Published online Oct 10, 2015. doi: 10.5306/wjco.v6.i5.142
Peer-review started: April 5, 2015
First decision: May 13, 2015
Revised: July 7, 2015
Accepted: July 24, 2015
Article in press: July 27, 2015
Published online: October 10, 2015
Colorectal liver metastasis (CRLM) is the major cause of death in patients diagnosed with colorectal cancer. The gold standard treatment of CRLM is surgical resection. Yet, in the past, more than half of these patients were deemed unresectable due to the inadequate future liver remnant (FLR). The introduction of efficient portal vein embolization (PVE) preoperatively allowed more resections of metastasis in CRLM patients by stimulating adequate liver hypertrophy. However, several experimental and clinical studies reported tumor progression after PVE which critically influences the subsequent management of these patients. The underlying pathophysiological mechanism of tumor progression post-PVE is still not fully understood. In spite of the adverse effects of PVE, it remains a potentially curative procedure in patients who would remain otherwise unresectable because of the insufficient FLR. Currently, the challenge is to halt tumor proliferation following PVE in patients who require this technique. This could potentially be achieved by either attempting to suppress the underlying oncologic stimulus or by inhibiting tumor growth once observed after PVE, without jeopardizing liver regeneration. More research is still required to better identify patients at risk of experiencing tumor growth post-PVE.
Core tip: This article discusses the effect of portal vein embolization (PVE) on colorectal liver metastasis (CRLM) growth and the suggested methods of prevention. In addition to presenting the various experimental and clinical studies emphasizing the suggested tumoral enhancing effect of PVE, this article highlights the concept of reversal of chemotherapy response, a potential effect occurring after PVE. This observation may impact significantly subsequent patients’ management as it may affect the resectability state of patients. Moreover, potential methods to prevent tumor growth are discussed in this article, indicating the need for further research in this field and highlighting the complex interaction between CRLM and liver regeneration milieu.
- Citation: Al-Sharif E, Simoneau E, Hassanain M. Portal vein embolization effect on colorectal cancer liver metastasis progression: Lessons learned. World J Clin Oncol 2015; 6(5): 142-146
- URL: https://www.wjgnet.com/2218-4333/full/v6/i5/142.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i5.142
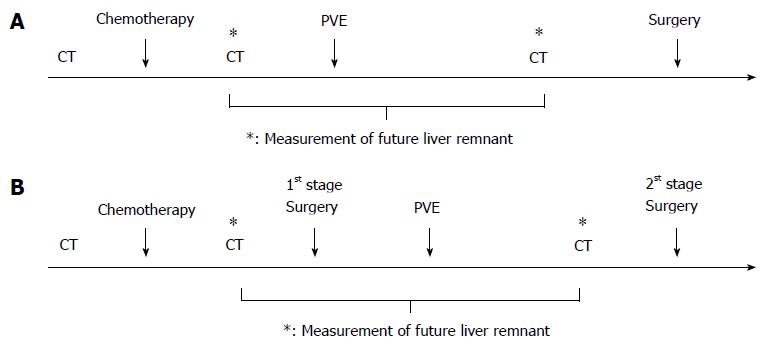
The most common site for colorectal cancer (CRC) metastasis is the liver which occurs in approximately 50% of patients during their disease course[1,2]. The 5-year survival rate of patients with local CRC is 90.3%, yet, survival drops ominously to 12.5% when remote metastases ensue in these patients[3]. In fact, colorectal liver metastasis (CRLM) is the leading cause of death in CRC patients with an overall median survival of 6-12 mo if not treated[4]. Surgical resection remains the gold standard and potentially curative treatment for CRLM[2,4]. In the past, only 15%-20% of patients with CRLM were candidates for liver resection because of insufficient future liver remnant (FLR), which puts patients at risk of hepatic dysfunction and post-operative morbidity and mortality[5-8]. Therefore, to increase resectability rate, the effective and relatively safe preoperative portal vein embolization (PVE) technique was introduced aiming to maximize the remnant liver volume before major hepatectomy[9]. The experience with preoperative PVE was first described more than 20 years ago by Makuuchi et al[10] in patients with hilar cholangiocarcinoma to induce ipsilateral hepatic atrophy and contralateral residual liver hypertrophy. The authors reported no major complications or liver failure in the 14 patients included in the study[10]. This successful and relatively safe technique allowed more liver metastases removal[6,9]. In addition to being recommended prior to major hepatectomy when the preoperative FLR is insufficient (< 25% of total liver volume), PVE is also part of the two-stage hepatectomy strategy, with the PVE being performed before the second stage resection, thereby facilitating resection in patients with bilateral CRLM[11]. Figure 1 provides a general overview of the clinical settings in which PVE is utilized.
An estimated FLR of less than 25% in patients with normal livers is a general indication for PVE prior to intended hepatectomy[12]. However, several studies reported the possible complications of PVE, namely inadequate FLR growth, higher disease recurrence and tumor growth acceleration in both embolized and non-embolized liver lobes[13-24]. Notably, rapid tumor progression following PVE remains a major concern for clinicians as it critically influences the clinical outcome and overall survival of CRLM patients[12,13]. As a matter of fact, local or distal tumor progression post-PVE may even lead to unresectable disease in a proportion of patients as observed in some studies[19,24]. Studies reporting tumor progression post PVE are summarized in Table 1. The exact mechanisms stimulating the hepatic atrophy-hypertrophy complex along with increased tumor volume post-PVE remains unclear. However, three mechanisms have been suggested to explain this occurrence: up-regulation of cytokines and growth factors stimulated by liver regeneration, compensatory increase in hepatic arterial blood perfusion and evoked cellular host response promoting local tumor growth[25].
| Ref. | No. of CRLM patients undergoing PVE | Percentage change in tumor volume and/or TGR and/or percentage of patients developing tumor progression after PVE |
| Simoneau et al[13] | n = 109 | 33.4% increase in TV in the right lobe (P < 0.001) and 49.9% increase in TV in the left lobe (P = 0.022) post-PVE |
| Elias et al[14] | n = 48 | 60% to 970% increase in TV post-PVE |
| Kokudo et al[15] | n = 18 | +20.8% (P = 0.016) increase in TV and 18.5% (P = 0.014) increase in percent tumor volume post-PVE |
| Mueller et al[19] | n = 53 | 80.9% (n = 17/53) of patients were unresectable due to tumor progression post-PVE |
| Pamecha et al[21] | n = 36 | 33% (n = 12/36) of patients had tumor progression post-PVE |
| Hoekstra et al[22] | n = 28 | 25% (n = 7/28) of patients developed new lesions in FLR and 42% of patients (n = 8/19) had tumor recurrence in the liver on follow up post-PVE |
| Pamecha et al[23] | n = 22 | TGR post-PVE was 0.36 ± 0.68 mL/d (-1) (P = 0.06) |
| Lindner et al[24] | n = 19 | 21% of patients developed tumor progression post-PVE |
The increasing body of evidence emphasizing the superior contribution of PVE to the observed tumoral growth triggered interest during the past decade. Hoekstra et al[26] examined the relationship between PVE and enhanced tumor growth in a rabbit model, which mirrored the clinical setting of CRLM patients. They concluded that a higher tumor growth rate occurred in the PVE group compared to non-PVE cohort group; however there was no significant difference between both groups in terms of markers of liver regeneration (IL-6, tumor necrosis factor alpha, growth factor hepatic growth factors and TGFB 1). Additionally, Maggiori et al[27] observed the same tumoral enhancing effect of PVE and ligation in their experimental rat model. Interestingly, this study showed that PVE increased tumor growth in the contralateral nonoccluded liver while decreasing it in the occluded liver portion. Similar results of diminished tumor volume in the embolized liver were also reported in another experimental animal study[28]. An additional experimental study conducted in an in-vivo rabbit model reported similar results of augmented tumor growth in the nonembolized liver whereas no effect was seen in the embolized liver[29]. Moreover, multiple clinical studies described concordant observations of tumor progression and higher recurrence rates in CRLM patients undergoing PVE[13-24]. A clinical study conducted by Pamecha et al[23] in 2009 was the first to correlate post-PVE tumor volumes measured by imaging with proliferative activity of cancer-cells observed on immunohistochemistry in two matched comparative groups. The authors confirmed the increased tumor growth rate was related to the proliferative activity post-PVE. Taking together all these experimental and clinical studies provides substantial evidence that PVE may play a critical role in promoting tumor growth.
Simoneau et al[13] attempted to further investigate this in one of the largest published observational studies. A total of 109 patients were included in the PVE group vs 11 patients in the no-PVE control group. Patients in the PVE group were further subdivided into bevacizumab group and non-bevacizumab group so as to evaluate the effect of pre-embolization chemotherapy given concurrently with bevacizumab on tumor progression and liver regeneration. Pre-embolization chemotherapy combined with anti-angiogenic therapy did not compromise liver regeneration as both groups had similar degrees of hepatic hypertrophy[13]. The study also showed a positive tumor growth rate (+0.07 cm3/d) in the PVE group compared to a negative growth rate (-0.06 cm3/d) in the control (no PVE) group (P < 0.001), suggesting that PVE may be associated with tumor progression in some patients despite an initial response to chemotherapy. The results of the authors thereby introduced of the hypothesis that PVE may, in some instances, stimulate tumor growth and actually reverse the chemotherapeutic response. This suggests that the effect of PVE may overcome the downsizing chemotherapy effect in a subset of patients, who may be more susceptible to progress after such a stimulus. Overall, the data derived from these observational studies on PVE and tumor growth raise some questions that not only have significant clinical value in the management of CRLM patients, but also shed some light on the complexity of liver metastasis biology, progression and resistance to therapy.
Understanding the mechanisms of liver regeneration and tumor growth post-PVE and identifying common factors stimulating both pathways may help to develop methods to inhibit tumor growth. Liver regeneration is regulated at the molecular level by a wide variety of growth factors and cytokines, such as tumor necrosis factor, interleukin-6, hepatocyte growth factor (HGF), transforming growth factor (TGF), vascular endothelial growth factor and epidermal growth factor[30]. Current evidence suggests that the up-regulation of these factors is common to stimulation of tumor pathways, and this was suggested as a possible theory explaining tumor growth after PVE[25,31]. Notably, it was postulated in one experimental study that HGF may be a key regulator, as it is a key factor for both hepatocyte regeneration and cancer cells proliferation. The investigators have observed a significant increased serum HGF after PVE compared to controls[29]. Thus, it has been suggested that the administration of anti-inflammatories or growth factor inhibitors at the time of PVE could potentially help in inhibiting tumor progression. To date, this still remains a theoretical concept as no targeted therapy that would prevent tumor progression without compromising liver hypertrophy has been demonstrated in clinical studies.
In another perspective, some investigators have focused on clinical strategies that would limit post-PVE tumor progression. Several approaches have been suggested in literature although a general consensus is still lacking. A preoperative period of 2-4 wk was suggested by Abdalla et al[12] to allow for adequate hepatic regeneration, while minimizing the time between PVE and resection is also recommended to reduce risk of tumor progression during the interval between end of chemotherapy and the procedure[22,24-25,32]. In addition, transarterial chemoembolization pre- and post-PVE was shown to be effective in preventing tumor growth in patients with hepatocellular carcinoma. Although its use for CRLM patients has not been reported, such an intervention may be a potentially promising strategy[25]. In addition, radio-embolization in the hepatic artery (for example with Yttrium-90), one of the current modalities of treatment of CRLM, may also hypothetically decrease the risk of tumor progression by decreasing the tumor arterial blood supply, with minimal effect to the normal adjacent liver parenchyma, but presently there is no evidence supporting its use post-PVE[25,33]. Lastly, the use of systemic therapy (neoadjuvant or adjuvant chemotherapy) is now widely used in the management of these patients[25,34]. In fact, chemotherapy may protect against tumor progression post-PVE without disturbing liver hypertrophy especially in patients who initially respond adequately[6,25,35]. Fischer et al[6] reported in an observational study that the administration of chemotherapy after embolization significantly reduced the rate of progression. Whether systemic or loco-regional therapy, many existing strategies have been and continue to be investigated as potential strategies to diminish tumor progression after embolization.
Despite the potential adverse effects of PVE, it remains an essential procedure done in the preoperative setting prior to major hepatectomy, allowing for resectability in patients who otherwise would remain unresectable due to insufficient FLR. More research is required to better stratify patients and identify those at increased risk of developing tumor growth post-PVE. Further research should focus on identifying tumors more responsive to a stimulatory environment and more prone to progress, to provide insight on the complex tumor biology of colorectal hepatic metastasis and to promote the development of personalized treatment strategies.
P- Reviewer: Edeline J, Shi Z S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 2. | Liu LX, Zhang WH, Jiang HC. Current treatment for liver metastases from colorectal cancer. World J Gastroenterol. 2003;9:193-200. [PubMed] [Cited in This Article: ] |
| 3. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 2025] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 4. | Konopke R, Roth J, Volk A, Pistorius S, Folprecht G, Zöphel K, Schuetze C, Laniado M, Saeger HD, Kersting S. Colorectal liver metastases: an update on palliative treatment options. J Gastrointestin Liver Dis. 2012;21:83-91. [PubMed] [Cited in This Article: ] |
| 5. | Smith JJ, D’Angelica MI. Surgical management of hepatic metastases of colorectal cancer. Hematol Oncol Clin North Am. 2015;29:61-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Fischer C, Melstrom LG, Arnaoutakis D, Jarnagin W, Brown K, D’Angelica M, Covey A, DeMatteo R, Allen P, Kingham TP. Chemotherapy after portal vein embolization to protect against tumor growth during liver hypertrophy before hepatectomy. JAMA Surg. 2013;148:1103-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery. 1990;108:572-580. [PubMed] [Cited in This Article: ] |
| 8. | Treska V, Skalicky T, Sutnar A, Vaclav L, Fichtl J, Kinkorova J, Vachtova M, Narsanska A. Prognostic importance of some clinical and therapeutic factors for the effect of portal vein embolization in patients with primarily inoperable colorectal liver metastases. Arch Med Sci. 2013;9:47-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, Lemoine A, Bismuth H. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 363] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] [Cited in This Article: ] |
| 11. | Abdalla EK, Bauer TW, Chun YS, D’Angelica M, Kooby DA, Jarnagin WR. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements. HPB (Oxford). 2013;15:119-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 341] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Simoneau E, Aljiffry M, Salman A, Abualhassan N, Cabrera T, Valenti D, El Baage A, Jamal M, Kavan P, Al-Abbad S. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastases. HPB (Oxford). 2012;14:461-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Elias D, De Baere T, Roche A, Mducreux J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999;86:784-788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, Ohta K, Yamaguchi T, Matsubara T, Takahashi T. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 16. | Barbaro B, Di Stasi C, Nuzzo G, Vellone M, Giuliante F, Marano P. Preoperative right portal vein embolization in patients with metastatic liver disease. Metastatic liver volumes after RPVE. Acta Radiol. 2003;44:98-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 366] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Heinrich S, Jochum W, Graf R, Clavien PA. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. J Hepatol. 2006;45:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Mueller L, Hillert C, Möller L, Krupski-Berdien G, Rogiers X, Broering DC. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol. 2008;15:1908-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, van Delden OM, Laméris JS, Gouma DJ. Controversies in the use of portal vein embolization. Dig Surg. 2008;25:436-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Pamecha V, Glantzounis G, Davies N, Fusai G, Sharma D, Davidson B. Long-term survival and disease recurrence following portal vein embolisation prior to major hepatectomy for colorectal metastases. Ann Surg Oncol. 2009;16:1202-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Hoekstra LT, van Lienden KP, Doets A, Busch OR, Gouma DJ, van Gulik TM. Tumor progression after preoperative portal vein embolization. Ann Surg. 2012;256:812-817; discussion 812-817;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Pamecha V, Levene A, Grillo F, Woodward N, Dhillon A, Davidson BR. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100:617-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Lindnér P, Cahlin C, Friman S, Hafstrom L, Klingenstierna H, Lonn L, Olausson M, Rizell M. Extended right-sided liver resection for colorectal liver metastases--impact of percutaneous portal venous embolisation. Eur J Surg Oncol. 2006;32:292-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | de Graaf W, van den Esschert JW, van Lienden KP, van Gulik TM. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Hoekstra LT, van Lienden KP, Verheij J, van der Loos CM, Heger M, van Gulik TM. Enhanced tumor growth after portal vein embolization in a rabbit tumor model. J Surg Res. 2013;180:89-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Maggiori L, Bretagnol F, Sibert A, Paradis V, Vilgrain V, Panis Y. Selective portal vein ligation and embolization induce different tumoral responses in the rat liver. Surgery. 2011;149:496-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Bretagnol F, Maggiori L, Zappa M, Sibert A, Vilgrain V, Panis Y. Selective portal vein embolization and colorectal liver metastases in rat: a new experimental model for tumor growth study. J Surg Res. 2011;171:669-674. [PubMed] [Cited in This Article: ] |
| 29. | Zou RH, Li AH, Han F, Hong J, Li BK, Huang W, Huang L, Yuan YF. Liver hypertrophy and accelerated growth of implanted tumors in nonembolized liver of rabbit after left portal vein embolization. J Surg Res. 2012;178:255-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Böhm F, Köhler UA, Speicher T, Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Mol Med. 2010;2:294-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 31. | Christophi C, Harun N, Fifis T. Liver regeneration and tumor stimulation--a review of cytokine and angiogenic factors. J Gastrointest Surg. 2008;12:966-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 32. | Matela J, Zabavnik Z, Jukić T, Jukić D, Glavina K, Tusek-Bunc K, Pavan-Jukić D. Selective portal vein embolization as introduction in major surgery. Coll Antropol. 2005;29:163-167. [PubMed] [Cited in This Article: ] |
| 33. | Sutcliffe RP, Bhattacharya S. Colorectal liver metastases. Br Med Bull. 2011;99:107-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287-4299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 35. | Covey AM, Brown KT, Jarnagin WR, Brody LA, Schwartz L, Tuorto S, Sofocleous CT, D’Angelica M, Getrajdman GI, DeMatteo R. Combined portal vein embolization and neoadjuvant chemotherapy as a treatment strategy for resectable hepatic colorectal metastases. Ann Surg. 2008;247:451-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |