Published online Feb 10, 2016. doi: 10.5306/wjco.v7.i1.122
Peer-review started: May 30, 2015
First decision: August 16, 2015
Revised: October 16, 2015
Accepted: December 8, 2015
Article in press: December 11, 2015
Published online: February 10, 2016
Processing time: 246 Days and 19.9 Hours
Adjuvant therapies for breast cancer have achieved great success in recent years and early breast cancer is now a curable or chronic disease. Targeted therapies, including endocrine therapy and human epidermal growth factor receptor-2 targeted therapy, marked a new era of breast cancer treatment. However, except for chemotherapy, an efficient drug treatment to improve the overall survival of breast cancer patients is still lacking for triple negative breast cancer. Furthermore, a certain proportion of breast cancer patients present with resistance to drug therapy, making it much more difficult to control the deterioration of the disease. Recently, altered energy metabolism has become one of the hallmarks of cancer, including breast cancer, and it may be linked to drug resistance. Targeting cellular metabolism is becoming a promising strategy to overcome drug resistance in cancer therapy. This review discusses metabolic reprogramming in breast cancer and the possible complex mechanism of modulation. We also summarize the recent advances in metabolic therapy targeted glycolysis, glutaminolysis and fatty acids synthesis in breast cancer.
Core tip: Breast cancer cells display distinct metabolic characteristics according to different molecular phenotypes. There may be crosstalk with the estrogen receptor and human epidermal growth factor receptor-2 signal pathways in the metabolic regulation in breast cancer cells that make it more complex to evaluate the efficiency of an anti-metabolic drug. On the other hand, the research on target metabolism in breast cancer will also largely help us to understand the complicated mechanism by which an anti-metabolic drug improves the efficacy of cancer therapy or overcomes drug resistance.
- Citation: Long JP, Li XN, Zhang F. Targeting metabolism in breast cancer: How far we can go? World J Clin Oncol 2016; 7(1): 122-130
- URL: https://www.wjgnet.com/2218-4333/full/v7/i1/122.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i1.122
Breast cancer now has the highest incidence of cancer in women. This is attributed to the molecular classification of breast cancer based on the hormonal receptor and human epidermal growth factor receptor-2 (HER-2), targeted therapy and other adjuvant therapies that prolong the overall survival and greatly decrease the mortality of this disease. However, mortality remains high for locally advanced and metastatic cancer. We still lack effective methods for treatment when drug resistance occurs and recurrence and metastasis develop, especially in triple negative breast cancer (TNBC).
Females have a specific energy metabolic pattern compared to males[1]. Estrogens, progesterone-to-estrogen ratio and androgen levels affect the energy material transporter and metabolic enzyme expressions in cells[2]. Estrogens may increase the expression of peroxisome proliferator activated receptor, Akt and activate AMP-activated protein kinase (AMPK), which consequently influence the metabolic process, including glucose utility, lipid uptake, storage, lipogenesis and lipid oxidation[3,4]. Endocrine therapy plays a pivotal role in estrogen receptor (ER) positive breast cancer treatment. Rapamycin, which inhibits the mammalian target of rapamycin (mTOR), is a downstream target of Akt and enhances the susceptibility of breast cancer cells to endocrine therapy[5]. However, there is still a certain proportion of breast cancer patients that present with primary resistance to endocrine therapy and some patients could develop secondary resistance which makes it much more difficult to control the disease progress[6]. A similar condition occurs in chemotherapy and HER-2 targeted therapy in breast cancer. Therefore, researchers are looking for new strategies or compounds to reduce drug resistance and enhance the efficacy of therapy.
Metabolic reprogramming is the primary and basic factor during cell transformation[7,8]. Foreign stress forces tumor cells to accommodate new circumstances through metabolic reprogramming caused by epigenetic change and gene mutation. Altered energy metabolism has become one of the hallmarks of cancer[7]. Mounting evidence also attributes the drug resistance to dysregulated cellular metabolism[9,10]. Recently, much more interest has focused on targeting metabolic enzymes for cancer therapy or reversing drug resistance[11-13]. Cancer cells have distinct metabolic properties, including enhanced aerobic glycolysis, fatty acid synthesis and glutaminolysis, to sustain immortal proliferation[7,14]. This review will discuss the metabolic reprogramming and advances in metabolic targeted therapy in breast cancer.
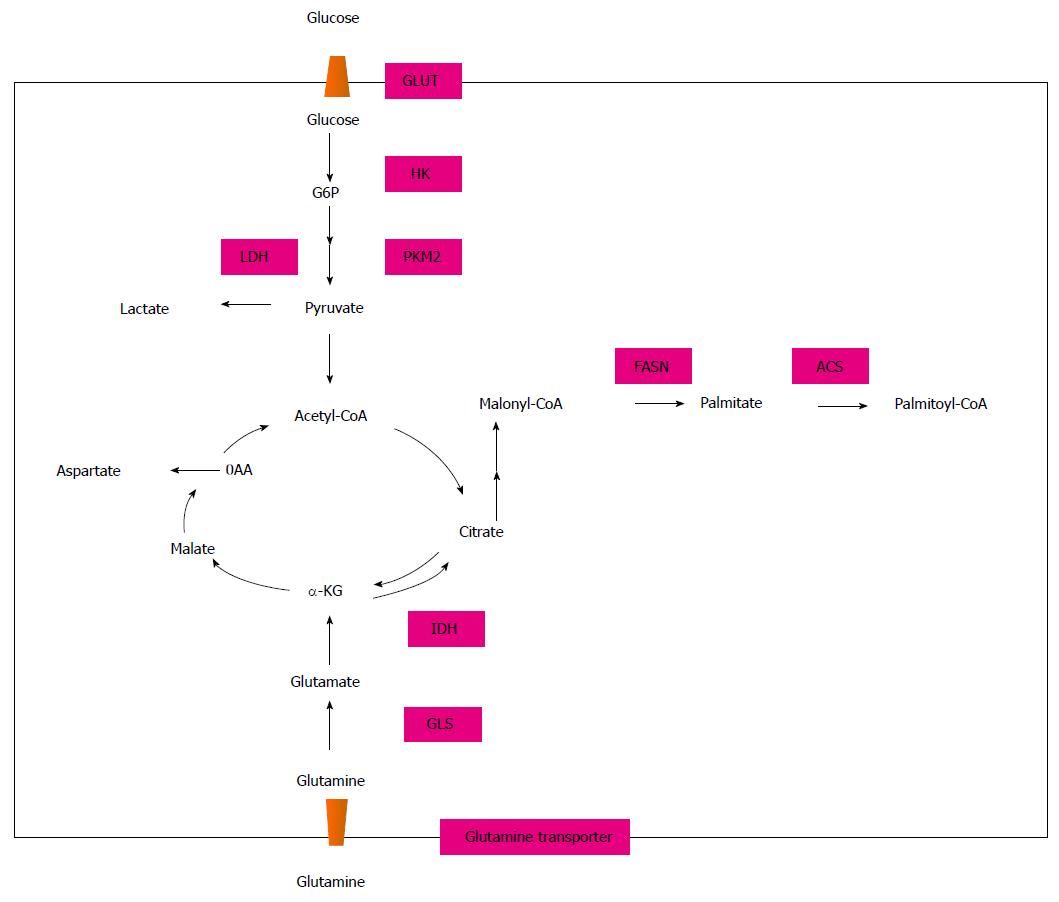
To meet the abundant requirement of energy and materials for proliferation, most malignant cells present with increased aerobic glycolysis, fatty acid synthesis and glutaminolysis, which are distinctive from normal cells[15] (Figure 1). In 1956, Warburg[16] first postulated that cancer cells had a significantly higher rate of glycolysis than normal cells to produce ATP for proliferation. He also hypothesized that due to the defective function of mitochondria (this was proved wrong afterwards), pyruvate produced from glycolysis was converted to lactate more than acetyl CoA through the tricarboxylic acid (TCA) cycle. This phenomenon is now called the Warburg effect and it exists regardless of oxygen availability. For the adaption of the Warburg effect, cancer cells exhibit altered expression of different glucose transporters and glycolysis enzymes. Glucose crosses the plasma membrane via glucose transporter proteins (GLUTs) and fourteen types have been identified. Although little is known about the role of glucose transporters in cancer biology, GLUT1, GLUT2, GLUT3, GLUT4, GLUT5 and GLUT12 have been detected in breast cancer cells[17-20]. Different expression patterns of GLUT isoforms in breast cancer may have an association with pathological grade, cancer cell differentiation and prognosis. According to the molecular subtype of invasive breast cancer, HER-2 positive and TNBC mostly exhibit higher levels of glycolysis which need higher levels of expression of GLUT[21]. As the most invasive type in breast cancer, TNBC had the highest expression of GLUT-1 when compared to other types[21]. Increased activity of enzymes involved in glycolysis, like hexokinase (HK) and lactate dehydrogenase-A (LDHA), have also been studied and their expression may affect cancer cell growth[22,23].
Increased glutamine metabolism is another alternative energy origin for cancer cells, including breast cancer, and is thought to be a central metabolic pathway cooperating with glycolysis[24,25]. Most cancer cells cannot proliferate without a glutamine supply and glutamine addiction provides intermediates for amino acid and lipid synthesis[26]. Under hypoxic conditions, proliferating cells, including breast cancer cells, mostly employ reductive metabolism of glutamine-derived alpha-ketoglutarate to synthesize acetyl CoA for lipid synthesis that normally enters into the canonical TCA cycle. That pathway is isocitrate dehydrogenase 1 dependent[27,28]. Intermediate metabolites derived from glutamine metabolism, such as antioxidants NADH, glutathione and ammonia, could change the reduction-oxidation status in cancer cells, promote stromal cell autophagy and increase tumor growth and drug resistance[25,29]. Cell studies showed that a high glutamine supply protected MCF7 cells from tamoxifen-induced apoptosis[30]. Amino acid transporter-2, glutaminase 1 (GLS) and glutamate dehydrogenase are three key enzymes involved in glutamine metabolism[31]. Immunohistochemical staining of breast cancer tissues indicated that HER-2 positive and TNBC exhibited the most frequent expression of glutamine metabolism related proteins than other types[32]. Glutamine produces glutamate under the catalytic effect of glutaminase, thus the ratio of glutamate to glutamine may indicate the glutamine metabolic activity[33]. Asiago et al[34] reported that an elevated level of glutamate was associated with disease outcome in breast cancer patients. Metabolomic analysis of 270 clinical breast cancer samples and 97 normal breast samples showed that breast cancer cells had a higher glutamate-to-glutamine ratio than normal cells, particularly ER-tumor cells[35]. A cell study showed that highly invasive and drug-resistant breast cancer cells were characterized by increased glutamine metabolism with an increased glutamate-to-glutamine ratio and greater expression of glutaminase compared with noninvasive breast cancer cells[36].
Under normal conditions, breast cells utilize circulating lipids for the synthesis of new structural lipids, while breast cancer cells mostly synthesize fatty acids by themselves. The biosynthetic enzyme fatty acid synthase (FASN) is the key enzyme required for the synthesis. FASN expression in breast cancer was first explored during the 1980s when its expression was increased after progestin treatment[37]. Recently, FASN expression has been recognized as an oncogene for its role in carcinogenesis. Upregulation of FASN has been reported in many different tumors, including breast cancer, and it may be associated with tumor development, recurrence and prognosis[38]. Immunohistochemistry staining revealed the highest FASN expression in HER-2 breast tumors and lowest in TNBC tumors, with the studies in breast cancer cells obtaining the same results[39,40]. Vazquez-Martin et al[41] postulated a ‘‘HER2-FASN axis’’ that indicated the bidirectional regulation mechanism between FASN and HER2 which could enhance cancer cell proliferation, survival, chemoresistance and metastasis in breast carcinomas.
Breast cancer is classified into four molecular subtypes: Luminal A, luminal B, HER-2 overexpression and basal types, with type luminal A accounting for about 70%[42]. The estrogen and HER-2 signal pathways play critical roles in breast cancer carcinogenesis, progression and prognosis. They may interact with each other as well as other signal pathways. Since most cancer cells have a high nutrition intake requirement to accommodate cell proliferation and altered metabolism may be a hallmark of cancer development, different molecular subtypes of breast cancer should exhibit distinct metabolic phenotypes. However, to date, we still know much less about the modulation mechanism of tumor-specific metabolic changes, especially in breast cancer[43]. We also know less about how these changes may change molecular phenotypes of breast cancer and affect response to drug treatment.
Although scientists are trying hard to find how signal pathways control the energy metabolism of cancer cells, little is known about the complex network. Hypoxia-inducible factors (HIF) and the proto-oncogene c-Myc are two major regulators in energy metabolism, including glucose, protein and fatty acid metabolisms[44]. Other genes, including Akt, Ras, Raf, Src and EGFR, may also be involved in glycolysis and activating these genes could cause increased glucose uptake. mTOR inhibitor rapamycin may inhibit cancer cell glucose metabolism by downregulating pyruvate kinase M2 and restoring the susceptibility of breast cancer cells to tamoxifen treatment effectively may be one mechanism of rapamycin[45]. On the other hand, estrogen-induced HIF-1 accumulation in breast cancer cells stimulates glucose uptake via the PI3K/Akt signaling pathway[19,46] which also leads to increased mTOR phosphorylation[47]. Another clinical study found that HIF-1 had the highest expression in HER-2 positive breast cancer[21]. It indicated that HIF-1 has crosstalk with the ER and HER-2 signal pathways.
The c-MYC gene controls cancer cell glutaminolysis through several targeted genes. MYC is overexpressed in 30%-50% of high-grade breast tumors[48,49]. Increased MYC expression often indicates increased dependency on glutamine and glucose for survival, may have a correlation with drug resistance in breast cancer cells and inhibition of MYC could reverse the drug resistance[50-52]. In antiestrogen resistant breast cancer cells, MYC could activate an unfolded protein response through glucose-regulated protein-78 (GRP78/HSP5A/BiP) and inositol-requiring enzyme-1α (IRE1α/ΕRΝ1) and increase c-Jun N-terminal kinase activation and spliced X-box protein-1 to support cell survival[45]. The inhibition of MYC was shown to decrease glutaminase activity, although there were different results in drug resistant breast cancer cells and other cells[50,53,54]. Inhibition of glutaminase reversely could decrease MYC expression[51]. Activation of the Akt/mTOR signal pathway also stimulates uptake of glutamine through increased glutaminase activity[55] and the underlying mechanism may be through eIF4B dependent control of c-Myc translation[56]. In both ER and HER-2 positive breast cancer cells, upregulation of HER-2 is one possible mechanism for endocrine treatment resistance. The crosstalk between ER and HER2 may regulate MYC-mediated glutamine metabolism[52]. ER downregulator fulvestrant may decrease glutamine consumption through inhibition of MYC and glutaminase and consistent expression of MYC may abrogate the effect of rapamycin on glutaminase[52,56], although the highest glutamine metabolic activity was seen in HER2-type breast cancer, which meant a possible correlation between glutamine activity and the HER-2 signal pathway[32].
Although the mechanism of overexpression of FASN in breast cancer cells is still uncertain, it has been proved that the potent lipogenic transcription factor sterol regulatory element-binding protein 1 (SREBP-1), can regulate FASN expression through binding with the site of the FASN promoter with co-activating transcription factors such as NF-γ Sp1 and Spot14[57,58]. Dietary polyunsaturated fatty acids suppress FASN expression through the modulation of NF-γ binding to the FASN promoter by SREBP-1c[59]. PI3k-Akt and the MAPK signal transduction pathway are also thought to be involved in FASN modulation[60,61]. Under hypoxic conditions, FASN gene is upregulated via the activation of Akt followed by the induction of the SREBP-1 gene[62]. Inhibition of MAP kinase also decreases transcription from the FASN promoter and reduces FASN expression in MCF7 cells[63]. The mTOR inhibitor rapamycin may also inhibit FASN in breast cancer cells[64]. Recently, a “HER2-FASN axis” is thought to exist which indicates the bidirectional regulation mechanism between FASN and HER2. The highest level of FASN expression in the HER-2 positive breast cancer type also confirms this hypothesis. FASN could also be regulated by estrogen in ER-positive breast cancer cells. Estrogen stimulates FASN expression and inhibiting FASN augments E2-stimulated transcriptional activity and enhances the E2-mediated ER expression synergistically[65].
As a basic energy resource for cancer cells, many enzymes are involved in glucose metabolism. The efficiency of target metabolism therapy has been proved in enhancing anticancer treatments or overcoming drug resistance in breast cancer cells, including chemotherapy resistance, endocrine therapy resistance and HER-2 targeted therapy resistance. Besides searching for a new agent to block glucose metabolism or induce a switch from glycolysis to mitochondrial respiration, researchers are also making much effort to find the underlying effect of existing agents on metabolic changes. Sorafenib is a multikinase inhibitor and may downregulate GLUT-1 expression in breast cancer cells through AMPK-dependent inhibition of the mTORC1 pathway, inhibit cell proliferation and induce apoptosis[66].
The glucose transporter family consists of 14 sodium-independent facilitative glucose transporters (SLC2A1-14 or GLUT1-14). GLUT1 appears to be the predominant glucose transporter in many types of cancer cells, including breast cancer[67]. A small compound, WZB117, has shown its inhibitory activity on GLUT1 in MCF-7 breast cancer cells[68]. Synergistic anticancer effects of combined WZB117 with other anticancer drugs, cisplatin or paclitaxel, were also observed. Added to the mitochondrial inhibitor, WZB117 was more efficient in inhibiting cell proliferation, which indicated WZB117 may be more effective in aggressive cancer cells that invariably had mitochondrial dysfunction[68].
HK-2, the first regulatory enzyme in glycolysis, has an important role in glycolysis. 2-DG, a glucose analog, binds with HK competitively and inhibits glycolysis. Although as a single agent the antitumor effect was not significant, a study showed that 2-DG combined with trastuzumab inhibited trastuzumab-sensitive and resistant breast cancers in in vitro and in vivo models of HER-2 positive breast cancers with more efficient inhibition of glycolysis via downregulation of heat shock factor 1 and LDHA[69].
LDHA is the enzyme that catalyzes the conversion of pyruvate to lactate. LDHA knockdown stimulates the switch of HER-2-initiated breast cancer cells to mitochondrial oxidative phosphorylation, decreases cell proliferation to hypoxic conditions and interferes with tumorigenicity[70]. Dichloroacetate (DCA), an inhibitor of pyruvate dehydrogenase kinase (PDK), may activate pyruvate dehydrogenase, which is governed by PDK, and facilitate the conversion of pyruvate to acetyl Co-A, which demonstrates the antiproliferative properties in highly metastatic diseases of DCA[71]. The inhibitor of LDH-A selectively inhibits the growth of HER-2-overexpressing cells and enhances the sensitivity of trastuzumab-resistant breast cancers to trastuzumab treatment[69,23]. Furthermore, downregulation of LDH-1 by oxamate shows a synergistical inhibitory effect on taxol-resistant breast cancer cells by promoting apoptosis when combined with taxol[9].
In many cancer cells, glutamine is used to replenish the TCA cycle and oxidative phosphorylation instead of glucose to produce enough ATP to support cell proliferation[72]. Glutamine addiction is a common strategy for some cancer cells like breast cancer cells to escape drug treatment. Glutamine transporters or glutaminolysis are becoming a potential pharmacological target to revert resistant cancer cells to respond to the initial therapy. An amino acid transporter SLC6A14, also known as ATB0,+, is upregulated specifically in ER-positive breast cancer. Blockade of SLC6A14 in ER-positive breast cancer cells could inhibit mTOR activity, cause cell apoptosis and activate autophagy[73].
Glutaminase, the enzyme that catalyzes glutamine to glutamate has attracted much interest for targeted cancer therapy recently. Two novel glutaminase inhibitors have been discovered: CB-839[74] and 968[51]. CB-839 showed the most potent antiproliferative activity in a TNBC cell line, while no antiproliferative activity was observed in an ER–positive cell line. In xenograft models, CB-839 displayed significant antitumor activity, both as a single agent and in combination with paclitaxel. Compound 968 showed the greatest cytotoxic effect in MDA-MB-231 breast cancer cells. Genome analysis proved that compound 968 could induce changes in many anti-apoptotic and/or promote metastasis-related gene expression and histone modifications as well, which subsequently activate apoptosis and decrease the invasiveness of MDA-MB-231 cells. It also enhanced chemotherapy sensitivity of breast cancer cells when combined with the chemotherapeutic drug doxorubicin.
FASN is the key biosynthetic enzyme in the fatty acid synthesis pathway that synthesizes long-chain fatty acids palmitate from malonyl-CoA. Acetyl-CoA carboxylase (ACC) carboxylates acetyl-CoA to malonyl-CoA. Upregulation of FASN has been reported both in premalignant lesions and most human cancers. In normal cells, fats are absorbed freely and FASN is downregulated, except in the lactating breast and cycling endometrium. The unique distribution of FASN in different tissues makes FASN an attractive target for cancer therapy. The inhibition of FASN causes depletion of the end product long chain fatty acids and the accumulation of the substrate malonyl-CoA. Evidence showed that inhibition of ACC did not induce cancer cell apoptosis, which meant the accumulation of malonyl-CoA may be the reason for the antitumor effect of FASN inhibition[75,76].
A bidirectional regulation mechanism between FASN and HER2 was illustrated[41,77]. FASN blockade suppresses HER2 overexpression at the transcriptional level with the upregulation of the expression of PEA3, a transcriptional repressor of HER-2. HER-2 overexpression stimulates FASN expression and fatty synthesis and this HER-2 mediated induction can be inhibited by trastuzumab. The combination of FASN inhibitor and trastuzumab stimulates MDA-MB-231/HER-2 cell apoptosis and re-sensitizes trastuzumab-resistant breast cancer through the downregulation of HER-2 expression[78,79]. Menendez et al[77] hypothesized that FASN inhibition would result in major changes in the synthesis of phospholipids, which should increase the degradation of HER-2 and enhance the action of the anti-HER-2 antibody trastuzumab.
Furthermore, FASN inhibitor cerulenin demonstrated a strong synergism with docetaxel in HER-2 overexpressing and docetaxel-resistant SK-Br3 cells, which indicated the role of FASN in HER-2-induced breast cancer chemotherapy resistance[80]. FASN blockade also could induce a synergistic chemosensitization of breast cancer cells to other chemotherapy agents, such as paclitaxel, adriamycin, 5-FU and vinorelbine[81-84].
Breast cancer is a heterogeneous group of neoplasms originating from epithelial cells that can be divided into various molecular phenotypes. Targeted therapy, such as endocrine therapy and HER-2 targeted therapy, has achieved great success in breast cancer treatment. However, like chemotherapy resistance, resistance to endocrine therapy and HER-2 targeted therapy can produce discouraging results. Recently, cancer research has focused on dysregulated metabolism in cancer cells and metabolic reprogramming is now considered a hallmark of cancer. More and more evidence supports the idea that dysregulated cellular metabolism may be associated with drug resistance in cancer therapy. In breast cancer, many agents that target specific enzymes in the metabolic pathways, including glycolysis, glutaminolysis and fatty acid synthesis, have been developed or proposed. Some of them have shown the ability to enhance the efficacy of current therapies and resensitize resistant cancer cells and have now been progressed to clinical trials. However, to date, none have been put into routine clinical practice for a couple of reasons. The main reason may be the extremely complex modulation of metabolism and their crosstalk with other signal pathways. Hence, there are three key problems that need to be elucidated: (1) energy pathways may be employed by cancer cells as well as normal cells. The influence or toxicity of metabolic drugs on normal cells should be evaluated carefully besides its antitumor effect. This question is prominent when combining metabolic drugs targeting different pathways to avoid insufficient effects or drug resistance; (2) for breast cancer, different molecular types may possess a specific metabolic phenotype. Even a “good” molecular type of breast cancer, like luminal A, may have recurrent metastasis caused by drug resistance in a relatively short period and so it is critical to find which specific enzymes for specific molecular phenotypes could be promising targets. This understanding will help us better distinguish which altered metabolic phenotypes may have a poorer prognosis and higher invasiveness than other types; (3) it has been postulated that metabolic regulation may have crosstalk with ER and HER-2 signal pathways. The genetic regulators such as c-myc, PI3k/Akt /mTOR and MAPK regulate metabolism as well as ER and HER-2 signal pathways. They form a complex framework, like the “FAS-HER-2 axis” and “c-myc-mTOR axis”, which determines the growth, apoptosis and drug resistance of cancer cells. Completely understanding the framework for breast cancer is still a challenge for developing a successful metabolic therapy. Nevertheless, much effort and progress has been made in this field and we hope that, in the near future, targeting tumor metabolic pathways may become an important component of the comprehensive treatment of breast cancer.
P- Reviewer: Matés JM, Song CJ, Tang SY S- Editor: Gong XM L- Editor: Roemmele A E- Editor: Jiao XK
| 1. | Varlamov O, Bethea CL, Roberts CT. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne). 2014;5:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 2. | Rune A, Salehzadeh F, Szekeres F, Kühn I, Osler ME, Al-Khalili L. Evidence against a sexual dimorphism in glucose and fatty acid metabolism in skeletal muscle cultures from age-matched men and post-menopausal women. Acta Physiol (Oxf). 2009;197:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58:803-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 4. | D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983-35991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | de Graffenried LA, Friedrichs WE, Russell DH, Donzis EJ, Middleton AK, Silva JM, Roth RA, Hidalgo M. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res. 2004;10:8059-8067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 301] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Milani A, Geuna E, Mittica G, Valabrega G. Overcoming endocrine resistance in metastatic breast cancer: Current evidence and future directions. World J Clin Oncol. 2014;5:990-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 46765] [Article Influence: 3340.4] [Reference Citation Analysis (4)] |
| 8. | Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 942] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 9. | Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 667] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 11. | Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 721] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 12. | Tennant DA, Durán RV, Boulahbel H, Gottlieb E. Metabolic transformation in cancer. Carcinogenesis. 2009;30:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 834] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 14. | Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Matés JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci USA. 2011;108:8674-8679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 15. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11682] [Article Influence: 730.1] [Reference Citation Analysis (0)] |
| 16. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9868] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 17. | Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, García Mde L, Medina RA, Carrasco M, Barberis S, Castro T. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol. 2006;207:614-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Rogers S, Docherty SE, Slavin JL, Henderson MA, Best JD. Differential expression of GLUT12 in breast cancer and normal breast tissue. Cancer Lett. 2003;193:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Garrido P, Morán J, Alonso A, González S, González C. 17β-estradiol activates glucose uptake via GLUT4 translocation and PI3K/Akt signaling pathway in MCF-7 cells. Endocrinology. 2013;154:1979-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res. 2012;18:721-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Choi J, Jung WH, Koo JS. Metabolism-related proteins are differentially expressed according to the molecular subtype of invasive breast cancer defined by surrogate immunohistochemistry. Pathobiology. 2013;80:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Hennipman A, van Oirschot BA, Smits J, Rijksen G, Staal GE. Glycolytic enzyme activities in breast cancer metastases. Tumour Biol. 1988;9:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Zhao YH, Zhou M, Liu H, Ding Y, Khong HT, Yu D, Fodstad O, Tan M. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689-3701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Cao MD, Lamichhane S, Lundgren S, Bofin A, Fjøsne H, Giskeødegård GF, Bathen TF. Metabolic characterization of triple negative breast cancer. BMC Cancer. 2014;14:941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 1008] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 26. | Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1408] [Cited by in RCA: 1332] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 27. | Leonardi R, Subramanian C, Jackowski S, Rock CO. Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J Biol Chem. 2012;287:14615-14620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1434] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 29. | Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Ko YH, Lin Z, Flomenberg N, Pestell RG, Howell A, Sotgia F, Lisanti MP, Martinez-Outschoorn UE. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011;12:1085-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Martín-Rufián M, Nascimento-Gomes R, Higuero A, Crisma AR, Campos-Sandoval JA, Gómez-García MC, Cardona C, Cheng T, Lobo C, Segura JA. Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. J Mol Med (Berl). 2014;92:277-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Kim S, Kim do H, Jung WH, Koo JS. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr Relat Cancer. 2013;20:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | de la Rosa V, Campos-Sandoval JA, Martín-Rufián M, Cardona C, Matés JM, Segura JA, Alonso FJ, Márquez J. A novel glutaminase isoform in mammalian tissues. Neurochem Int. 2009;55:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Asiago VM, Alvarado LZ, Shanaiah N, Gowda GA, Owusu-Sarfo K, Ballas RA, Raftery D. Early detection of recurrent breast cancer using metabolite profiling. Cancer Res. 2010;70:8309-8318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 35. | Budczies J, Pfitzner BM, Györffy B, Winzer KJ, Radke C, Dietel M, Fiehn O, Denkert C. Glutamate enrichment as new diagnostic opportunity in breast cancer. Int J Cancer. 2015;136:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Simpson NE, Tryndyak VP, Beland FA, Pogribny IP. An in vitro investigation of metabolically sensitive biomarkers in breast cancer progression. Breast Cancer Res Treat. 2012;133:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Chalbos D, Chambon M, Ailhaud G, Rochefort H. Fatty acid synthetase and its mRNA are induced by progestins in breast cancer cells. J Biol Chem. 1987;262:9923-9926. [PubMed] |
| 38. | Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 39. | Kim S, Lee Y, Koo JS. Differential expression of lipid metabolism-related proteins in different breast cancer subtypes. PLoS One. 2015;10:e0119473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Wang YY, Kuhajda FP, Li J, Finch TT, Cheng P, Koh C, Li T, Sokoll LJ, Chan DW. Fatty acid synthase as a tumor marker: its extracellular expression in human breast cancer. J Exp Ther Oncol. 2004;4:101-110. [PubMed] |
| 41. | Vazquez-Martin A, Ortega-Delgado FJ, Fernandez-Real JM, Menendez JA. The tyrosine kinase receptor HER2 (erbB-2): from oncogenesis to adipogenesis. J Cell Biochem. 2008;105:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736-1747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2597] [Cited by in RCA: 2773] [Article Influence: 198.1] [Reference Citation Analysis (0)] |
| 43. | Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 650] [Cited by in RCA: 631] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 44. | Iqbal MA, Bamezai RN. Resveratrol inhibits cancer cell metabolism by down regulating pyruvate kinase M2 via inhibition of mammalian target of rapamycin. PLoS One. 2012;7:e36764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Ko BH, Paik JY, Jung KH, Lee KH. 17beta-estradiol augments 18F-FDG uptake and glycolysis of T47D breast cancer cells via membrane-initiated rapid PI3K-Akt activation. J Nucl Med. 2010;51:1740-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Sudhagar S, Sathya S, Lakshmi BS. Rapid non-genomic signalling by 17β-oestradiol through c-Src involves mTOR-dependent expression of HIF-1α in breast cancer cells. Br J Cancer. 2011;105:953-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Matés JM, Alonso FJ, Wang C, Seo Y. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 532] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 48. | Blancato J, Singh B, Liu A, Liao DJ, Dickson RB. Correlation of amplification and overexpression of the c-myc oncogene in high-grade breast cancer: FISH, in situ hybridisation and immunohistochemical analyses. Br J Cancer. 2004;90:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Deming SL, Nass SJ, Dickson RB, Trock BJ. C-myc amplification in breast cancer: a meta-analysis of its occurrence and prognostic relevance. Br J Cancer. 2000;83:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 50. | Shajahan-Haq AN, Cook KL, Schwartz-Roberts JL, Eltayeb AE, Demas DM, Warri AM, Facey CO, Hilakivi-Clarke LA, Clarke R. MYC regulates the unfolded protein response and glucose and glutamine uptake in endocrine resistant breast cancer. Mol Cancer. 2014;13:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 51. | Simpson NE, Tryndyak VP, Pogribna M, Beland FA, Pogribny IP. Modifying metabolically sensitive histone marks by inhibiting glutamine metabolism affects gene expression and alters cancer cell phenotype. Epigenetics. 2012;7:1413-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Chen Z, Wang Y, Warden C, Chen S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J Steroid Biochem Mol Biol. 2015;149:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1736] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 54. | Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci USA. 2012;109:8983-8988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 381] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 55. | Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153:840-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 455] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 56. | Csibi A, Lee G, Yoon SO, Tong H, Ilter D, Elia I, Fendt SM, Roberts TM, Blenis J. The mTORC1/S6K1 pathway regulates glutamine metabolism through the eIF4B-dependent control of c-Myc translation. Curr Biol. 2014;24:2274-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 57. | Xiong S, Chirala SS, Wakil SJ. Sterol regulation of human fatty acid synthase promoter I requires nuclear factor-Y- and Sp-1-binding sites. Proc Natl Acad Sci USA. 2000;97:3948-3953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Donnelly C, Olsen AM, Lewis LD, Eisenberg BL, Eastman A, Kinlaw WB. Conjugated linoleic acid (CLA) inhibits expression of the Spot 14 (THRSP) and fatty acid synthase genes and impairs the growth of human breast cancer and liposarcoma cells. Nutr Cancer. 2009;61:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Teran-Garcia M, Adamson AW, Yu G, Rufo C, Suchankova G, Dreesen TD, Tekle M, Clarke SD, Gettys TW. Polyunsaturated fatty acid suppression of fatty acid synthase (FASN): evidence for dietary modulation of NF-Y binding to the Fasn promoter by SREBP-1c. Biochem J. 2007;402:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes (Lond). 2008;32 Suppl 4:S36-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 61. | Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 2190] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 62. | Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 316] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 63. | Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp Cell Res. 2002;279:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Yan C, Wei H, Minjuan Z, Yan X, Jingyue Y, Wenchao L, Sheng H. The mTOR inhibitor rapamycin synergizes with a fatty acid synthase inhibitor to induce cytotoxicity in ER/HER2-positive breast cancer cells. PLoS One. 2014;9:e97697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Menendez JA, Oza BP, Atlas E, Verma VA, Mehmi I, Lupu R. Inhibition of tumor-associated fatty acid synthase activity antagonizes estradiol- and tamoxifen-induced agonist transactivation of estrogen receptor (ER) in human endometrial adenocarcinoma cells. Oncogene. 2004;23:4945-4958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Fumarola C, Caffarra C, La Monica S, Galetti M, Alfieri RR, Cavazzoni A, Galvani E, Generali D, Petronini PG, Bonelli MA. Effects of sorafenib on energy metabolism in breast cancer cells: role of AMPK-mTORC1 signaling. Breast Cancer Res Treat. 2013;141:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 67. | Young CD, Lewis AS, Rudolph MC, Ruehle MD, Jackman MR, Yun UJ, Ilkun O, Pereira R, Abel ED, Anderson SM. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One. 2011;6:e23205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 68. | Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 430] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 69. | Zhao Y, Liu H, Liu Z, Ding Y, Ledoux SP, Wilson GL, Voellmy R, Lin Y, Lin W, Nahta R. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011;71:4585-4597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 70. | Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1220] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 71. | Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989-28994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 223] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 72. | Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. 2013;9:712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 73. | Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy-Thandavan S, Gurav A, Gnanaprakasam JP, Singh N, Schoenlein PV. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830-31838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 74. | Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 783] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 75. | Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213-218. [PubMed] |
| 76. | Zhou W, Simpson PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, Pinn ML, Ronnett GV, Kuhajda FP. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003;63:7330-7337. [PubMed] |
| 77. | Menendez JA, Vellon L, Lupu R. Targeting fatty acid synthase-driven lipid rafts: a novel strategy to overcome trastuzumab resistance in breast cancer cells. Med Hypotheses. 2005;64:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, Lupu R. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci USA. 2004;101:10715-10720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 79. | Vazquez-Martin A, Colomer R, Brunet J, Menendez JA. Pharmacological blockade of fatty acid synthase (FASN) reverses acquired autoresistance to trastuzumab (Herceptin by transcriptionally inhibiting ‘HER2 super-expression’ occurring in high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells. Int J Oncol. 2007;31:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Menendez JA, Lupu R, Colomer R. Inhibition of tumor-associated fatty acid synthase hyperactivity induces synergistic chemosensitization of HER -2/ neu -overexpressing human breast cancer cells to docetaxel (taxotere). Breast Cancer Res Treat. 2004;84:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Menendez JA, Vellon L, Colomer R, Lupu R. Pharmacological and small interference RNA-mediated inhibition of breast cancer-associated fatty acid synthase (oncogenic antigen-519) synergistically enhances Taxol (paclitaxel)-induced cytotoxicity. Int J Cancer. 2005;115:19-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Liu H, Liu Y, Zhang JT. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol Cancer Ther. 2008;7:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 83. | Vazquez-Martin A, Ropero S, Brunet J, Colomer R, Menendez JA. Inhibition of Fatty Acid Synthase (FASN) synergistically enhances the efficacy of 5-fluorouracil in breast carcinoma cells. Oncol Rep. 2007;18:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |