INTRODUCTION
Glioblastoma (GBM) is one of the aggressive primary brain tumors with survival period falls from one to three years upon treatment[1,2]. Today’s available treatments (chemotherapy, radiation and surgery) have little success and alternative or combinational approaches to control gliomas are in need[3]. In alternative treatments, gene therapy has shown promise due to development of various tools such as lentivirus, adenovirus, adeno-associated virus, conditional replicating viruses and tumor specific promoters[3-5]. In a gene therapy approach, therapeutic or suicidal genes or oncolytic viruses are delivered into the tumor cells to suppress or eliminate tumor growth[3,6]. Numerous studies have shown the ability of oncolytic viruses to suppress the tumor cells and have been proven to be safe in clinical trials[4,5,7]. The efficacy of the oncolytic agents, however, may be compromised due to their limited infection efficiency, mode of delivery to the tumors, and distribution into the entire tumor area[6]. Currently, different oncolytic viral vectors are being administered directly into the tumor sites by multiple intratumor injections[4,8,9]. Intratumor injection is advantageous compared with systemic injection of a virus to control entry into the circulation and to avoid unexpected side effects[10]. Strategies for improved gene delivery are highly desirable to overcome some of these short comings. In this context, genetically transformed cells are being considered as delivery vehicles to deliver genes or viruses directly into different tumors[11-14]. Due to their unique property to migrate to the pathological lesions, stem cells are considered a unique choice to be the delivery vehicle for therapeutic genes to the tumors, especially for glioma[15-17]. Endothelial progenitor cells (EPCs) are a subpopulation of pluripotent hematopoietic stem cells (HSC), which show active migration and incorporation into the neovasculatures of glioma when administered locally or systemically[18-21]. Based on the characteristics of EPCs, it is possible to use these cells as vehicles for the delivery of therapeutic genes to gliomas (using viral vectors)[14]. In addition, EPCs can be collected from a patient’s own peripheral blood and bone marrow, which in turn eliminate the possibility of immune response[22].
Locally administered transfected EPCs can act as delivery vehicles for genes, viral vectors or both. For example, transgenic EPCs can carry either a therapeutic gene, such as growth inhibitory or inflammatory factors, or a suicidal gene, such as HSV-tk for antiviral drugs or human sodium iodide symporter (hNIS) for I-131 (radioiodine) or both[7,23,24]. The delivery of genes from transfected EPCs into adjacent tumor cells can be achieved by using replication competent viral vectors that will enable the virus to grow inside the EPCs, shed, and transfect the adjacent tumor and neovasculature cells[25]. Upon effective infection of the tumor and adjacent neovasculature cells, anti-tumor treatment can be started, especially if suicidal genes are used[5,7,23,24]. Local administration of virus-transfected EPCs will have advantages over the direct injection of viral particles to the tumors for the following reasons: (1) EPCs will not allow quick release of viral vectors to the circulation through blood brain barrier; (2) local administration will prevent accidental transfection of cells in non-target organs or tissues; and (3) locally administered EPCs may migrate not only to the periphery but also to the center of the tumor and incorporate into neovasculatures[14,25-27]. Therefore, there is a higher chance for the extensive transfection of tumor cells during the migration of transfected EPCs. That would enable the subsequent anti-tumor treatment (by targeting suicidal gene for example) to be more effective[7,23,24].
Reporter gene systems have been increasingly used for monitoring gene therapy in various tumor models to in vivo determine the delivery and expression of transgene products. hNIS is an intrinsic transmembrane glycoprotein that mediates the transport of iodides into the thyroid follicular cells[28,29]. A number of studies have demonstrated that active iodide uptake can be induced in a variety of cells[21,30,31]. This transport system also transports Tc-99m and can be imaged by gamma camera[32-34]. The expression of hNIS in the transfected cells is obviously higher than the non-transfected cells and higher signal associated with hNIS expression can be monitored with SPECT imaging. In our recent report we also showed the importance of hNIS in detecting in vivo gene expression[14].
The purposes of this study was to determine: (1) whether EPCs transfected with replication competent adenoviral vectors carrying hNIS can migrate to other parts of a tumor following injection into orthotopic glioma; and (2) whether migration of EPCs and the delivery of the gene product to the tumor cells can be determined by in vivo imaging.
MATERIALS AND METHODS
CD133+ cell collection and isolation
Human cord blood was collected under Henry Ford Health System Institutional Review Board (IRB) approved protocol. The cord blood was collected from placenta using published method with minor modifications[35]. In brief, placental blood directly collected into 50 mL tube contains 1 × PBS (with penicillin/streptomycin and ethylene diamine tetraacetic acid) by opening the clamps. Collected cord blood samples were maintained in ice until reaching the laboratory. Once reaching the laboratory cord blood CD133+ cells were isolated using our published method[14,21,36]. The mononuclear fraction from cord blood was separated using Ficoll density gradient centrifugation. Immunomagnetic isolation kit (Miltenyi, CA) was used to isolate the CD133+ cells. Isolated CD133+ cells were maintained as suspension cultures using CellGenix SCGM media (CellGenix, Germany) supplemented with 40 ng/mL of stem cell factor (SCF), 40 ng/mL of FLT3 and 10 ng/mL of thrombopoietin (TPO) (Prospec, United States).
Production of replication competent viral vector
Replication competent adenovirus carrying hNIS gene was a gift from Dr. Barton, Henry ford Hospital[7]. The full details of the construction of the adenovirus were described in the published work of Dr. Barton[7]. The adenoviral construct used in this study was Ad5-yCD/mutTK(SR39)rep-hNIS (replication-competent adenovirus)[7]. The adenoviral vector carries therapeutic genes and reporter genes. The E1 region contains therapeutic gene [yeast cytosine deaminase (yCD) and mutant herpes simplex virus thymidine kinase mutTK (SR39) fusion gene] and the E3 region contains reporter gene (hNIS)[7]. Transgenes were expressed under the control of human cytomegalovirus (CMV) promoter[7]. We used adenovirus under approved Institutional Recombinant DNA Biosafety Committee (IRDBC) protocol. Adenovirus was produced using published method with minor modifications[37]. To produce adenovirus, 293 cells were seeded at a density of 1.6 × 106 per T75 flask. When they reached confluence, cells were split into three T75 flasks. When cells reached 80% confluence, the media was replaced with 5 mL of serum free Dulbecco’s modified Eagle’s medium (DMEM) containing adenovirus (2.6 × 109 viral particles per milliliter) and incubated at 37 °C, 5% CO2. After one hour incubation, 10 mL of complete media was added and incubated at 37 °C, 5% CO2. After 3 d of incubation supernatant containing adenovirus was collected and concentrated using polyethylene glycol (PEG) solution. Viral supernatants were mixed with PEG solution and incubated overnight at 4 °C. After incubation, virus was collected by centrifugation at 1500 g for 30 min. Concentration of viral particles were measured using ultraviolet-visible (UV) spectrometer. In brief, 5 μL of sample (adenovirus) was added to 495 μL of 0.1% SDS solution. After vortex optical density (OD) was measured using UV spectrometer at 260 and 280 nm wavelength. Number of viral particle in concentrated samples were calculated using following formula: 1 OD260 = 1012 viral particles per milliliter[37].
Transfection of EPCs
We used adenovirus in transfection experiments according to approved IRDBC protocol (Henry Ford Health system institutional recombinant DNA and biosafety committee). Adenoviral transfection of EPCs was performed according to published method with minor modifications[26]. To develop transfected EPCs, adenovirus carrying hNIS gene was added to sterile 1.5 mL microcentrifuge tube in 1:2000 ratio (cell:viral particle) and incubated for 1 h at 37 °C, 5% CO2. After one hour incubation, 1-3 mL of fresh media was added and transferred to a 6-well plate and further incubated for 24 h.
Tc-99m uptake assay
To test expression of hNIS gene in transfected EPCs, a Tc-99m uptake assay was performed according our published method[14]. In brief, 10 μCi of Tc-99m (Mallinckrodt, United States) was added to around 1 to 1.5 × 106 cells (in serum free media) and incubated at 37 °C fo-r 30 min followed by washing twice with phosphate buffered saline (PBS) and cell associated activity in pellet was measured using gamma counter (Wizard 1420, PerkinElmer, United States). We used the same method to test the Tc-99m uptake in iron labeled transfected EPCs.
Labeling of cells with ferumoxides
EPCs were labeled with ferumoxides using our published method[21,36]. In brief, ferumoxides (Fe) (Berlex Laboratories, United States) and protamine sulfate (Pro) were added to the cell suspension followed by 15 min incubation at 37 °C, 5% CO2. Upon incubation, complete stem cell media was added and incubated for 4 h followed by washing with PBS. Finally cells were resuspended in serum free media at the desired concentration for injection.
For cell viability, 100 μL cell suspension were mixed with trypan blue dye and observed under a microscope to determine cell viability. There were three types of cell preparations, which were injected in three separate groups of tumor bearing animals: (1) non-transfected non-FePro labeled (control cells); (2) transfected, FePro labeled; and (3) transfected, non-FePro labeled.
Animal model
Animal experiments in this study was approved by animal care and user committee at Henry Ford Health System. Human glioma cells (U-251, gift from Dr. Steve Brown, HFHS) were cultured with DMEM supplemented with 10% FBS, penicillin (100 IU/mL), and streptomycin (100 μg/mL). Upon reaching confluent, cells were collected and made a cell suspension (4 × 105 cells/5 μL) in serum free media before implanting in rat brain. Athymic nude rats 6-8 wk of age and 150-170 g of weight (Charles River Laboratory, Inc.) were used for the implantation[14,21,38]. Firstly, animals were anesthetized by intraperitoneal injection using ketamine/xylazine mixture (100 mg/kg ketamine, 10 mg/kg xylazine) and tumor cells were implanted according to our published methods[14,21,38]. There were at least 3 animals for each condition.
Intratumor injection of transfected EPCs
Intratumor injection was performed according to published method with modifications[39]. After 14 d of post implantation of U251 cells in the rat brain, animals were anesthetized and their skulls were exposed. Using a dental drill a hole was made at 3 mm to the right and
1 mm anterior to the bregma, exactly at the site of tumor implantation, and a 10 μL micro-syringe fitted with 26 s gauge-needle loaded with EPCs (around one or two million) in 5 μL was lowered to the depth of 4 mm, then raised to the depth of 3 mm. Either transfected labeled, transfected non-labeled EPCs or control EPCs were injected stepwise at a rate of 0.5 μL/30 s until the entire volume had been injected and syringe was withdrawn at one millimeter per minute. Bone wax was used to seal the drilled hole and finally the overlying skin was sutured.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) imageries were obtained using a 3.0 Tesla clinical system (Signa Excite, GE health) using 50 mm diameter small animal imaging coil (Litzcage small animal imaging system, Doty Scientific Inc, Columbia, SC) according our published method[14]. Rats were anesthetized with 1.5%-2.0% isoflurane in oxygen and secured in a small animal imaging coil. MRI images were acquired before and 7 d post intratumor injection of EPCs carrying adenoviral vectors. Images were obtained with three dimensional (3D) isotropic Fast Imaging Employing Steady sTate Acquisition with parameters repetition time = 11.4 ms, echo time = 5.6 ms, using a 200 × 200 matrix, field of view = 60 mm and number of excitation = 2, effective slice thickness was 0.3 mm[14].
SPECT imaging
SPECT images were acquired based on our published method[14]. In brief, animals were anesthetized using ketamine/xylazine (100/10 mg/kg) and 1mCi of Tc-99m was injected through tail vein. After one hour of tail vein injection animals were subjected for SPECT imaging. Ketamine/xylazine (100/10 mg/kg) was used to achieve continuous state of anesthesia during l imaging period. SPECT was acquired with a dedicated PRISM3000 gamma camera fitted with mutli-pinhole rat collimators, 360 degree rotation with 36 degree increments, 180 s per projection, using 256 × 256 matrices, with a field of view of 4 cm × 6 cm[14]. We scanned the animals for 30 min to acquire SPECT images on the tumor area[14].
Histological analysis
Animals were euthanized and whole brain samples were collected as described in our previous publications[14,21,38]. For histological analysis, brain samples were fixed and processed for the frozen sections. The sections were strained with Prussian blue for the detection of iron labeled cells[14]. For the detection of transgene expression, sections were stained with anti-hNIS antibody (Genetex, TX, United States). For further analysis, we used anti-vWF antibody for the detection of endothelial cells and anti-EGFR (epidermal growth factor receptor) antibody for the detection of tumor cells. Some sections were double stained to determine double expression of hNIS/vWF (hNIS expression in administered EPCs) or hNIS/EGFR (hNIS expression in surround tumor cells)[14].
Statistical analysis
All data are expressed as mean ± SD. A P value of < 0.05 was considered significant.
RESULTS
Reporter gene (hNIS) expression, viability and proliferation
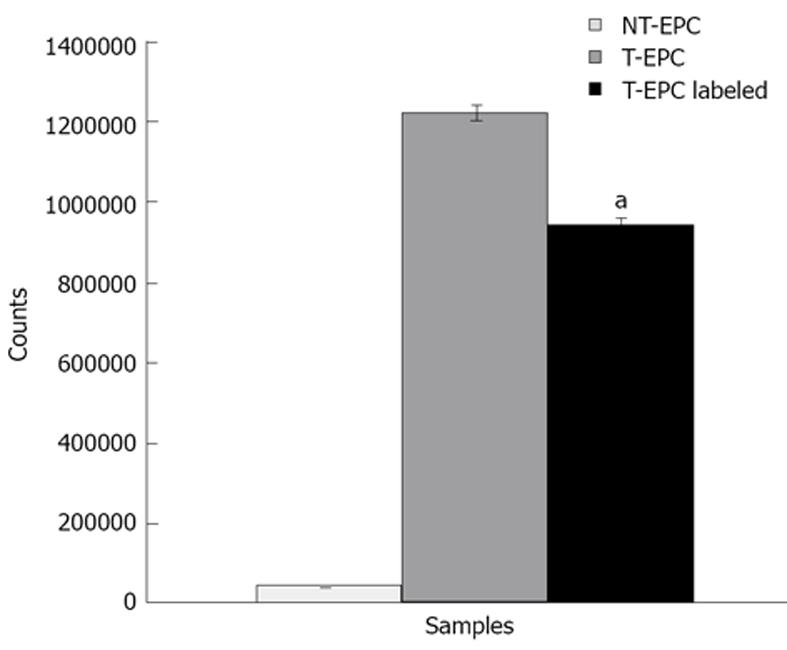
EPCs were transfected with adenovirus to study the ability of EPCs as vehicle to deliver the adenovirus into glioma. Firstly, we optimized the transfection efficiency without compromising the cell viability, which is important to determine the cell to viral dose. We studied the viral dose versus cell viability by transfecting cells with different doses of viral particles. We determined cell to viral ratio (1:2000) was optimal dose. We further studied the expression of transgene in EPCs using Tc-99m uptake assay. Figure 1 shows Tc-99m uptake was significantly higher in transfected EPCs as well as in FePro labeled transfected EPCs compared to the control EPCs. These results indicate the functional expression of hNIS gene in the EPCs.
Figure 1 Transgene expression study using TC-99m uptake assay.
Transfected and control endothelial progenitor cells (EPCs) were subjected to Tc-99m uptake assay to determine the transgene expression. Tc-99m uptake assay was performed with three type of conditions: (1) Non transgenic, non-labeled (without iron) EPC (control); (2) transgenic, non-labeled EPC; (3) transgenic and labeled (with iron) EPCs. Transfected EPCs showed higher Tc-99m uptake compared to control non transfected EPCs, which clearly indicates the functional expression of transgene. Data was indicated with Mean ± SD. aP < 0.05 vs control cells.
MRI and SPECT imaging
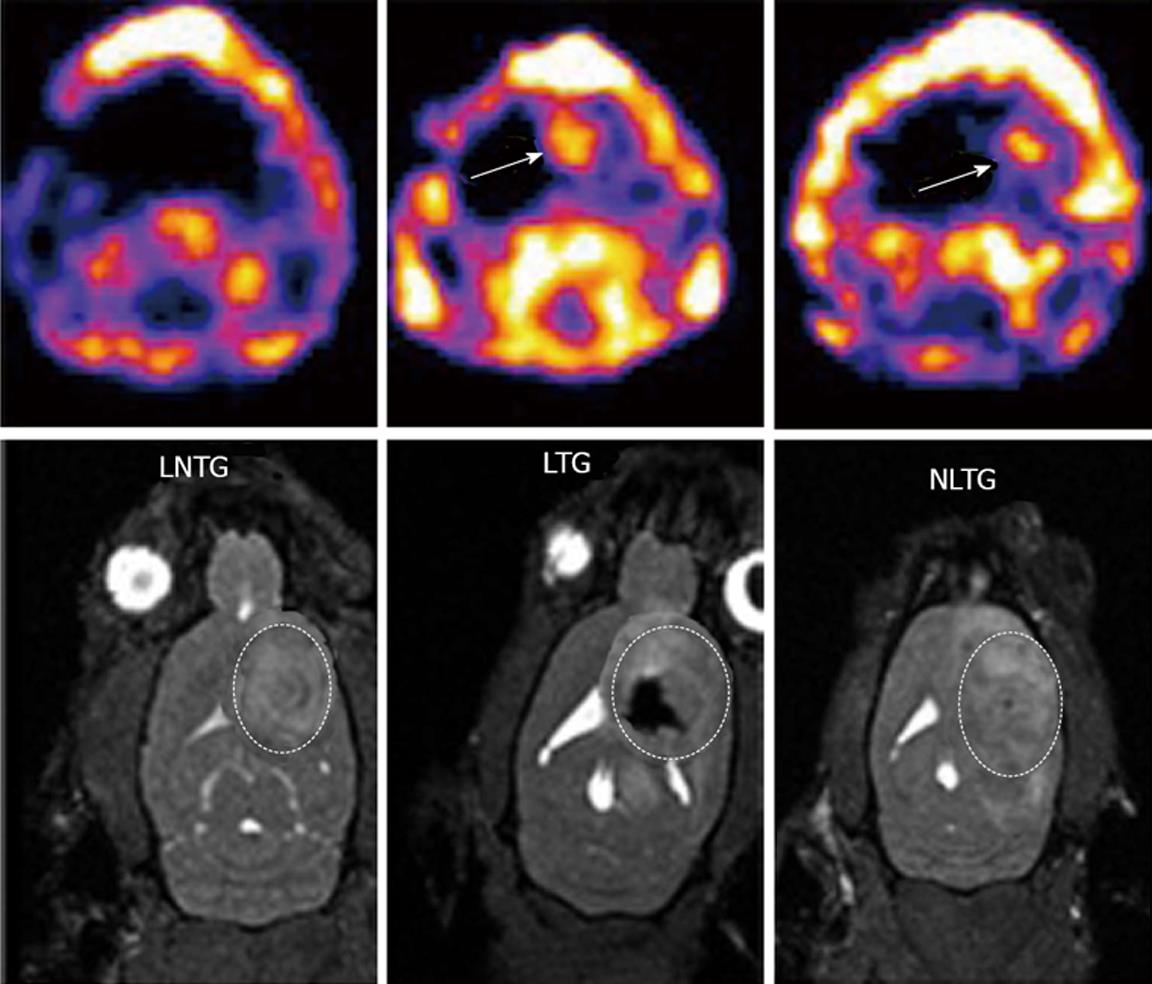
To study whether EPCs can deliver transgene to glioma, we injected transfected FePro labeled EPCs directly (intratumor) into glioma. Animals underwent MRI before and seven days after intratumor injection of EPCs. Figure 2 shows animals that received Fepro labeled EPCs into the tumor showed low signal intensity on MRI inside the tumor. All animals that received either labeled or non-labeled transfected EPCs showed accumulation of Tc-99m in the tumor. On the other hand, animals that received non-labeled non-transduced EPCs (control) showed neither low signal intensity nor Tc-99m activity in the tumor (Figure 2).
Figure 2 Magnetic resonance imaging and single photon emission computed tomography images for tracking of intratumor injected endothelial progenitor cells and transgene expression.
Expression of transgene (hNIS) was detected by single photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI) was used to detect the cell migration in the tumor. Upper panels (SPECT images): Left: Animals received non transgenic, non-labeled endothelial progenitor cells (EPCs) (control); Middle: Animals received transgenic EPCs labeled with iron; Right: Animals received non labeled transgenic EPCs. Corresponding MRI images are shown in the lower panels. Animals received labeled transgenic EPC or non-labeled transgenic EPC showed higher activities of Tc-99m in the tumors compared to control animals that received non-transgenic EPCs. MRI images clearly indicate the presence of iron labeled transgenic EPCs (lower panel, middle).
Histological and immunological analysis
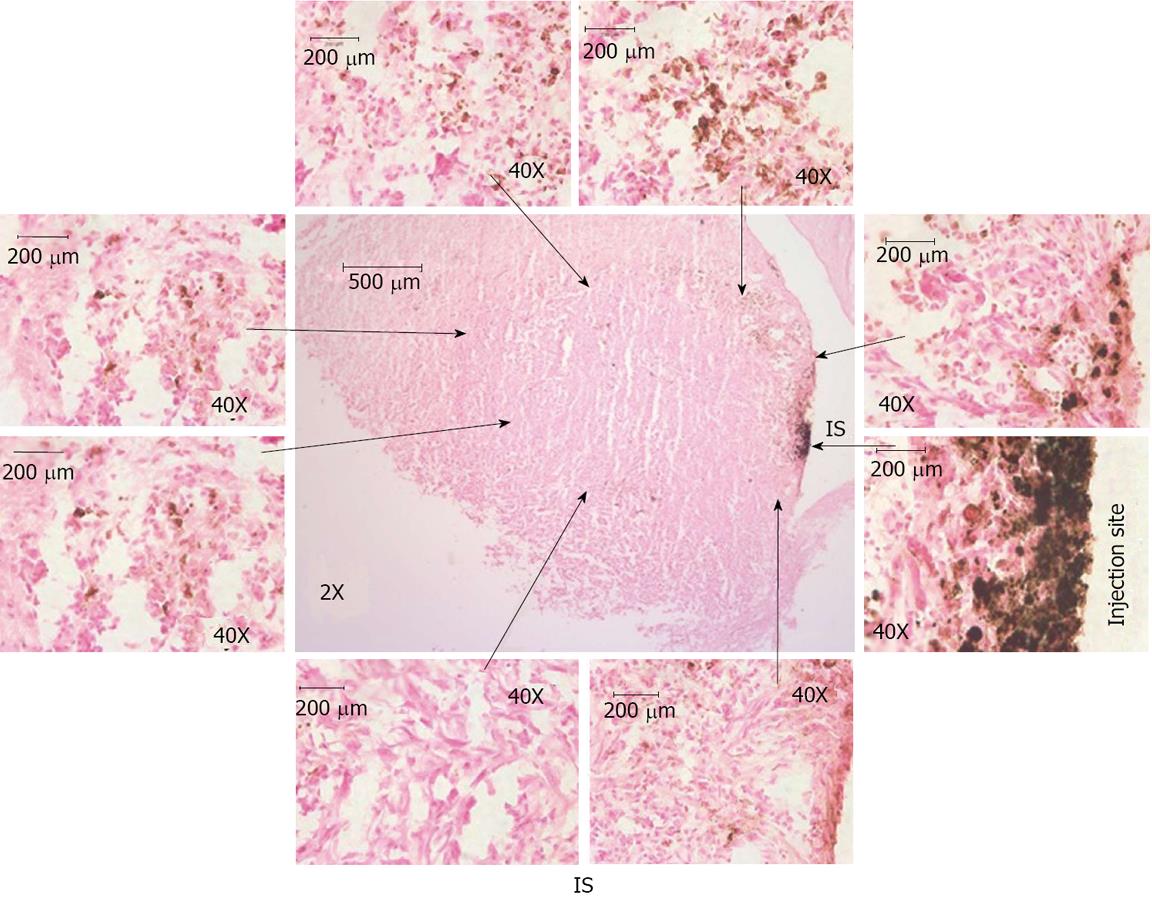
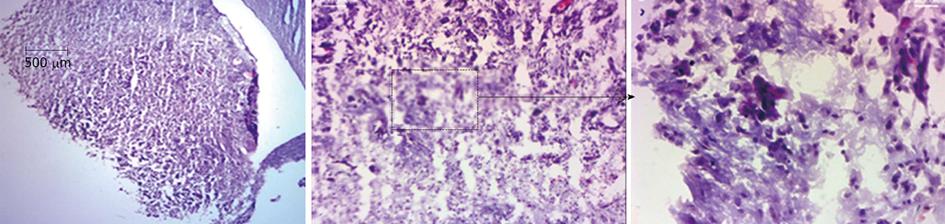
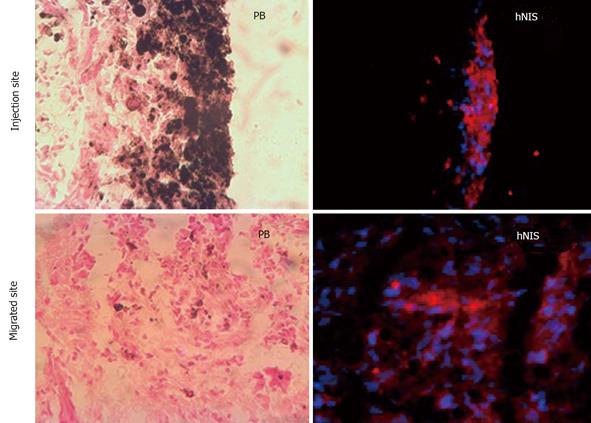
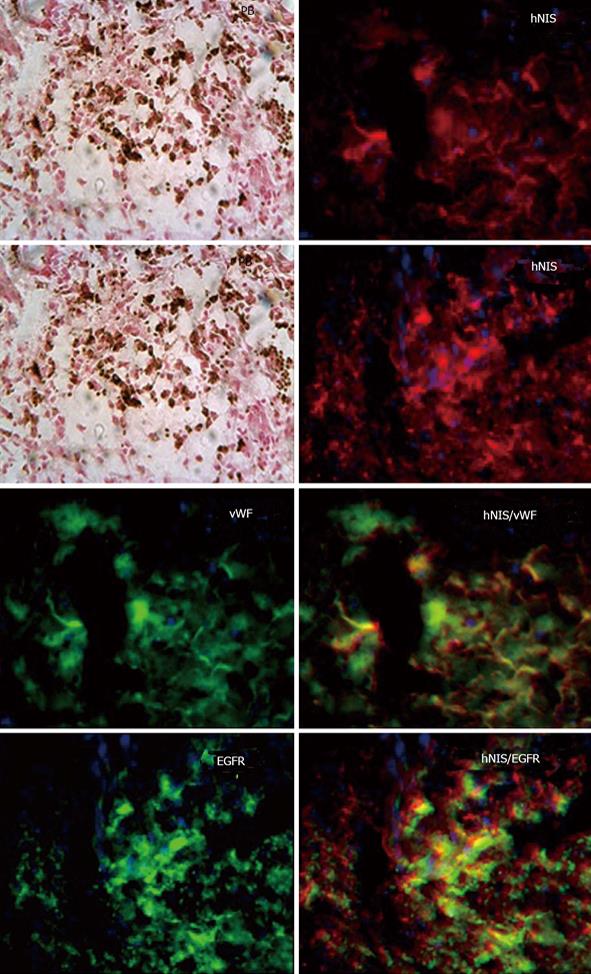
Migration of intratumor injected iron labeled EPCs in and around the tumor was detected using prussian blue staining (Figure 3). These results indicate that the EPCs are migrating from the injection sites to the periphery as well as center of the tumors. The sections also stained with HE to observe the necrotic cells, we observed necrotic cells at periphery and central regions of the tumors (Figure 4). Immunohistochemistry staining revealed the expression of transgene (hNIS) at the injection site and at distal areas where injected EPCs migrated (Figure 5). Double labeling of sections with vWF and hNIS showed that endothelial cells migrated and expressed the transgene (Figure 6). To find out whether adenovirus also transfected the adjacent tumor cells, sections were also double stained for EGFR and hNIS. EGFR is highly expressed in glioma cells and can be used as a marker to detect the tumor cells. In double labeling, some of the hNIS positive cells also stained for the EGFR marker which indicates that adenovirus has transfected tumor cells (Figure 6). This indicates EPCs carrying replication competent adenoviral vector can transmit viral vector to the surrounding tumor cells and can act as delivery vehicles.
Figure 3 Histological analysis of intratumor injection and migration of cells.
Animal brain sections were stained with Prussian blue and observed under light microscope. Center image (magnification × 2) shows the iron positive cells at injection site and migration around periphery and inside the tumor. Images × 2 shows the whole tumor area and injection sites and images × 40 shows iron labeled cell at injection site and around the tumor. Images × 40 were linked to images × 2 with arrow to show the region × is 40 near to the arrows (not exact match). These images indicate the migration of transgenic endothelial progenitor cells (EPCs) to periphery of the tumor from the injection site. Some of the EPCs were also migrated to the center of the tumor from the injection site. IS: Injection site.
Figure 4 Hematoxylin and eosin staining of brain sections.
Animal brain sections were stained with hematoxylin and eosin, In images × 2 show whole tumor area and images × 10 and images × 40 showing some the necrotic cells at injection site as well as at others regions of the tumor.
Figure 5 Immunohistochemical analysis of transgene expression at the site of injection and migrated areas.
Red fluorescence (hNIS positive) is observed at site of injection of transgenic iron labeled endothelial progenitor cells (EPCs) and areas where injected EPCs migrated.
Figure 6 Analysis of transgene delivery to tumor cells by double labeling.
Sections were double with hNIS/vWF or hNIS/EGFR. Sections stain with hNIS/vWF clearly shows some of the red hNIS positive cells also express green vWF positive markers [positive for endothelial progenitor cells (EPC)] indicating the administered EPCs. While hNIS/EGFR double staining clearly shows some of the red positive cells (hNIS positive cells) also express green EGFR positive markers indicating expression of transgenes in glioma cells. Prussian blue staining shows the iron positive cells at the corresponding sites.
DISCUSSION
In this study, we used cord blood derived EPCs to deliver transgenes directly into glioma in rat models. Replication competent adenovirus was used to transfect EPCs and the transfected EPCs were directly injected into the tumors to deliver the transgene to glioma. Adenovirus has been used in gene therapy for the treatment of glioma due to various advantages[27,40,41]. Adenoviral vectors have capability to deliver therapeutic genes to tumor cells and at same time replicate in the tumor cells where by it destroys tumor cells[41]. However, the efficacy of using adenoviral vectors in tumor therapy is hampered due to volumes of distribution and blood brain barrier[42,43]. The migratory ability of the tumor cells and their infiltration into the normal brain parenchyma is the main limiting factor for adenoviral penetration and gene delivery[44,45]. New methods are warranted to enhance delivery of therapeutic genes to glioma via adenovirus for improved gene delivery. In this context, cord blood derived EPCs have qualities such as their unique ability to self-renew, are easy to extract, give less immune response making them as candidates for gene delivery and recently several genetic tools have been developed to manipulate them to carry transgenes further, which enhance their case[14,26,36]. Our recent studies suggest that cord blood derived EPCs can be used as therapeutic gene delivery vehicles to brain tumors in animal models[14]. In our previous report, we showed systemic injection of lentivector transduced EPCs to carry transgene to glioma. Where we used a replication deficit lentivirus to integrate the reporter gene and showed their deliver capacity of transgenes into tumors[14]. In this present study, we have used replication competent adenovirus to transfect EPCs and deliver transgenes directly into tumors via intratumor injections. The advantage with this delivery system is one way it can deliver transgenes to tumors and secondly it can destroy the tumor cells by its self-replication properties[7,23]. Intratumor administration of cells helps in direct loading of therapeutic genes into tumors and avoids distribution of transfected EPCs to other organs following administration. In addition, adenovirus transfected EPCs can help self-replication of the virus, which helps release a large volume of transgenes into glioma[41,42,44,46]. To generate transgenic EPCs, we have transfected EPCs with adenovirus carrying the hNIS gene. We tested the transgene expression using Tc-99m uptake assay.
The route of administration of transgenic EPCs is important for the safe and optimal gene delivery. When transduced (by lentivector) EPCs were injected intravenously, most of the transgenic cells accumulate in the liver, spleen, and bone marrow[14]. These are vital organs and if it were by replication competent adenoviral vector mediated transfection, adenovirus could have infected the cells in some of these organs[47,48]. On the other hand, intratumor injection of EPCs would less likely go beyond the margin of the tumor or the primary organ that contains the tumor. In tumor conditions, tumor cells releases several factors which signals the migration of endothelial cells from bone marrow towards the glioma[49]. In this context, intratumor injected EPCs would migrate to the margin of tumors by similar signal mechanisms. In our previous studies we already showed that the locally implanted EPCs migrated towards the periphery of the tumor[50]. Several other studies indicate the success of cells delivering viral vectors to tumor, which include mouse fibroblasts, neural precursor cells, T-lymphcytes and MSCs[51-54]. To take the cell therapy to clinics it is also important to monitor the migration of the administered cells away from the site of injection using in vivo imaging tools. MRI is a non-invasive, high resolution imaging tool for tracking the migration of administered cells in vivo[55]. Tracking of viral vectors is important aspect in gene therapy to analyze their biodistribution around the glioma and to study their targeting of infiltrative tumor cells[42]. Adenoviral vectors targeting glioma has been covalently tagged with super-paramagnetic iron oxide nanoparticles and monitored using MRI[42]. We have developed efficient labeling methods to label EPCs using superparamagnetic iron oxide nanoparticles (SPION) and tracked these ferumoxides labeled EPCs in vivo[14,21,36]. In this study, we infected the EPCs and labeled them with SPIONs and migration and homing of labeled cells was monitored with MRI. We labeled the EPCs instead of labeling the virus, which might have an advantage to generate high signal and easy detection compared to direct labeling. In addition, adenovirus is free from the conjugation since EPC is labeled with iron particles which help the virus to stay in native form and might increase viral infection ability. High labeling efficiency can be achieved by incubation with SPIONs particles which is rather simple when compared with conjugation[36]. In addition, cell labeled with iron oxide as delivery vehicles can be translated to the clinics since SPIONs are FDA approved agents[56]. Ahmed et al[57] used neural stem cells to deliver oncolytic adenovirus to glioma and their results showed NSCs based delivery increased adenovirus survival rate compared with the adenovirus alone. These studies clearly indicate the advantage of using stem cells to deliver the oncolytic adenovirus to tumors[57]. To our knowledge, this is the first report to use the cord blood EPCs as adenovirus delivery vehicles to glioma. In addition, we used clinical MRI (3 T) for the monitoring of the distribution of intratumor injected transgenic EPCs which further help in direct translation into clinics. One of the major disadvantage with iron labeling is that it cannot differentiate the dead and live cells, since iron positive signal can be generated from the dead cells, thus it is important to monitor the cell fate in gene therapy approach[58]. Moreover, it is important to determine the distribution kinetics of virus at glioma and to determine therapeutic effect as well as subsequent dose calculations[58]. To facilitate these qualities, we chose hNIS as reporter gene to help in monitoring viable cells, quantitate EPCs engraftment, and to determine the viral load[58].
SPECT imaging was used to track injected cells in vivo, and most of the works used In-111 oxine labeling to monitor the cells[59]. However, this approach has drawbacks due to radioisotope’s (In-111 oxine) half-life and the signal goes down with time, which leads to short term monitoring (up to 7 d) of the injected cells. These short comings can be overcome by using reporter genes such as hNIS, which allows repeated detection of injected cells for long periods of time[7]. The first report on the use of hNIS as a reporter to monitor the delivery of oncolytic adenovirus was the path barker in monitoring and optimizing oncolytic viral therapy[7]. Barton et al[7] showed adenovirus delivery of therapeutic genes (cytosine deaminase/thymidine kinase) along with reporter genes (NIS) to monitor the gene therapy. These studies further indicate safety and efficacy of the adenoviral base gene delivery[5,7,23]. Most of the work on utilizing hNIS to monitor the adenovirus delivery was done on cardiac, prostate, and cystic fibrosis models[58] and not much data is available on NIS based monitoring of cell based therapies in glioma models. In this study, we transfected EPCs with adenovirus and injected them into the glioma to deliver the transgene (hNIS). SPECT imaging detects the uptake of Tc-99m (radioisotope) as long as the cells are alive and express hNIS[7,14,60]. We used dual imaging modalities to monitor the transgenic EPCs’ ability to deliver transgenes and to determine the intratumor homing and migration of administered cells by SPECT and MRI, respectively. For transgene delivery, we observed radiotracer (Tc-99m) uptake in the tumor site. Histological staining of brain sections revealed presence of iron labeled cells in the tumor not only at the site of injection but also away from the site of injection. We also stained consecutive sections with anti-hNIS antibody and visualized the transgene expression. Double staining of the hNIS stained sections with either vWF or EGFR showed that cells positive for hNIS expression also expressed either vWF or EGFR. These findings indicate that not only endothelial cells, (vWF positive) but also tumor cells (EGFR positive) expressed the transgenes, and this is only possible due the transfected EPCs’ ability to deliver the adenoviral vectors in the surrounding tumor cells.
In a conclusion, this study is an exploration of EPCs’ capacity to deliver transgenes in glioma upon intratumor administration. We showed transfected EPCs can be tracked once implanted in tumors using MRI imaging. We successfully monitored the transgene delivery by EPCs to tumor cells using SPECT imaging and immhunohistochemistry. This study showed the usefulness of EPCs as delivery vehicles of adenoviral vector to deliver therapeutic genes to glioma and act as imaging probe.