Published online Aug 10, 2014. doi: 10.5306/wjco.v5.i3.194
Revised: April 28, 2014
Accepted: May 29, 2014
Published online: August 10, 2014
Ductal carcinoma in situ (DCIS) is a non-obligate precursor of invasive breast cancer with a variable biological behavior which is difficult to accurately predict using the current clinico-pathological parameters. Randomized controlled trials have demonstrated that adjuvant radiotherapy (RT) reduces the risk of local recurrence after adequate local excision of DCIS. Tamoxifen may be considered as an adjuvant endocrine treatment in patients with high risk estrogen receptor positive disease. There is however a growing consensus that RT can be safely omitted in a subgroup of patients with favorable biological features in order to avoid overtreatment. The sentinel node biopsy is not routinely indicated but should be considered in women undergoing mastectomy for DCIS. The discovery of molecular signatures that accurately predict the biological behavior of this common malignancy will facilitate a personalized treatment approach in the future.
Core tip: Localized ductal carcinoma in situ (DCIS) is treated with adequate local excision followed by radiotherapy (RT) in most cases whereas extensive disease is treated with mastectomy (± immediate reconstruction). RT may be safely omitted in some patients with adequately excised low risk DCIS.
- Citation: Choy C, Mokbel K. Towards optimal treatment of ductal carcinoma in situ. World J Clin Oncol 2014; 5(3): 194-196
- URL: https://www.wjgnet.com/2218-4333/full/v5/i3/194.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i3.194
Ductal carcinoma in situ (DCIS) represents an intra-ductal epithelial proliferation of malignant cells and is considered to be a non-obligate precursor of invasive breast cancer. It currently accounts for approximately one fifth of newly-diagnosed breast cancers and its incidence has been rising due to the wider adoption of screening mammography and the introduction of high spatial resolution magnetic resonance imaging (MRI)[1,2]. DCIS usually presents as mammographic micro-calcifications or non-mass enhancement (with segmental distribution) on MRI. The latter is more sensitive imaging modality than mammography in detecting intermediate and high grade DCIS and is more accurate in estimating the disease extent[2]. Symptomatic DCIS is much less common nowadays and clinically presents as a palpable mass or nodularity, pathological nipple discharge or occasionally found as an incidental pathological finding during surgery for other reasons such as reduction mammoplasty. Furthermore symptomatic DCIS is associated with higher rates of local recurrence (LR) after treatment compared with screen-detected disease[1].
The overall risk of DCIS progressing to invasive breast cancer has been reported to range from 14% to 75% depending upon the nuclear grade[1]. This indicates that a significant proportion of DCIS cases are not life -threatening and do not require any treatment. The challenge however is to accurately identify such cases in order to ovoid overtreatment. Unfortunately the current clinico-pathological parameters used in clinical practice are unable to identify clinically less relevant disease and therefore all DCIS lesions require at least surgical excision.
Optimal treatment of DCIS requires adequate surgical excision of the lesion with tumor-free surgical margins[1]. The surgical treatment may consist of breast conservation surgery (BCS) or mastectomy with or without immediate breast reconstruction.
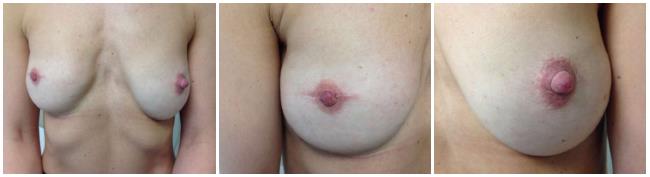
The sentinel node biopsy (SNB) is not routinely indicated for pure DCIS and should be reserved for patients undergoing mastectomy[1]. There is a growing consensus that a 2 mm tumor-free margin represents an adequate surgical margin[1]. The skin-sparing mastectomy (SSM) technique (with or without nipple-areola preservation) facilitates immediate reconstruction with improved aesthetic outcomes (Figure 1) in women opting to have immediate reconstruction[3].
Having addressed the issue of surgical treatment and the need for complete removal of DCIS lesions in the light of current knowledge, the next issue to address is the need for adjuvant treatments.
Patients undergoing mastectomy for DCIS have an excellent prognosis and do not usually require further treatment. Post-mastectomy radiation should be considered for extensive high grade DCIS with significant involvement of the surgical margins[1]. If the DCIS is ER positive, then adjuvant endocrine therapy can be considered in such cases (extensive disease involving surgical margins) and in the context of chemoprevention of malignancy in the contra-lateral breast.
For women undergoing BCS, all randomized controlled trials (RTCs) have demonstrated that adjuvant radiotherapy (RT) reduces the risk of LR after adequate local excision of localized disease[4-6].
A recent update from the NSABP B-17 and NSABP B-24 trials[4] has demonstrated that adjuvant RT is associated with a significantly lower LR rate after a median follow up of 15 years. Approximately one half (54%) of the recurrences were invasive, and for these patients the overall survival (OS) was significantly lower [hazard ratio (HR) of death = 1.75, 95%CI: 1.45 to 2.96, P < 0.001].
The EORTC 10853 randomized trial also showed that RT reduced the risk of any LR by 48% (HR = 0.52; 95%, P < 0.001) after a median follow up of 15 years[5].
The UK/ANZ DCIS trial investigated the effect of adjuvant treatment with tamoxifen after BCS and RT for DCIS[6]. After a median follow-up of 12.7 years, tamoxifen use was associated with a significant reduction in LR and the incidence of contra-lateral breast cancer (HR = 0.71, 95%; P = 0.002).
A combined analysis of the UK/ANZ DCIS and B-24 trials revealed that the addition of tamoxifen to BCS and RT for DCIS reduced the risk of invasive LR and the incidence of in situ disease in the contralateral breast regardless of age with no improvement in OS[7]. Taken together these studies suggest that tamoxifen is not routinely indicated after BCS for ER+ DCIS, but can be considered in selected cases at an increased risk of LR. The results of these trials should be communicated to patients in order to help them make informed decisions in the context of adverse effects of tamoxifen. The NSABP B-35 and IBIS II trials are currently evaluating whether aromatase inhibitors are more effective than tamoxifen as an adjuvant endocrine treatment after BCS for DCIS.
It is clear that adjuvant treatments following BCS for DCIS significantly reduce the risk of LR which is invasive in 50% of LR cases. Although adjuvant therapy has not been shown to improve OS, it is reasonable to assume that reduction in invasive LR will translate into an OS benefit over time. The uncertainty remains however whether adjuvant treatments especially RT could be safely omitted in certain subgroups of patients at a low risk of LR.
Numerous prospective and retrospective studies have reported the lesion size, nuclear grade, patient’ age, the presence/absence of necrosis, margin’s width and the comedo morphology to be significantly associated with the risk of[8]. The use of these factors in combination can guide adjuvant treatment recommendations in order to minimize overtreatment in the context of multidisciplinary management. For example a woman who had complete excision of a low grade DCIS measuring less than 2 cm with a tumor-free margin of at least 1 cm does not require any further treatment, whereas a premenopausal woman who had BCS for a 3 cm high grade ER+ DCIS will benefit from RT and tamoxifen. The Van Nuys prognostic index includes tumor size, nuclear grade and margin width. Patients with low Van Nuys scores can avoid adjuvant treatment after BCS[9].
There is a consensus however that the above predictive factors are crude and that there is a need to develop more accurate predictors of DCIS behavior based on the molecular profile of the tumor. The Oncotype-DX-DCIS genomic score has been recently introduced to guide RT recommendations after BCS[10]. This multi-gene expression assay calculates a score based on 7 cancer-related genes and 5 reference genes. It was validated using formalin-fixed paraffin embedded tumor tissues and clinical outcome data from the ECOG 5194 trial which included patients treated with BCS with or without adjuvant RT[11]. A low score (< 39) indicates a very low risk of LR and suggests that adjuvant RT can be safely omitted.
The expression of αvβ6 on myoepithelial cells of DCIS has been recently reported to predict disease progression to invasive malignancy and LR. This protein up-regulates MMP-9 through TGFβ causing tumor progression[12].
Further research focused on molecular and biological profiling is likely to facilitate a personalized treatment approach to patients with newly-diagnosed DCIS in order to optimize the clinical outcome while minimizing harm from overtreatment. However such research is likely to be complicated by intra-tumor molecular heterogeneity and the role of the microenvironment in tumor development and progression[13].
P- Reviewer: Yokoyama Y S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Lambert K, Patani N, Mokbel K. Ductal carcinoma in situ: recent advances and future prospects. Int J Surg Oncol. 2012;2012:347385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Baur A, Bahrs SD, Speck S, Wietek BM, Krämer B, Vogel U, Claussen CD, Siegmann-Luz KC. Breast MRI of pure ductal carcinoma in situ: sensitivity of diagnosis and influence of lesion characteristics. Eur J Radiol. 2013;82:1731-1737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Reefy S, Patani N, Anderson A, Burgoyne G, Osman H, Mokbel K. Oncological outcome and patient satisfaction with skin-sparing mastectomy and immediate breast reconstruction: a prospective observational study. BMC Cancer. 2010;10:171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, Land SR, Margolese RG, Swain SM, Costantino JP. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 5. | Donker M, Litière S, Werutsky G, Julien JP, Fentiman IS, Agresti R, Rouanet P, de Lara CT, Bartelink H, Duez N. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054-4059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 6. | Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, Forbes JF, Bishop H, Fentiman IS, George WD. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 374] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 7. | Petrelli F, Barni S. Tamoxifen added to radiotherapy and surgery for the treatment of ductal carcinoma in situ of the breast: a meta-analysis of 2 randomized trials. Radiother Oncol. 2011;100:195-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Wang SY, Shamliyan T, Virnig BA, Kane R. Tumor characteristics as predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Breast Cancer Res Treat. 2011;127:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 9. | Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186:337-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 11. | Motwani SB, Goyal S, Moran MS, Chhabra A, Haffty BG. Ductal carcinoma in situ treated with breast-conserving surgery and radiotherapy: a comparison with ECOG study 5194. Cancer. 2011;117:1156-1162. [PubMed] [Cited in This Article: ] |
| 12. | Allen MD, Thomas GJ, Clark S, Dawoud MM, Vallath S, Payne SJ, Gomm JJ, Dreger SA, Dickinson S, Edwards DR. Altered microenvironment promotes progression of preinvasive breast cancer: myoepithelial expression of αvβ6 integrin in DCIS identifies high-risk patients and predicts recurrence. Clin Cancer Res. 2014;20:344-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Cowell CF, Weigelt B, Sakr RA, Ng CK, Hicks J, King TA, Reis-Filho JS. Progression from ductal carcinoma in situ to invasive breast cancer: revisited. Mol Oncol. 2013;7:859-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |