Yu WN, Shen HY, Wan LQ, Jia XF, Wang YS, Zhao Y. Study of recombinant human interleukin-12 for treatment of complications after radiotherapy for tumor patients. World J Clin Oncol 2017; 8(2): 158-167
- URL: https://www.wjgnet.com/2218-4333/full/v8/i2/158.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i2.158
Interleukin-12 (IL-12) is an immunoregulatory protein produced by macrophages, B cells, mononuclear cells, keratinocytes and dendritic cells, and its target point lies in early undifferentiated pluripotent hematopoietic stem cells. The studies of IL-2 trace back to early in 1986. Subsequently, many studies have confirmed that IL-12 can contribute to enhancing immunity. For example, Zhang et al[1] found a cytokine which can promote secretion of cytotoxic T cells (CTLs) and lymphatic factor-activated killer cells (LAK) in synergy with IL-2. In 1989, Bellone and Trinchieri[2] found a cytokine called natural killer cell-stimulating factor (NKSF), which can stimulate the production of IFN-γ. Eventually, it became known that the two cytokines were the same substance, now known as IL-12.
Based on subsequent research studies, IL-12 seems to serve as an immunoregulatory anti-cancer agent for oncology patients. However, the adverse events related to IL-12, including fever, chills, decreased peripheral blood cells and organ dysfunction, have limited the clinical application of IL-12[3]. Recombinant human interleukin-12 (rhIL-12) is an immunoregulatory protein produced by gene engineering technology. RhIL-12 has similar biological activity to IL-12. With the advantage of high purity (> 98%), high activity and low therapeutic dose, rhIL-12 became the only agent which could not only restore hematopoietic function but also improve immune function[4].
The basic experimental studies have found that the recovery and reconstitution of hematopoiesis system after radiotherapy is helpful to avoid the rapid increase of single blood cells, which lead to high fever, conjunctival hemorrhage, abnormal immune response, embolism and other detrimental side effects[5]. But a large number of studies are only based on animal experiments. The aim of our study, then, was to explore the interventional effects of rhIL-12 in tumor patients receiving radiotherapy, including the complications after radiotherapy, the curative effects on hematopoietic function and immune function as well as dose-effect relationship, and to provide scientific basis for clinical application.
To observe 100 patients with mid-advanced tumors who were treated with Cyber knife or image-guided radiotherapy (IGRT) in the People’s Liberation Army 107th Hospital, from October 2014 to June 2016. Inclusion criteria were: (1) Tumor confirmed by pathology, CT or MRI diagnosis, for which the clinical staging criteria were III-IV according to the World Health Organization (WHO); (2) ECOG score of 1 to 4 points; (3) Postoperative recurrence or lymphocytes invasion and metastasis, and need for radiosurgery; and (4) Provision of written informed consent for research and therapy. Exclusion criteria were: (1) Illness combined with tuberculosis or serious failure of important organs, such as heart, liver, kidney, lung, etc.; (2) Presence of benign tumors; (3) History of organ transplantation or allergies; and (4) Pregnant or lactating women or women of childbearing age. The experimental study was approved by the hospital’s ethics committee.
In the treatment group, 34 of the subjects (68%) were males and 16 (32%) were females; the mean age was 58.5-year-old (range: 27-83 years). Fifty subjects (30%) had lung cancers, 12 (24%) had liver cancers, 8 (16%) had head tumors, 1 (2%) had pancreatic cancer and 14 (28%) had other tumors. Solid relapse and metastasis tumor, for which size of the nidus could be assessed, accounted for 98% (n = 49) of the cases. Diffuse invasive metastatic tumor accounted for 2% (n = 1) of the cases, and the tumor diameter ranged from 3 cm to 16 cm. The patients who were classified as recurrent after the surgery or with more than 2 lesions accounted for 96% (n = 48) of the cases. In the control group, 28 of the subjects (56%) were males and 22 (44%) were females; the mean age was 57.4-year-old (range: 25-79 years). Twenty subjects (40%) had lung cancers, 15 (30%) had liver cancers and 15 (30%) had pancreatic cancers.
Equipment: Cyber knife, third-generation model produced by a United States’ accuracy company; IGRT, Eiekta Synergy model produced by a Swedish medical company; Flow cytometer produced by the United States’ BD Biosciences; Enzyme-mark instrument produced by a United States’ automation company; Chemiluminescence apparatus and automatic biochemistry analyzer produced by Roche Company; Hematology analyzer produced in Japan.
Drugs and reagents: RhIL-12 (for injection) produced by Qingdao Litai Kang Pharmaceutical Co. Ltd. Antibodies used in the study were purchased from BD Biosciences, including anti-human-IgG-FITC, anti-human-CD45-FITC, anti-human-CD56-PE and anti-human-CD3-PerCP-CD4-FITC-CD8-PE. Hemolysin was produced by an American research and development company.
Methods: After being admitted to hospital, all patients’ data were recorded for the three routine( liver and kidney function, heart function, bleeding and clotting time) and the imaging examination (such as electrolytes, color Doppler, CT and IMT), as well as adverse reactions, etc. All patients signed “consent form of precise radiotherapy”, “consent form of experimental study” and “agreement about the clinical application of rhIL-12 for prevention and treatment of malignant tumor radiotherapy complications”.
According to the research program, the patients were divided into 50, 100, 150, 200 and 250 ng/kg different-dose subgroups, and injected with rhIL-12 subcutaneously. Peripheral blood samples (for red blood cell (RBC), white blood cell (WBC) and platelet (PLT) assessment) and the immunophenotypes (CD4/8, CD45 and CD56) were collected before dosing (0 d) and at 12 h, 3 d, 7 d, 10 d, 14 d, 21 d, 28 d after dosing respectively. The effects on hematopoietic function and immune function were observed, as well as the dose-effect relationship. The control group used symptomatic supportive treatment.
Test evaluation: The patients who accepted high-dose and short-course of accurate radiotherapy, such as cyber knife and IGRT, could experience induction of decrease of peripheral blood cells and decline or imbalance of immune function. In this study, rhIL-12 was given to explore the interventional effects on radiation oncology surgery patients, including effects on hematopoietic function and immune function as well as dose-effect relationship and to provide scientific basis for drug development and clinical application.
WHO objective evaluation criteria: Complete remission (CR) indicated all symptoms and signs disappearing for 4 wk; partial remission (PR) indicated the tumor size was estimated to have reduced by more than 50% for at least 4 wk. No change (NC) indicated the patient’s condition had no obvious change for at least 4 wk, the tumor size has increased less than 25% and decreased less than 50%. PD (worsen) indicated new lesions having appeared or lesions had increased by more than 25%.
Zubrod-ECOG-WHO score: 0 score stood for normal activities; 1 score stood for mild symptoms and almost normal activities; 2 score stood for the time staying in bed as less than 50% of the daytime; 3 score stood for the time staying in bed as more than 50% of the daytime; 4 score stood for completely bedridden; 5 score stood for death. Total efficiency = (CR + PR)/total number of cases × 100%.
SPSS16.0 software was used for statistical analysis. Continuous variables are expressed as a mean and standard deviation; the mean differences between the groups were compared by independent t-test and ANOVA, and χ2 test was used to compare classified variables. Two values of data used two distribution tests. P < 0.05 indicated that the difference was statistically significant. Charts and tables were made by Prism GraphPad 4 software.
All of the 100 patients completed the study.
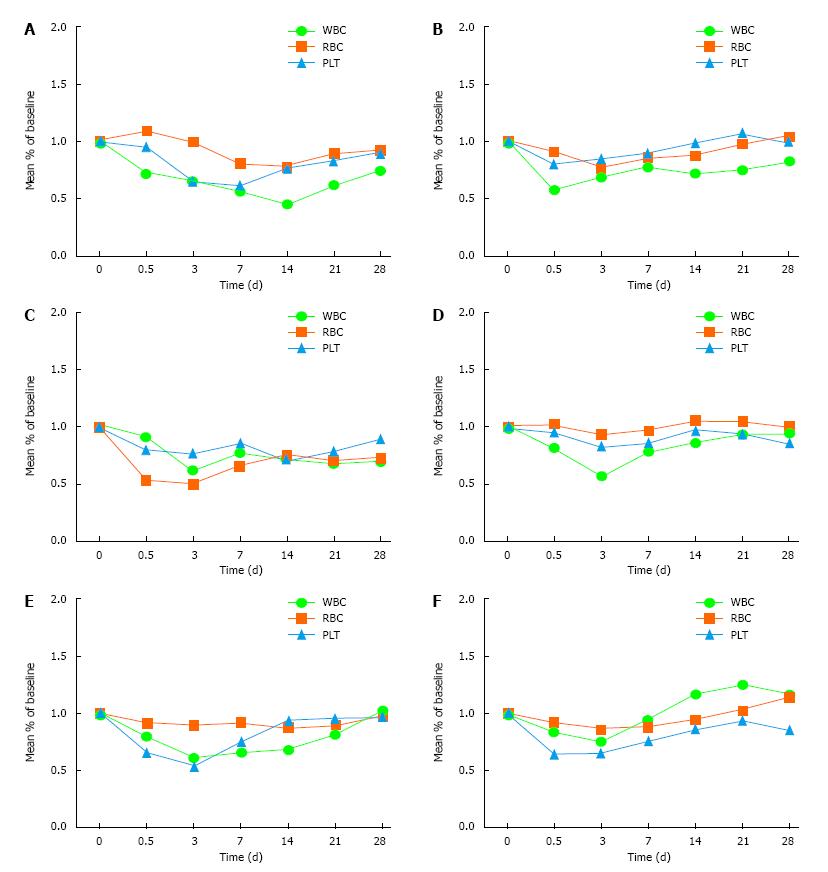
Treatment group: There was a transient decline of WBC and PLT within 12 h after treatment and by 3 d the lowest level was reached; the recovery rate decreased after 7 d, and the trend became stable after 21 d until the end of observation. This trend was relatively significant for WBC.
Control group: The whole blood cells declined on 3 d after radiotherapy, decreased significantly after 7 d, and reached the lowest point on 14 d. The degree of decrease was related to the radiation dose and tumor size. The difference between the treatment group and the control group was statistically significant (Table 1 and Figure 1).
| Group | Indicator | 0 d | 12 h | 3 d | 7 d | 14 d | 21 d | 28 d |
| Control | WBC (× 109/L) | 7.66 ± 0.82 | 5.5 ± 0.67 | 4.2 ± 0.39 | 4.3 ± 0.48 | 4.21 ± 0.62 | 4.69 ± 0.38 | 4.89 ± 0.63 |
| RBC (× 1012/L) | 3.92 ± 0.31 | 4.26 ± 0.43 | 3.9 ± 0.41 | 3.93 ± 0.22 | 4.38 ± 0.36 | 3.86 ± 0.34 | 3.89 ± 0.28 | |
| PLT (× 109/L) | 358 ± 0.43 | 339 ± 31.45 | 232 ± 20.43 | 258 ± 19.2 | 275 ± 0.31 | 296 ± 0.29 | 321 ± 0.26 | |
| 50 ng/kg | WBC (× 109/L) | 6.31 ± 0.59 | 3.6 ± 0.35 | 4.27 ± 0.46 | 4.85 ± 0.35 | 4.52 ± 0.42 | 4.72 ± 0.39 | 5.18 ± 0.52 |
| RBC (× 1012/L) | 5.43 ± 0.54 | 4.92 ± 0.53 | 4.16 ± 0.38 | 4.59 ± 0.82 | 4.73 ± 0.32 | 5.26 ± 0.37 | 5.63 ± 0.41 | |
| PLT (× 109/L) | 231 ± 20.81 | 185 ± 18.24 | 195 ± 18.97 | 205 ± 18 | 226 ± 18.2 | 246 ± 17.5 | 229 ± 19.4 | |
| 100 ng/kg | WBC | 8.4 ± 0.45 | 7.7 ± 7.89 | 5.3 ± 10.64 | 6.6 ± 0.98 | 6.1 ± 0.64 | 5.73 ± 0.47 | 5.9 ± 0.21 |
| RBC (× 1012/L) | 6.3 ± 0.72 | 3.37 ± 0.43 | 3.2 ± 0.34 | 4.2 ± 0.37 | 4.78 ± 0.46 | 4.5 ± 0.42 | 4.6 ± 0.34 | |
| PLT (× 109/L) | 231 ± 27.2 | 185 ± 10.7 | 178 ± 12.6 | 195 ± 12.8 | 166 ± 10.9 | 182 ± 12.5 | 205 ± 15.3 | |
| 150 ng/kg | WBC (× 109/L) | 6.6 ± 0.73 | 5.4 ± 0.76 | 3.8 ± 0.35 | 5.2 ± 0.37 | 5.7 ± 0.42 | 6.2 ± 0.54 | 6.3 ± 0.54 |
| RBC (× 1012/L) | 3.4 ± 0.36 | 3.5 ± 0.37 | 3.2 ± 0.29 | 3.3 ± 0.28 | 3.6 ± 0.24 | 3.6 ± 0.26 | 3.44 ± 0.21 | |
| PLT (× 109/L) | 367 ± 35.75 | 352 ± 32.45 | 306 ± 30.12 | 316 ± 16 | 357 ± 17 | 348 ± 26 | 317 ± 16 | |
| 200 ng/kg | WBC (× 109/L) | 5.3 ± 0.8 | 4.2 ± 0.3 | 3.2 ± 0.1 | 3.5 ± 0.32 | 3.6 ± 0.27 | 4.3 ± 0.31 | 5.4 ± 0.43 |
| RBC (× 1012/L) | 4.6 ± 0.51 | 4.2 ± 0.41 | 4.1 ± 0.45 | 4.2 ± 0.3 | 4 ± 0.2 | 4.12 ± 0.34 | 4.5 ± 0.42 | |
| PLT (× 109/L) | 278 ± 36 | 183 ± 19 | 149 ± 14 | 208 ± 22 | 259 ± 24 | 267 ± 25 | 271 ± 21 | |
| 250 ng/kg | WBC (× 109/L) | 3.6 ± | 3 ± 0.37 | 2.7 ± 0.24 | 3.4 ± 0.4 | 4.2 ± 0.3 | 4.5 ± 0.4 | 4.2 ± 0.4 |
| RBC (× 1012/L) | 3.6 ± 0.3 | 3.3 ± 0.2 | 3.1 ± 0.2 | 3.2 ± 0.3 | 3.4 ± 0.3 | 3.7 ± 0.2 | 4.1 ± 0.3 | |
| PLT (× 109/L) | 364 ± 35 | 235 ± 21 | 240 ± 20 | 276 ± 22 | 314 ± 19 | 342 ± 21 | 312 ± 20 |
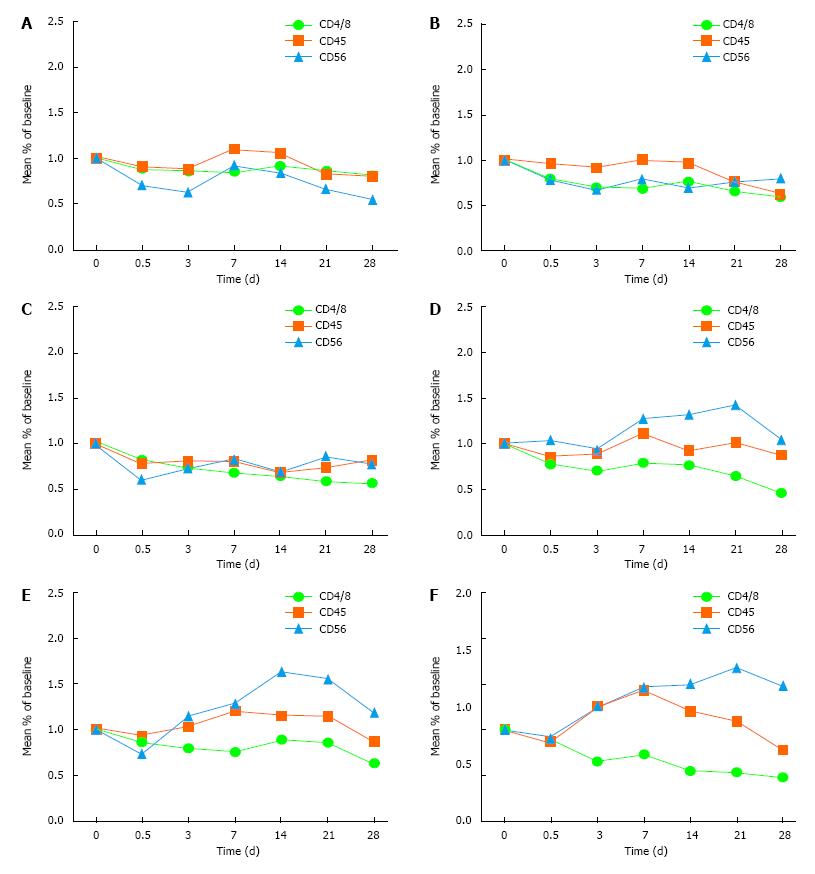
The aim was to observe the immune indexes, including CD4/8, CD45 and CD56.
Treatment group: There was a transient decline of CD4/CD8 within 12 h in the 150, 200 and 250 ng/kg subgroups. There was volatility rise between 3-14 d but the level remained below the pre-medication level, and went down after 21 d. There was a transient decline of CD45 and CD56 within 12 h, which rose after 3 d and went down after 21 d. The overall recovery improvement trend was obvious.
Control group: The trend of the immune indexes showed rebound on 3 d and a continuous downward trend after 7 d. There was no significant difference in these immune indexes between the other two groups (50 and 100 ng/kg subgroups) and the control group (Table 2 and Figure 2).
| Group | Indicator | 0 d | 12 h | 3 d | 7 d | 14 d | 21 d | 28 d |
| Control | CD4/8 | 20.4 ± 2.6 | 18 ± 1.2 | 17.5 ± 1.2 | 17.8 ± 1.3 | 18.4 ± 1.2 | 17.3 ± 1.4 | 16.5 ± 0.4 |
| CD45 | 75.2 ± 7.5 | 68.1 ± 5.2 | 65.4 ± 4.2 | 83.3 ± 5.2 | 79.3 ± 3.7 | 62.4 ± 5.1 | 60.3 ± 3.6 | |
| CD56 | 12.3 ± 1.2 | 8.7 ± 0.6 | 7.6 ± 0.5 | 11.4 ± 1.3 | 10.4 ± 0.9 | 8.2 ± 0.6 | 6.7 ± 0.5 | |
| 50 ng/kg | CD4/8 | 28.2 ± 1.8 | 22.4 ± 1.6 | 19.8 ± 1.6 | 19.6 ± 1.6 | 21.4 ± 1.8 | 18.8 ± 1.2 | 16.9 ± 1.4 |
| CD45 | 81.9 ± 2.4 | 78.8 ± 5.1 | 75.2 ± 3.6 | 82.9 ± 3.8 | 79.8 ± 5.6 | 62.4 ± 4.6 | 51.8 ± 4.3 | |
| CD56 | 27.9 ± 2.2 | 21.9 ± 1.8 | 19.2 ± 1.2 | 22.1 ± 1.8 | 19.7 ± 2.1 | 21.6 ± 1.3 | 22.4 ± 0.9 | |
| 100 ng/kg | CD4/8 | 15.4 ± 1.5 | 12.7 ± 1.2 | 11.5 ± 1.1 | 10.6 ± 1.2 | 10.1 ± 0.3 | 9.2 ± 0.4 | 8.7 ± 0.2 |
| CD45 | 84.3 ± 2.6 | 66.1 ± 3.8 | 68.7 ± 3.5 | 67.9 ± 4.4 | 57.6 ± 4.6 | 63.1 ± 3.6 | 70.2 ± 3.1 | |
| CD56 | 14.8 ± 1.8 | 8.9 ± 0.6 | 10.8 ± 0.9 | 12.3 ± 1.5 | 10.4 ± 4.6 | 12.9 ± 0.3 | 11.6 ± 0.4 | |
| 150 ng/kg | CD4/8 | 16.5 ± 1.2 | 12.8 ± 1.2 | 11.6 ± 0.9 | 13.1 ± 4.6 | 12.6 ± 1.5 | 10.8 ± 0.9 | 7.6 ± 0.5 |
| CD45 | 74.2 ± 3.6 | 63.2 ± 3.2 | 66.1 ± 4.8 | 82.7 ± 5.2 | 68.1 ± 4.6 | 75.2 ± 4.2 | 65.1 ± 4.1 | |
| CD56 | 6.2 ± 0.3 | 6.5 ± 0.3 | 5.9 ± 0.3 | 7.9 ± 0.6 | 8.2 ± 7.4 | 8.8 ± 0.9 | 6.5 ± 0.1 | |
| 200 ng/kg | CD4/8 | 9.6 ± 0.4 | 8.3 ± 0.61 | 7.6 ± 0.6 | 7.2 ± 0.9 | 8.6 ± 4.6 | 8.2 ± 1.2 | 6.1 ± 0.2 |
| CD45 | 64.5 ± 4.2 | 60.7 ± 4.2 | 65.8 ± 4.4 | 76.6 ± 5.9 | 74.3 ± 4.6 | 73.2 ± 5.2 | 55.9 ± 5.0 | |
| CD56 | 3.8 ± 0.2 | 2.8 ± 0.2 | 4.4 ± 0.4 | 4.9 ± 0.9 | 6.2 ± 0.6 | 5.9 ± 0.3 | 4.5 ± 0.1 | |
| 250 ng/kg | CD4/8 | 6.4 ± 0.2 | 5.7 ± 0.2 | 4.2 ± 0.3 | 4.7 ± 0.3 | 3.5 ± 0.3 | 3.4 ± 0.9 | 3.1 ± 0.9 |
| CD45 | 46.5 ± 3.9 | 40.2 ± 3.2 | 57.9 ± 4.1 | 66.5 ± 3.8 | 54.9 ± 4.2 | 50.4 ± 4.9 | 35.9 ± 3.2 | |
| CD56 | 4.3 ± 0.2 | 3.9 ± 0.2 | 5.4 ± 0.4 | 6.3 ± 0.5 | 6.6 ± 0.3 | 7.2 ± 0.6 | 6.4 ± 0.8 |
The remission rate in the treatment group (84%) was obviously higher than that in the control group (60%), and the difference was statistically significant (P < 0.05). During the observation period, there were no recurrence, metastasis or death, and the survival time of patients was significantly prolonged. There were 2 patients in the 250 ng/kg subgroup that had low fever after administration, 1 in the 200 ng/kg subgroup and 2 in the 250 ng/kg subgroup that had mild impairment of liver function during the observation period. There was no other adverse event found (Table 3).
The aim was to observe the ECOG score for a period of a month after rhIL-12 intervention.
Treatment group: There were 26 patients (52%) with normal activities after treatment, and the difference was statistically significant as compared with the 10 cases (20%) before treatment (P < 0.05). The life quality of patients was significantly improved.
Control group: There were 20 patients (40%) with normal activities after treatment, and the difference was not statistically significant as compared with the 12 cases (24%) before treatment (P > 0.05) (Table 4).
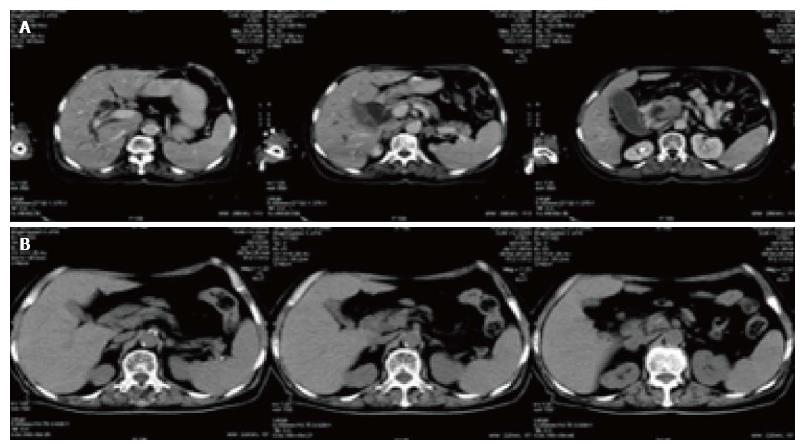
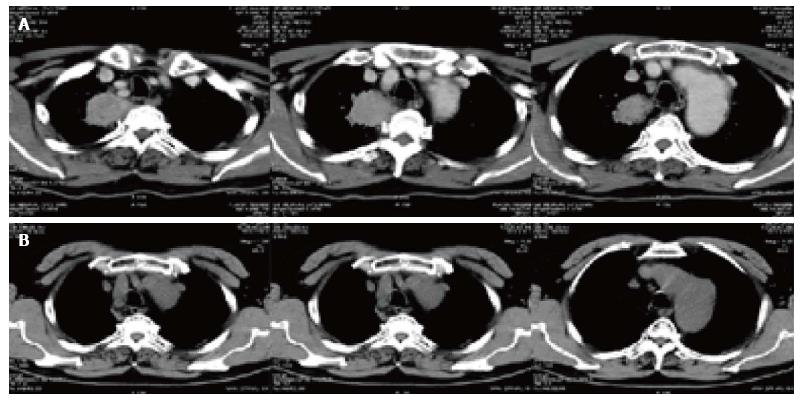
There were two CT pictures, including 1 case of pancreatic cancer and 1 case of lung cancer in the treatment group, before and after treatment. The results showed that the original lesion was significantly reduced after treatment and no new lesions appeared (Figures 3 and 4).
The incidence of cancer is rising, and cancer has become one of the main causes of death[6]. In recent years, radiotherapy of malignant tumors has developed rapidly, especially for accurate radiotherapy. However, the clinical curative effect for patients who have larger or more numerous tumor lesions are often reduced due to adverse reactions after radiotherapy, such as immune injury and myelosuppression. Under normal circumstances, the immune systems maintain the physiological balance and stabilization of the body.
Immune cells are the first line of the anti-tumor system. Immune regulatory factors or cytokines participate in immune regulation by means of signal transduction[7]. Studies have shown that tumor cells can escape immunosurveillance through a number of special mechanisms. The immune system is critical to the body’s surveillance against cancer.
The immune function of about 86% of patients has been shown to be on the decline in the early stage of cancer, and to further decline after treatment, which is the main cause of tumor metastasis and recurrence[8]. What’s more, the complications including infection and bleeding that are caused by the decrease of peripheral blood cells counts are also common causes of death in patients with cancer.
Therefore, improving immune function and reducing myelosuppression are indispensable auxiliary treatments in the process of tumor radiotherapy. At present, the treatment of cancer has entered into the era of personalized multidisciplinary treatment. The research shows that the combination of radiotherapy and immunotherapy has a unique advantage[9]. In this respect, IL-12 has received more and more attention.
IL-12 is an immunoregulatory protein produced by macrophages, B cells, mononuclear cells, keratinocytes and dendritic cells. IL-12 mainly functions to mediate cellular immunity, and it can induce the differentiation of T helper cell 1 (Th1), as well as promote the proliferation of NK cells and T cells, further stimulate IFN-γ secretion, and enhance the ability to kill target cells. IL-12 also can promote the formation of interferon-inducible protein-10 (IP-10). IP-10 can prevent the formation of tumor blood vessels, thereby reducing and blocking the nutrition source of tumor cells and inhibiting their growth[10,11].
It has been nearly 30 years since IL-12 was discovered, and a large amount of the related research is still at the stage of basic study and animal study. Many studies have found its abilities to improve immunity capability, adjust the immune function and inhibit the production of tumor blood vessels, but the adverse reactions such as chills and fever, nausea and vomiting, pulmonary edema and allergic reactions limit its clinical application and temper its promotion.
RhIL-12 is a new kind of biological agent secreted by Chinese hamster ovary cells through gene engineering. Its biological activity is similar to that of IL-12. The advantage of rhIL-12, however, is its high purity (> 98%), high activity (≥ 10000 IU/μg), low therapeutic dose and dosage that can be tolerated. Preliminary experimental study[12-14] found that rhIL-12 is currently the only biological agent with the advantage of comprehensive recovery of hematopoietic function, regulation of the
body’s immunity, inhibition of tumor angiogenesis and inhibition of tumor growth, thereby improving the life quality of patients with cancer[15]. As an immune regulatory factor, it plays an important role in both primary and secondary immunity[16]. Especially for radiotherapy patients who present with larger or more numerous tumor targets (more than two), it has important research value.
In our study, there were 100 patients who had larger or more numerous tumor s (more than two) and who received precision radiotherapy (Cyber knife or IGRT). The following conclusions are drawn. In the treatment group, the whole blood cells showed a transient decline within 12 h after treatment. The reason for this may be that the cell changes into the microcirculation or the bone marrow microenvironment, which may affect the proliferation of hematopoietic stem cells. The whole blood cells reached the lowest level at 3 d. People have always stopped treatment at this time, which represents a misunderstanding of the early research. The recovery rate decreased after 7 d, and the trend became stable after 21 d until the end of observation. This trend is relatively significant for WBC. Compared with the control group, the difference was significant, which indicated that the rhIL-12 was effective.
Observation of the immune indexes, including CD4/8, CD45 and CD56, showed a transient decline of CD4/CD8 within 12 h in the 150, 200 and 250 ng/kg subgroups in the treatment group, with volatile rises between 3-14 d, but remaining below the pre-medication level, and then decreasing after 21 d. There was a transient decline of CD45 and CD56 within 12 h, which rose up after 3 d and went down after 21 d. Compared with the control group, the difference was significant. The improvement tendency was obvious, which suggested that rhIL-12 could promote the immune function of the patients after radiotherapy.
From our observations of the clinical manifestations, 2 patients in the 250 ng/kg subgroup showed low fever after administration, which could be returned to normal after physical cooling. One patient in the 200 ng/kg subgroup and 2 in the 250 ng/kg subgroup showed mild injury of liver function during the observation period, which could be returned to normal after liver-protecting therapy. The injury related to radiation or rhIL-12 needs to be further studied.
In addition, this study explored the relationship between biological activity and concentration. The result showed that there was no statistical difference among the 50 ng/kg subgroup, the 100 ng/kg subgroup and the control group for immune response. What’s more, the adverse reactions were mainly concentrated in the 200 and 250 ng/kg subgroups, which suggests that the suitable clinical dosage concentration in our study is 150 ng/kg. The anti-tumor activity of rhIL-12 has been shown in clinical trials, but its toxicity to some extent limits the application. Therefore, rhIL-12 still needs to be further researched.
In conclusion, rhIL-12 can prevent radiation damage, improve hematopoietic function, regulate immunity, reduce the side effect of radiotherapy and improve the quality of life of patients. It has good clinical application value and good development prospect as tumor auxiliary treatment.
Interleukin-12 (IL-12) is an immunoregulatory protein produced by macrophages, B cells, mononuclear cells, keratinocytes and dendritic cells, and its target point lies in early undifferentiated pluripotent hematopoietic stem cells. However, the adverse events related to IL-12, including fever, chills, decrease of peripheral blood cells and organ dysfunction, have limited its clinical application. Recombinant human interleukin-12 (rhIL-12) is an immunoregulatory protein produced by gene engineering technology. RhIL-12 has similar biological activity to IL-12, but with the advantage of high purity (> 98%), high activity and low therapeutic dose. RhIL-12 has become the only available agent which can not only restore hematopoietic function but also improve immune function. Basic experimental study has found that the recovery of hematopoietic function after radiotherapy is helpful to avoid the rapid increase of single blood cells, which can lead to high fever, conjunctival hemorrhage, abnormal immune response, embolism and other detrimental side effects. But a large number of studies are basic in nature and based on animal experiments. The aim of the study was to explore the interventional effects of rhIL-12 on tumor patients receiving radiotherapy, including the complications after radiotherapy, the curative effects on hematopoietic function and immune function as well as dose-effect relationship, and to provide scientific basis for drug development and clinical application.
Some studies have shown that rhIL-12 can stimulate various kinds of cytokines through stimulating the bone marrow microenvironment, either directly or indirectly, further promoting long-term hematopoietic reconstitution progenitor cells’ differentiation and maturation, and instigate short-term hematopoietic reconstitution progenitor cells. These help to achieve a comprehensive recovery of hematopoietic function. In addition, in the circumstances of no supportive treatments, primate experiments demonstrated that using a low dose of rhIL-12 within 24 h after lethal dose irradiation could significantly improve (4-times) the animal’s survival rate. What’s more, rhIL-12 could promote the healing of skin wounds after radiation injury. As a radiation injury prevention drug, rhIL-12 is still effective at 24 h to 48 h after radiation. At the same time, a large number of animal experiments have shown that IL-12 can significantly inhibit the growth and metastasis of malignant tumors, prolonging the survival time of tumor-bearing animals. IL-12 can enhance the natural killer (NK) cell and cytotoxic T lymphocyte (CTL) cell response and the ability to induce production of IFN-C, which indicates that it may have anti-tumor activity. IL-12 enhances the binding ability of NK cells and K562 target cells and tumor cell monolayer, and enhances the cytotoxicity of NK cells to tumor cells. Because rhIL-12 and IL-12 have similar biological activities, some studies have shown that low dose of rhIL-12 can inhibit tumor cell growth, and rhIL-12 has synergistic anti-cancer effect on radiotherapy and chemotherapy, which needs further clinical validation.
The study found that low-dose rhIL-12 has the effect of prevention and treatment for the decrease of blood cells after radiotherapy, and could effectively improve the immune function and reduce the complications of radiotherapy.
RhIL-12 can prevent radiation damage, improve hematopoietic function, regulate immunity, reduce the detrimental side effects of radiotherapy and improve the quality of life of patients.
IL-12: Interleukin-12 is an immunoregulatory protein produced by macrophages, B cells, mononuclear cells, keratinocytes and dendritic cells, and its target point lies in early undifferentiated pluripotent hematopoietic stem cells; RhIL-12: Recombinant human interleukin-12 is an immunoregulatory protein produced by gene engineering technology; its biological activity is similar to IL-12.
The authors conducted an interesting clinical study on rhIL-12 for the prevention and treatment of complications after radiotherapy in patients with malignant tumors. The manuscript was well written. The methodology was clear and accurate. The results section was adequate.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu G, Ozyigit G, Xiao EH S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Zhang L, Feng D, Hu Y, Tsung K, Norton JA. IL-12 augments antitumor responses to cycled chemotherapy. J Immunother. 2015;38:137-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Bellone G, Trinchieri G. Dual stimulatory and inhibitory effect of NK cell stimulatory factor/IL-12 on human hematopoiesis. J Immunol. 1994;153:930-937. [PubMed] [Cited in This Article: ] |
| 3. | Lemoine S, Jaron B, Tabka S, Ettreiki C, Deriaud E, Zhivaki D, Le Ray C, Launay O, Majlessi L, Tissieres P. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J Allergy Clin Immunol. 2015;136:1355-1368.e1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Basile LA, Gallaher TK, Shibata D, Miller JD, Douer D. Multilineage hematopoietic recovery with concomitant antitumor effects using low dose Interleukin-12 in myelosuppressed tumor-bearing mice. J Transl Med. 2008;6:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1761] [Cited by in F6Publishing: 1687] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 7. | Pertl U, Luster AD, Varki NM, Homann D, Gaedicke G, Reisfeld RA, Lode HN. IFN-gamma-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J Immunol. 2001;166:6944-6951. [PubMed] [Cited in This Article: ] |
| 8. | Wadler S, Levy D, Frederickson HL, Falkson CI, Wang Y, Weller E, Burk R, Ho G, Kadish AS. A phase II trial of interleukin-12 in patients with advanced cervical cancer: clinical and immunologic correlates. Eastern Cooperative Oncology Group study E1E96. Gynecol Oncol. 2004;92:957-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N, Czapla J, Matuszczak S, Szala S. Combined Tumor Cell-Based Vaccination and Interleukin-12 Gene Therapy Polarizes the Tumor Microenvironment in Mice. Arch Immunol Ther Exp (Warsz). 2015;63:451-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8283] [Cited by in F6Publishing: 8159] [Article Influence: 509.9] [Reference Citation Analysis (0)] |
| 11. | Dhainaut M, Coquerelle C, Uzureau S, Denoeud J, Acolty V, Oldenhove G, Galuppo A, Sparwasser T, Thielemans K, Pays E. Thymus-derived regulatory T cells restrain pro-inflammatory Th1 responses by downregulating CD70 on dendritic cells. EMBO J. 2015;34:1336-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Robertson MJ, Soiffer RJ, Wolf SF, Manley TJ, Donahue C, Young D, Herrmann SH, Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779-788. [PubMed] [Cited in This Article: ] |
| 13. | Ribas A, Amarnani SN, Buga GM, Butterfield LH, Dissette VB, McBride WH, Glaspy JA, Ignarro LJ, Economou JS. Immunosuppressive effects of interleukin-12 coexpression in melanoma antigen gene-modified dendritic cell vaccines. Cancer Gene Ther. 2002;9:875-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994-5000. [PubMed] [Cited in This Article: ] |
| 15. | Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2687] [Cited by in F6Publishing: 2711] [Article Influence: 129.1] [Reference Citation Analysis (0)] |
| 16. | Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 501] [Cited by in F6Publishing: 501] [Article Influence: 22.8] [Reference Citation Analysis (0)] |