CLINICAL ASPECTS
Progress in the diagnosis and treatment of primary breast carcinoma (BrCa) has not led to an overall improvement in survival. The main reason is that residual disease always generates distant metastases, eventually leading to a substantial reduction in life expectancy. As revealed by autopsy studies, undiagnosed metastases in BrCa patients are more frequent than expected, and without an effective strategy aimed at reducing the spread of cancer cells, clinically relevant metastases are estimated to remain the main cause of death associated with BrCa. Although the presence of metastasis at diagnosis is uncommon in BrCa, the risk for this complication increases considerably within a few years after first treatment. Metastases involving multiple organs, mainly the lung, liver, and bone, are the most frequent situation, while the incidence of isolated metastasis is thought to be lower than 5%[1].
Large autopsy series have demonstrated that bone metastases are present in 54%-73% of BrCa patients who died of the cancer[2]. In addition, the high incidence of latent bone metastases at autopsy and the frequent detection of disseminated tumor cells in the bone marrow by sensitive methods suggest that bone colonization by BrCa cells is commonly associated with an indolent disease course and clinical dormancy[3]. For this reason, study of the molecular determinants associated with predominance of dormancy over aggressive metastatic growth will have an important impact on clinical management of BrCa patients.
Although BrCa is the most common primary tumor type associated with metastatic bone disease, bone-only metastasis is infrequent and about 2% of these patients are diagnosed at the time of initial treatment[4,5]. Also, according to autopsy records the chance of having bone-restricted metastasis is very low. Patients with disease confined to bone are more likely to be older at diagnosis and to have well differentiated tumors. Bone metastases are frequently associated with lung and/or liver metastases, and the cases with bone-only metastases are about 5%[1]. In a distinctive way, patients with bone metastasis are more likely to have metastases in the central nervous system (CNS, about 27%) with respect to patients without bone metastasis (about 10%)[6], and 18% of patients with CNS disease have bone as the initial site[7]. In addition, lung and liver metastases are frequently found in more widespread disease with respect to bone metastasis[6].
The predilection of BrCa cells for bone is confirmed by the fact that in more than 50% of BrCa patients, bone is the first site of distant relapse[5,8]. The median period from BrCa diagnosis to the development of first metastatic bone lesion is about 3 years. However, 49% of these relapsing patients tend to develop extraosseous metastases, and 56% of patients with solitary bone metastasis tend to develop multiple metastatic bone lesions.
Also, for BrCa patients with bone-only disease the presence of multiple bone metastases at first diagnosis is common (59%), and the more frequent (> 50%) anatomical sites are thoracic and lumbar spine, the sternum, and pelvis. The sternum was described as the site of solitary metastasis in a significant percentage of BrCa patients at the time of diagnosis and this could be explained by anatomical proximity with the primary tumor[5,9].
The median survival after the first recurrence of BrCa in bone is markedly higher with respect to the survival of those with first recurrence of cancer in extraosseous sites (20 mo vs 3 mo)[8]. Although bone-only metastasis demonstrates a relative good prognosis, skeletal involvement is frequently associated with considerable morbidity. This includes hypercalcemia, fractures, impaired mobility, and spinal cord compression and pain, which require higher and higher doses of analgesics.
According to the histological and clinical features, bone metastases can be classified as osteolytic, osteosclerotic, or mixed, the latter when both features coexist in the same metastatic district[10]. BrCa bone metastases are quite exclusively osteolytic, characterized by bone destruction due to an exacerbated activity of osteoclasts, the bone cells physiologically devoted to resorb bone matrix. Indeed, the first theories speculated that the osteolytic lesion was the result of physical pressure of the tumor on the bone or bone resorption activity of the tumor cells themselves. It has also been highlighted that cancer cells induce lymphocytes to produce factors such as prostaglandins, which in turn could stimulate destruction of the bone[11]. To date, more and more evidence has conclusively shown that cancer cells are not able to directly destroy the bone, but they release factors that directly or indirectly activate the formation and activity of osteoclasts[10].
Due to the prevalent osteolytic nature of BrCa bone metastasis, skeletal progression of the disease can be monitored by the measurement of specific biochemical markers derived from the breakdown of type I collagen[12]. These peptides include crosslinked C-terminal telopeptide isomers (CTX) and crosslinked N-terminal telopeptide (NTX), which can be measured in serum and urine.
MOLECULAR BASES
The high osteotropism of BrCa cells has been widely demonstrated in preclinical studies. The predilection of tumor cells for bone tissue could not be explained simply by anatomical features, and the so-called “seed and soil” theory, postulated by Steven Paget more than 100 years ago, is still valid today[13]. This theory emphasizes that the process of colonization requires an interaction between tumor cells, which represent the seed, and the bone microenvironment, a deposit of calcium and growth factors released in response to bone resorption that provides the fertile “soil” in which cancer cells can proliferate.
These considerations have supported an intense investigation of the molecular determinants of osteotropism. However, the frequent widespread presentation of metastases in BrCa patients indicates that the pure osteotropic signature is restricted to a limited number of bone-only cases. Today, histologic analysis of BrCa subtypes drives prognosis, and in many cases permit prediction of the metastatic propensity of the primary tumor[14]. Although much effort has been made to find a link between molecular profiles and metastatic site, this has not been fully established. In addition, molecular subtyping of the primary tumor is often not repeated in the metastases and the assumption that the metastatic tumor has identical marker expression as the primary tumor is currently debated[15]. Moreover, it has to be considered that the bone microenvironment, with its peculiar characteristics, may exert strong selective pressure on tumor cells, thus influencing the resulting phenotype of clinically relevant metastases. For example, parathyroid hormone-related protein (PTHrP), which was previously considered an effective predictor for identifying patients who are at high risk of developing bone metastases and which is expressed in the large majority of BrCa bone metastases and in 60% of primary BrCa, is thought to be stimulated in BrCa cells in response to transforming growth factor-β (TGF-β) released in the bone[16].
Estrogen receptor (ER) expression represents the best consolidated marker associated with the risk for bone metastasis[17]. ER+ breast tumors relapse preferentially to the bones over a delayed period[18]. However, because there is significant loss of ER expression at many metastatic sites including bone, the role of ER in driving pathogenesis of bone metastasis needs to be verified[6].
PHYSIOLOGY OF BONE REMODELING
Understanding the molecular mechanisms leading to cancer-induced bone metastases requires knowledge of the physiology of bone tissue which, in contrast to its “hard” feature, is extremely dynamic and undergoes continuous renewal throughout the life of each individual, through a process known as bone remodeling[28]. In particular, it has been estimated that about 10% of the bone is renewed every year[29]. This phenomenon guarantees the following crucial functions: (1) Regulation of calcium homeostasis; (2) Renewal of old bone; (3) Substitution of primary infantile bone with mechanically competent bone; and (4) Repair of ischemic and microfractured bone.
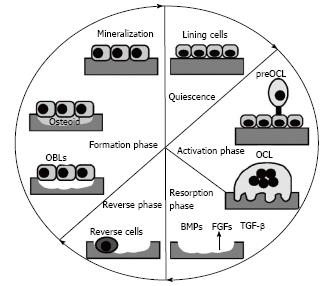
Bone remodeling is the result of a perfect balance between the function of bone resorption performed by osteoclasts and osteogenesis accomplished by osteoblasts. Both cell types are the principal cells of bone tissue. This balance is crucial for maintenance of a proper bone mass and the lack of synchrony between the two functions is the starting point for skeletal diseases. As described in Figure 1, bone remodeling takes place according to the following phases:
Figure 1 Phases of bone remodeling.
Starting from the quiescence phase, bone remodeling is triggered by different stimuli that lead to activation of lining cells, which increase surface expression of receptor activator of nuclear factor kappa-B ligand (RANKL). This cytokine, by binding to its receptor RANK, promotes osteoclast differentiation (activation phase). Next, mature osteoclasts resorb bone (resorption phase), thus allowing the release of factors usually stored in the bone matrix (BMPs, TGF-β and FGFs) that recruit osteoblasts in the reabsorbed area. Once recruited, osteoblasts form the bone matrix and ensure its mineralization (formation phase), completing the bone remodeling process. BMPs: Bone morphogenetic proteins; TGF-β: Transforming growth factor-β; FGFs: Fibroblast growth factor; OCL: osteoclast; OBL: osteoblast.
Activation phase
This phase is so called because the lining cells, which are quiescent osteoblasts, respond to different stimuli (i.e., growth factors, alteration of mechanical loading, and micro fractures) by increasing the expression of factors that stimulate osteoclast differentiation.
Resorption phase
Mature osteoclasts polarize on the bone surface, adhere to it and, by a process of acidification and subsequent release of proteolytic enzymes, such as the cathepsin K and the metalloproteinase (MMP)-9, degrade the bone matrix[30]. Once they have accomplished their function, osteoclasts undergo apoptosis, a physiologic consequence necessary to avoid exacerbated bone resorption.
Reverse phase
This phase is so called because of the presence of reverse cells, macrophage-like cells that are likely responsible for removing the debris produced during bone matrix degradation.
Formation phase
The players of this phase are osteoblasts, recruited by the growth factors that are usually stored in the bone matrix but that are released after its degradation by osteoclasts. Once recruited, osteoblasts produce a new bone matrix, initially not calcified (osteoid), and then they provide mineralization, thus completing the bone remodeling process.
PLAYERS IN BONE REMODELING
The principal cells of the bone involved in bone remodeling, osteoblasts and osteoclasts, also play a key role in the development of bone metastases.
Osteoclast formation and function
Osteoclasts arise from the monocyte/macrophage lineage[31] and are multinucleated cells formed by the fusion of mononuclear precursors[30]. Starting from a pluripotent hematopoietic stem cell, the transcription factor PU.1, along with macrophage colony stimulating factor (M-CSF), drives the commitment of a common progenitor for macrophages and osteoclasts. In particular, M-CSF stimulates proliferation of osteoclast precursors and upregulates receptor activator of nuclear factor kappa-B (RANK) expression, while PU.1 positively regulates the expression of c-Fms, the M-CSF receptor[32]. With the appearance of c-Fms and RANK receptors, the precursors become fully committed to an osteoclast lineage. The RANK/receptor activator of nuclear factor kappa-B ligand (RANKL) pathway is mandatory for osteoclast differentiation and function, although it is not the only player in correct osteoclastogenesis.
Once differentiated, multinucleated osteoclasts need to adhere to the bone matrix and polarize in order to resorb bone. The first step of resorption requires the dissolution of the inorganic component of the matrix, which is hydroxyapatite. This can be achieved by the release of hydrochloric acid into the area to be resorbed, called resorption lacuna[33], and also requires sealing of the underlined bone matrix, which is achieved through a cytoskeletal rearrangement and subsequent formation of an actin ring. This is a circumferential structure formed by several dynamic and dot-like structures called podosomes, each of which consists of an actin core surrounded by the αvβ3 integrin and associated cytoskeletal proteins[34].
Dissolution of mineral crystals allows digestion of the bone matrix organic component, which is performed by MMPs and lysosomial cathepsins. Among the latter, cathepsin K has a crucial role, as its deletion in mice leads to several skeletal diseases[35]. Regarding the MMPs, osteoclasts mainly produce the MMP-9 isoform and, to a lesser extent, MMP-14[36].
Osteoblasts
They arise from a mesenchymal stem cell (MSC) lineage consisting of pluripotent cells following a specific program of gene expression that may give rise to different tissue-specific cells including osteoblasts, chondrocytes, fibroblasts, myocytes, and adipocytes[37]. The initial step of osteoblastogenesis is the commitment of MSCs towards an osteo/chondro-progenitor, which relies on the activation of two principal pathways: the Wingless-int (Wnt) pathway and the pathway triggered by bone morphogenetic proteins (BMPs). One of the earliest factors mandatory for osteoblast differentiation is Runt-related transcription factor 2 (RUNX2), along with osterix (OSX), which is downstream of RUNX2[38]. Committed pre-osteoblasts are identifiable because they express alkaline phosphatase (ALP), one of the earliest markers of the osteoblast phenotype. As the pre-osteoblasts cease to proliferate, a key signaling event occurs for development of the large cuboidal differentiated osteoblasts. The active osteoblast is highly enriched in ALP and secretes bone matrix proteins such as collagen I and several non-collagenous proteins including osteocalcin, osteopontin, osteonectin, and bone sialoprotein II.
BONE REMODELING PERTURBATION AND THE ONSET OF THE VICIOUS CYCLE
The physiology of bone remodeling is drastically disrupted by tumor cells once they colonize the bone milieu, eventually leading to the so-called vicious cycle (Figure 2). Indeed, tumor cells produce factors that, directly or indirectly through osteoblasts, induce exacerbated osteoclastogenesis, which in turn increases bone resorption and osteolysis. This means that tumor cells are not able to destroy bone per se, but they constrain resident cells to support their growth by creating new spaces inside the bone and allowing the release of several growth factors stored herein, such TGF-β, VEGF, insulin-like growth factors, platelet-derived growth factor, and BMPs. Thus, synergy between osteoclasts and tumor cells is created that fuels the vicious cycle, with an inexorable increase in both bone destruction and tumor growth[10].
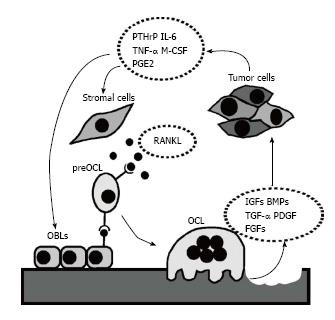
Figure 2 Schematic representation of the vicious cycle.
Under physiologic conditions, osteoblasts produce factors that regulate osteoclastogenesis (RANKL/OPG). Mature osteoclasts erode the bone matrix, allowing the release of factors (IGF-1, BMPs, TGF-β, PDGF, and FGFs). Tumor cells perturb this homeostasis by producing factors (PTHrP, IL-6, TNF-α, M-CSF, and PGE2) that favor osteoclastogenesis, with subsequent bone resorption and release of the growth factors stored in the bone matrix, which in turn enhance tumor growth. BMPs: Bone morphogenetic proteins; TGF-β: Transforming growth factor-β; FGFs: Fibroblast growth factor; IGFs: Insulin-like growth factors; PDGF: Platelet-derived growth factor; M-CSF: Macrophage colony stimulating factor; PGE2: Prostaglandin E2; OCL: osteoclast; OBL: osteoblast.
The ability of tumor cells to release in the bone microenvironment osteoclastogenic factors, usually produced by osteoblasts, further feeds the vicious cycle. Among the osteoclastogenic factors is PTHrP, produced by tumor cells under the stimulation of TGF-β, which in turn elicits RANKL expression and inhibits OPG production by bone marrow stromal cells and osteoblasts. Evidence for a role of PTHrP in this context arose some years ago from studies by Theresa Guise showing, in a mouse model of bone metastasis, that treatment with PTHrP-blocking antibody reduced BrCa cell-induced osteolysis as well as cancer growth. Moreover, tumor cells also produce M-CSF and prostaglandin E2, as well as several pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-11, and tumor necrosis factor α (TNFα), which directly stimulate osteoclast formation and function[41]. Finally, evidence from the vicious cycle tells us that, in order to fight it, we could act on two fronts: interfering with the release of specific factors by tumor cells or inhibiting aberrant osteoclastogenesis, and these two phenomena are strictly related.
THERAPEUTIC TARGETS
RANK/RANKL pathway
Given the crucial role of this pathway in osteoclast differentiation, it is not surprising that it has been considered one of the most promising therapeutic targets, leading to the development of denosumab, a human monoclonal antibody directed against membrane-bound and soluble RANKL. Denosumab prevents the binding of RANKL to its receptor, eventually leading to the inhibition of osteoclastogenesis. This drug is currently in clinical trials for the treatment of post-menopausal osteoporosis and bone metastases. In particular, denosumab administered subcutaneously every 4 wk at a dose of 120 mg showed a higher efficiency compared with zoledronic acid in delaying the onset of skeletal-related events in patients with BrCa and in reducing the levels of the bone resorption marker NTX[42]. However, there was no difference between the two drugs in terms of the effect on patient survival. With regard to the potential side effects, we should not forget the role played by RANKL in the immunologic contest. A few cases of osteonecrosis of the jaw[43] as well as frequent occurrence of hypocalcaemia[44] have been reported.
Cathepsin K
Cathepsins are a family of cysteine proteases with 11 members in humans (cathepsin B, C, H, F, K, L, O, S, V, W, and X/Z) with different functions, such as antigen presentation, apoptosis, and autophagy. In the pathological context, cathepsin has been positively correlated with tumor invasion and angiogenesis[45]. Indeed, B and L cathepsins are prognostic markers of different cancers, among them breast, where their increased expression is associated with a poor prognosis[46].
Among the different cathepsins, the K isoform has been identified as a good therapeutic target in bone metastasis treatment, due to its pivotal role in bone resorption. Recently, inhibition of cathepsin B was also proven to be effective at inhibiting bone and lung metastases in a mouse model of BrCa[47]. The crucial role of cathepsin K in osteoclast functions has been clarified by the evidence that a human mutant form of this gene determines a rare genetic disease called pycnodysostosis, characterized by an impairment of bone resorption. Preclinical data from animal models of BrCa bone metastases showed the ability of the cathespin K inhibitor AFG-495 to reduce osteolytic lesions as well as local tumor growth[48]. Another recent compound is odanacatib (MK-0822), a promising drug already used for osteoporosis treatment. A phase II clinical trial involving BrCa patients with bone metastases showed a reduction of bone resorption markers after 4 wk of treatment[49].
Metalloproteases
Due to their function of degrading extracellular matrix, MMPs are obviously involved in the general process of invasion and metastasis. Indeed, some of them play a specific role in the onset of bone metastases, such as MMP-7, which is able to cut membrane-bound RANKL, thus increasing its local activity and favoring bone metastasis development[50]. MMP-1, along with a disintegrin-like and metalloproteinase with thrombospondin motifs 1 (ADAMTS1), promotes proteolytic cleavage of epidermal growth factors (EGFs), which in turn inhibit osteoblast production of OPG, thus favoring osteoclastogenesis[51]. Nannuru et al[52] showed that treatment of mice with Cl66 mammary tumors with MMP13 antisense oligonucleotides led to a significant reduction in bone destruction and in the number of activated osteoclasts at the tumor-bone interface, likely by reducing MMP-9 and RANKL expression.
Vascular cell adhesion proteins
This family is also mainly involved in carcinogenesis and metastasis. Among them, vascular cell adhesion proteins (VCAMs) seem to give a specific contribution to the development of bone metastases, as demonstrated by a recent study. Indeed, by interacting with its integrin receptor α4β1, VCAM-1 promotes the recruitment of osteoclast precursors. Moreover, treatment of metastatic mice with VCAM-1-blocking antibody significantly reduced bone and lung metastases[53].
Mammalian target of rapamycin
Rapamycin is an immunosuppressive and antitumoral drug previously used to prevent graft rejection. Rapamycin inhibits mammalian target of rapamycin (mTOR), which is a serine-threonine kinase that stimulates cell survival and proliferation and whose deregulation is associated with the development of several tumors. What makes rapamycin an interesting target for bone metastases treatment is the evidence that the signal triggered by mTOR is important for the survival of osteoclasts[54]. Moreover, a recent study showed that treatment with rapamycin reduced both the incidence and the area of the osteolytic lesion, through the inhibition of osteoclast formation[55].
Integrins
Integrins include a large family of surface receptors that mediate cell-extracellular matrix interactions. Osteoclasts mainly express αvβ3 integrin, which plays a crucial role in osteoclast adhesion, a process that is mandatory for correct bone resorption[56]. Preclinical evidence shows that αvβ3 inhibition blocks osteolysis and tumor growth in animal models of bone metastases. These studies were the starting point for ongoing clinical trials that are testing the effectiveness of various αvβ3 antagonists in different bone metastatic cancers[57].
Erythroblastic leukemia viral oncogene homolog (ErbB) receptors
The ErbB family of receptor tyrosine kinases represents an attractive therapeutic target in carcinomas. In fact, ErbB kinases are frequently overexpressed in these cancers and regulate important aspects of cancer progression by activating several key intracellular signaling intermediates, including PhosphoInositide 3 Kinase (PI3K), Ras-Raf Mitogen Activated Protein Kinase (MAPK), c-Jun N-terminal Kinase (JNK) and PhosphoLipase C (PLC)[58]. The ErbB family comprises four members: ErbB1 [alias epidermal growth factor receptor (EGFR) and HER1]; ErbB2 (alias HER2 and Neu); ErbB3 (alias HER3); and ErbB4 (alias HER4). For EGFR and HER2, sufficient data have been collected to support their use as two of the five protein surrogate markers for assessing BrCa subtypes [ER, progesterone receptor (PgR), HER2, EGFR, and cytokeratin-5 (CK5)][59]. The role of the other ErbB kinases in BrCa progression remains controversial. In addition, only HER2 is found to be amplified in about 25% of primary BrCa cases. Although the data indicate that EGFR is not overexpressed in primary BrCa with respect to the normal breast epithelia[60], recent studies have suggested a significant association of EGFR expression with the aggressive basal-type BrCa[61] and with circulating BrCa cells[62,63].
Current evidence does not support the hypothesis that EGFR or HER2 expression can predict bone metastasis[17]. In contrast, ErbB-expressing BrCa shows the predilection to metastasize to visceral sites, including the brain, liver, and lung[64]. Nevertheless, numerous preclinical studies have proposed a leading role for ErbB kinases in the progression of bone metastases[65]. In particular, ErbB receptors may participate in the positive feedback underlying the vicious cycle. In fact, EGFR ligands present in the bone microenvironment are able to directly stimulate bone cells and osteolysis. Among the recognised ligands of the ErbB family, EGF, TGF-β, and amphiregulin (AREG) have been proposed as the main players in the bone microenvironment. EGF and TGF-βstimulation of osteoclastogenesis and bone resorption is accompanied by decreased OPG expression and sustained production of RANKL by bone stromal cells[56,66]. In addition, EGFR is expressed by osteoblasts, and its activation stimulates osteoblast proliferation and decreases mineralization[67]. EGFR ligands may also function by an autocrine loop, stimulating the release of cytokines by BrCa cells that directly influence osteoclastogenesis or indirectly stimulating EGFR signaling within bone cells. The key cytokine produced by BrCa cells in this way is PTHrP, which is able to stimulate RANKL expression and inhibit OPG expression in cells of the osteoblast lineage[68]. In addition, PTHrP induces the expression and shedding of AREG and TGF-β, increasing the availability of ErbB ligands in the bone microenvironment. In turn, osteoblasts release EGFR ligands and perpetuate the cycle of osteoclast activation via RANKL and thus bone destruction[65].
Src
In the last few years, Src, a nonreceptor tyrosine kinase, has attracted increasing interest due to its involvement in both tumorigenesis and bone metabolism. Src activity appears to be significantly associated with the development of bone metastases and late-onset relapse to bone. Using a bioinformatics approach, it was demonstrated that the role of Src was independent of the distinct molecular subtypes of human BrCa and also ER status[69].
In BrCa cells, Src is frequently overexpressed and overactivated, allowing the transduction of signaling pathways associated with proliferation, adhesion, invasion, and angiogenesis[70]. In particular, Src is a key mediator for several cell surface receptors, including EGFR and HER2[71]. Src-overexpressing tumors may have a specific survival advantage in the bone microenvironment. In fact, activated Src is required for Chemokine (C-X-C) motif Ligand 12 (CXCL12)/Stromal cell-Derived Factor (SDF)-mediated cell survival and abrogates TNF-Related Apoptosis-Inducing Ligand (TRAIL)-mediated apoptosis[69]. Preclinical studies confirmed that treatment with Src inhibitors effectively reduced BrCa growth compared with untreated cells[72]. In several mouse models of BrCa, inhibition of Src activity decreased metastases and improved survival[73,74]. Other in vivo studies have found inhibition of bone metastasis but not of metastases at visceral sites, confirming the specific role for Src in bone turnover[69]. Moreover, Src activity has been linked to resistance to anti-hormonal therapy[75]. That BrCa bone metastases are more frequent in ER+ than ER- tumors suggests the possibility that Src inhibition could overcome resistance to endocrine therapy and block metastatic growth. The results from a clinical trial recruiting ER+ and HER2+ BrCa seemed to confirm a better potential activity of an inhibitor of Src in ER+ tumors with respect to HER2+ cases[76].
At the same time, Src is a key signaling molecule in the physiology of bone, since osteoclast function appears to be dependent on the activation of Src[77]. Indeed, src-deficient mice develop osteopetrosis, with a significant reduction of bone resorption, although the number of osteoclasts was equal to that of wild-type (WT) littermates, thus indicating that Src is mandatory for osteoclast activity[78]. Significantly, when Src inhibitors were used in in vivo models of BrCa bone metastasis, it was reported that bone resorption as well as BrCa growth at the metastatic site were significantly inhibited[74]. There are currently several Src inhibitors in preclinical development or in clinical trials for the treatment of solid tumors[79]. One of these compounds, dasatinib, a dual Src/Abl inhibitor, approved for the treatment of leukemia, was successfully investigated in phase I/II clinical trials in patients with prostate and BrCa bone metastases. Dasatinib treatment was associated with a substantial decrease in bone resorption markers and with a lack of disease progression in a significant percentage of breast and prostate cancer patients[80,81]. However, although recent data of the Randomized Study Comparing Docetaxel Plus Dasatinib to Docetaxel Plus Placebo in Castration Resistant Prostate Cancer (READY) trial confirmed the reduction in bone resorption, they showed no overall survival improvement, suggesting the need for appropriate predictive biomarkers[82].
Chemokines
Chemokines constitute a family of small secreted cytokines. Their name comes from their ability to induce chemotaxis in adjacent responsive cells. Chemokines are generally divided into four classes according to the number and position of cysteine residues: CC, in which the residues are adjacent; CXC, in which the residues are separated by one amino acid; the family XC, which has only one cysteine residue; and CX3C, in which the cysteine residues are separated by three amino acids[83]. Some chemokines are pro-inflammatory, being able to induce an immune response consisting of attraction of immune cells at the site of infection, while others are involved in homeostasis, controlling the process of cell migration. These proteins exert their effect by interacting with specific transmembrane receptors called chemokine receptors, present on many different cell types[84]. The chemokine receptors belong to the family of G protein-coupled receptors (GPCRs), which have an extracellular region that binds to chemokines, seven transmembrane α-helices, and a cytoplasmic side associated with a G protein. Several studies highlighted the importance of CXCL12 (SDF-1) and CXCR4 in BrCa. The first studies on the role of CXCR4 in BrCa date back to 2001[85], and its specific involvement in the migration of BrCa cells from the primary site through the basement membrane was reported by Yagi et al[86] in 2011. Mutation at the COOH-terminal domain of CXCR4 also plays a role in receptor regulation during the process of epithelial-to mesenchymal transition[87]. It has also been shown that CXCR4 levels are high in bone metastasis, suggesting that the CXCL12/CXCR4 axis plays an important role in its pathogenesis. CXCL12 is a homeostatic chemokine constitutively expressed also in those organs that are the most common metastatic sites of BrCa, including bone marrow, but its secretion by damaged tissues is particularly abundant. CXCR4 expression is low or absent in normal breast tissue, while it is upregulated in neoplastic tissue; moreover, CXCR4 levels are related to the degree of tumor malignancy. However, additional studies are needed to confirm that the increase of CXCR4 expression can be a predictive marker for metastatic diffusion. Shim et al[88] showed that, in cultured cells, the binding of CXCL12 to CXCR4 induced CXCR4 translocation from the cytoplasm to the nucleus. After its translocation to the nucleus, CXCR4 works as a transcription factor[89], leading to upregulation of cytokines such as MCP-1 and IL-8 and metalloproteases such as MMP-2 and MMP-9. The importance of CXCR4 in BrCa progression was confirmed by the use of anti-CXCR4 antibody or specific siRNA, in vitro and in vivo, showing its ability to block the formation and the dissemination of metastasis.
Given the involvement of chemokines and chemokine receptors in tumor progression, many molecules have been developed to counteract their biological activity. These molecules belong to two categories: peptides and small molecules. Bicyclams are low-molecular-weight agents that have been shown to act as potent and selective CXCR4 antagonists. Bicyclam AMD 3100 (Plerixafor) is currently being investigated in clinical trials alone or in combination with bisphosphonates[83]. The 14-residue polypeptide 4F-benzoyl-TN14003 (BKT140) is a highly selective and unique CXCR4 antagonist[90]. Besides its ability to inhibit BrCa metastases by impairing migration, it could also be used as a diagnostic tool to identify CXCR4 receptor-positive tumor cells in culture as well as in paraffin-embedded clinical tumor samples[91]. Finally, in vivo studies have demonstrated that the synthetic peptide antagonist CTCE-9908 is able to reduce the incidence and extent of bone metastases. Unfortunately, results from the first clinical trials conducted in patients with advanced metastatic disease showed good tolerance but low response[92].
Transcription factors
The osteolytic phenotype is associated with the activity of a specific pattern of transcription factors, including glioma-associated oncogene family zinc finger 2 (GLI2), RUNX2, and hypoxia-inducible factor-1 (HIF-1). In particular, GLI2 stimulates PTHrP expression by tumor cells and is mainly involved in the development of melanoma-induced bone metastases[93,94]. With regard to RUNX2, it has been demonstrated that inactivating mutations of its gene in BrCa cells significantly inhibit bone metastasis development in animal models[95]. Finally, HIF-1, besides its pivotal role in hypoxia-associated tumor progression, also stimulates tumor-driven osteolysis (Figure 3). Transcriptional activity of HIF-1 is induced by the reduction in oxygen (O2) availability. HIF-1 is a heterodimeric protein composed of an O2-regulated HIF-1α subunit and a constitutively expressed HIF-1β subunit[96]. HIF proteins, when stabilized, trigger the transcription of numerous target genes involved in tumor growth and in promoting the feed-forward of the metastatic cycle[97]. Hypoxia is a frequent consequence of the growth of solid tumors. It is well known that many hypoxia-response genes regulated by HIF-1α are genes involved in controlling energy metabolism. These include VEGF, which permits increased O2 delivery to cells by stimulating angiogenesis, and glycolytic enzymes, which allow cells to survive O2 deprivation[98]. Paradoxically, therapeutic inhibition of tumor-induced angiogenesis could stimulate autocrine growth factor secretion downstream of HIF-1, thus producing a more aggressive phenotype in BrCa cell lines[99].
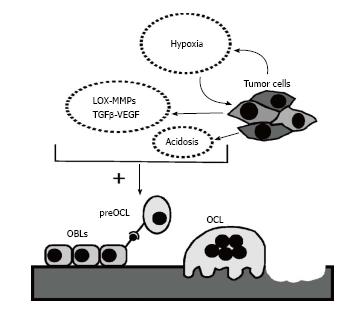
Figure 3 Potential pro-metastatic role of hypoxia in bone.
Hypoxia stimulates targeting of cancer cells to bone and facilitates their survival within the bone microenvironment. The presence of a hypoxic environment, further sustained by cancer growth, induces the secretion of hypoxia-inducible factors by cancer cells. These factors, in conjunction with parallel acidification of the microenvironment, are associated with osteoclastogenesis. TGF-β: Transforming growth factor-β.
Concerning the role of hypoxia in pre-metastatic niche formation, clinical studies have shown that expression levels of HIF-1α in BrCa patients increase proportionally with the severity of the pathologic stage[100]. As reported by Le et al[101] in 13 different types of human cancers, HIF-1α was overexpressed in two-thirds of the all regional lymph nodes and bone metastasis examined. Furthermore, increased levels of HIF-1α were associated with a poor prognosis[102,103]. Several data have indicated that factors stimulated by HIF-1α are associated with bone metastasis through their capacity to modify extracellular bone matrix and stimulate metastatic homing of cancer cells[85,104,105].
Some of the most well-known target genes of hypoxia, which are able to promote metastatic progression, include lysyl oxidase (LOX), LOX-like (LOXL) family proteins such as LOXL2 and LOXL4, TGF-β, MMP2, MMP9, CXCR4, SDF-1, and VEGF[106]. It was demonstrated that HIF-1, in hypoxic BrCa cells, could promote BrCa metastasis by inducing the expression of LOX proteins. This phenomenon is probably due to increased VEGF secretion by endothelial cells (ECs) and the modification of collagen molecules in the extracellular matrix (ECM)[107]. While the role of LOX in metastases was initially attributed to its capacity to remodel the ECM in the proximity of the primary tumor, subsequent studies revealed that LOX could remodel the ECM at distant sites and recruit bone marrow-derived cells to the metastatic niche[108].
In the bone microenvironment, overactivated HIF-1α can increase the development of osteolytic bone metastases via dysregulation of factors involved in the vicious cycle. Several findings have suggested that acidosis, which is caused by glycolytic metabolism of hypoxic cancer cells, has a negative effect on osteoblast differentiation as well as on osteoblast functions[109,110]. In contrast to the effect on osteoblast differentiation, hypoxia seems to stimulate osteoclast-like cell formation. Frick et al[111] demonstrated higher RANKL mRNA expression in bone cells caused by metabolic acidosis with respect to control. In addition, osteoblasts express components of the HIF-1 pathway, and hypoxia can upregulate the expression of VEGF-A, the major inducer of tumor angiogenesis[112].
Although HIF-1 is an attractive therapeutic target and several different strategies have been developed to directly target HIF-1, none of these inhibitors have been translated to the clinical setting. However, different chemical compounds and chemotherapeutic drugs that indirectly target HIF-1α, such as EGFR inhibitors, digoxin and other cardiac glycosides, antracyclines, geldanamycin and other heat shock protein 90 (HSP90) inhibitors and, recently, topotecan and topoisomerase I inhibitors, have been shown to counteract primary cancer progression, angiogenesis, and metastasis in mouse models[113-117]. Among these inhibitors, promising results were obtained with the HIF-1α inhibitor 2-methoxyestradiol (2ME2), which was able to decrease osteolytic lesion area and tumor burden in an in vivo model of bone metastasis[118,119].