Published online Feb 10, 2013. doi: 10.5306/wjco.v4.i1.4
Revised: January 29, 2013
Accepted: February 7, 2013
Published online: February 10, 2013
Processing time: 78 Days and 0.3 Hours
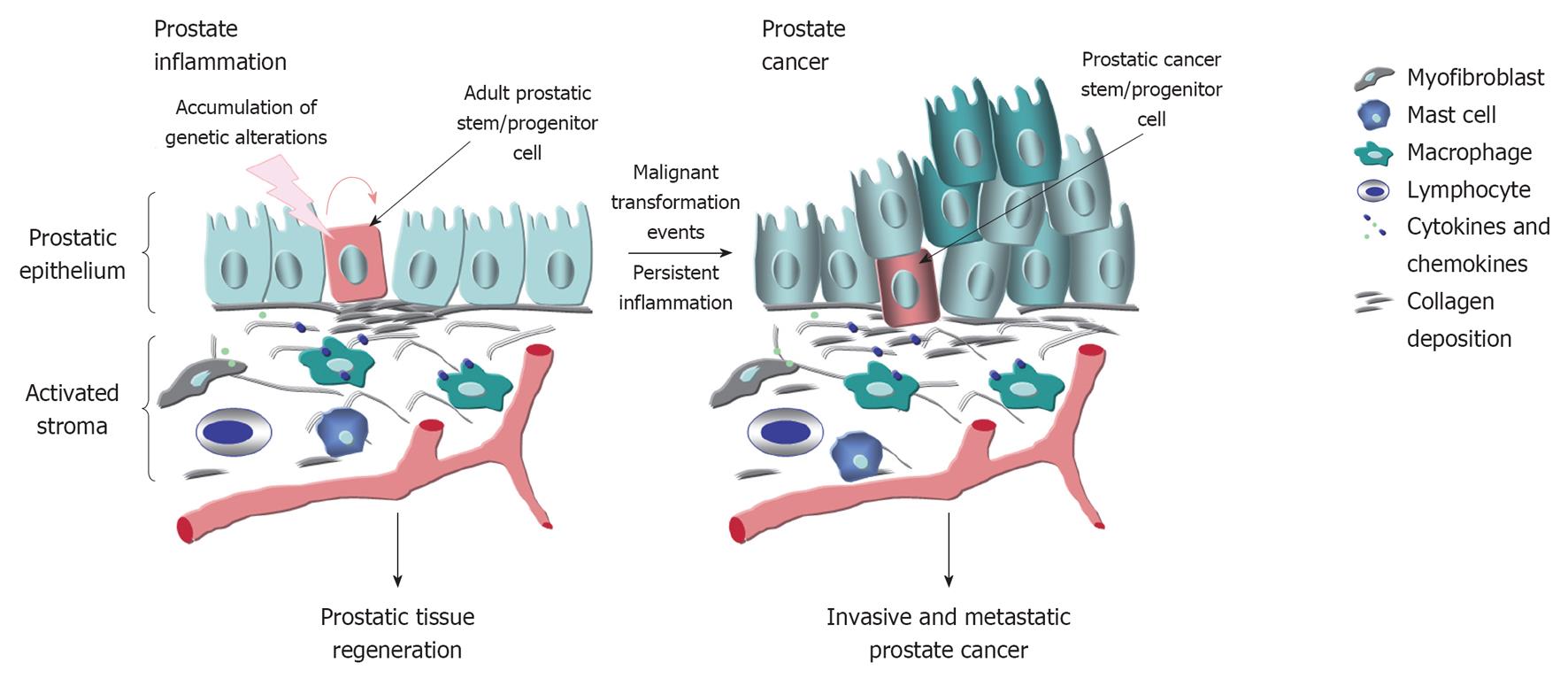
The characterization of animal models has indicated that the genetic, dietary and environmental factors and hormonal imbalance may influence the risk to develop prostate inflammatory lesions and prostate cancer (PC) confirming human epidemiologic data. It is now established that the prostate inflammatory response typically results in major changes in the local microenvironment of epithelial cells of the prostate gland, including an intense stromal remodeling, activation of fibroblasts, infiltration of immune cells such as mast cells, macrophages and B and T lymphocytes and collagen deposition. The immune cells recruited at prostate inflammatory lesions and myofibroblasts may contribute to the release of numerous pro-inflammatory cytokines and chemokines that in turn can promote the oxidative stress, genomic instability and proliferation of epithelial cells. The accumulation of additional genetic and/or epigenetic alterations in prostatic stem/progenitor cells may subsequently culminate to their malignant transformation and PC initiation and progression and more particularly with advancing age. The potential mechanistic relationships between the molecular events associated with the persistent inflammatory response and prostate carcinogenesis have important implications for optimizing the current therapies against different prostatic disorders and PCs.
- Citation: Mimeault M, Batra SK. Development of animal models underlining mechanistic connections between prostate inflammation and cancer. World J Clin Oncol 2013; 4(1): 4-13
- URL: https://www.wjgnet.com/2218-4333/full/v4/i1/4.htm
- DOI: https://dx.doi.org/10.5306/wjco.v4.i1.4
Prostate cancer (PC) is the most common malignancy and the second leading cause of cancer-related deaths in men in the United States[1-5]. Significant improvement of screening tests had led to a more effective therapeutic intervention for patients diagnosed with localized PCs[1,2,5-9]. Although this advance, the progression of organ-confined PCs to the locally advanced or metastatic castration-resistant PCs (CRPCs), which are resistant to conventional treatments by anti-hormonal therapy, radiotherapy and first-line systemic docetaxel-based chemotherapies, typically culminates in the death of patients after about 12 to 19 mo[1-6,8,10]. The molecular events responsible for PC initiation and progression to metastatic CRPCs, treatment resistance and disease relapse remain poorly understood. Consequently, the establishment of deregulated gene products in PC cells and the changes in their local tumor microenvironment that play critical functions for the prostate carcinogenesis, metastases, treatment resistance and disease recurrence is of major importance in developing novel molecular biomarkers and therapeutic targets.
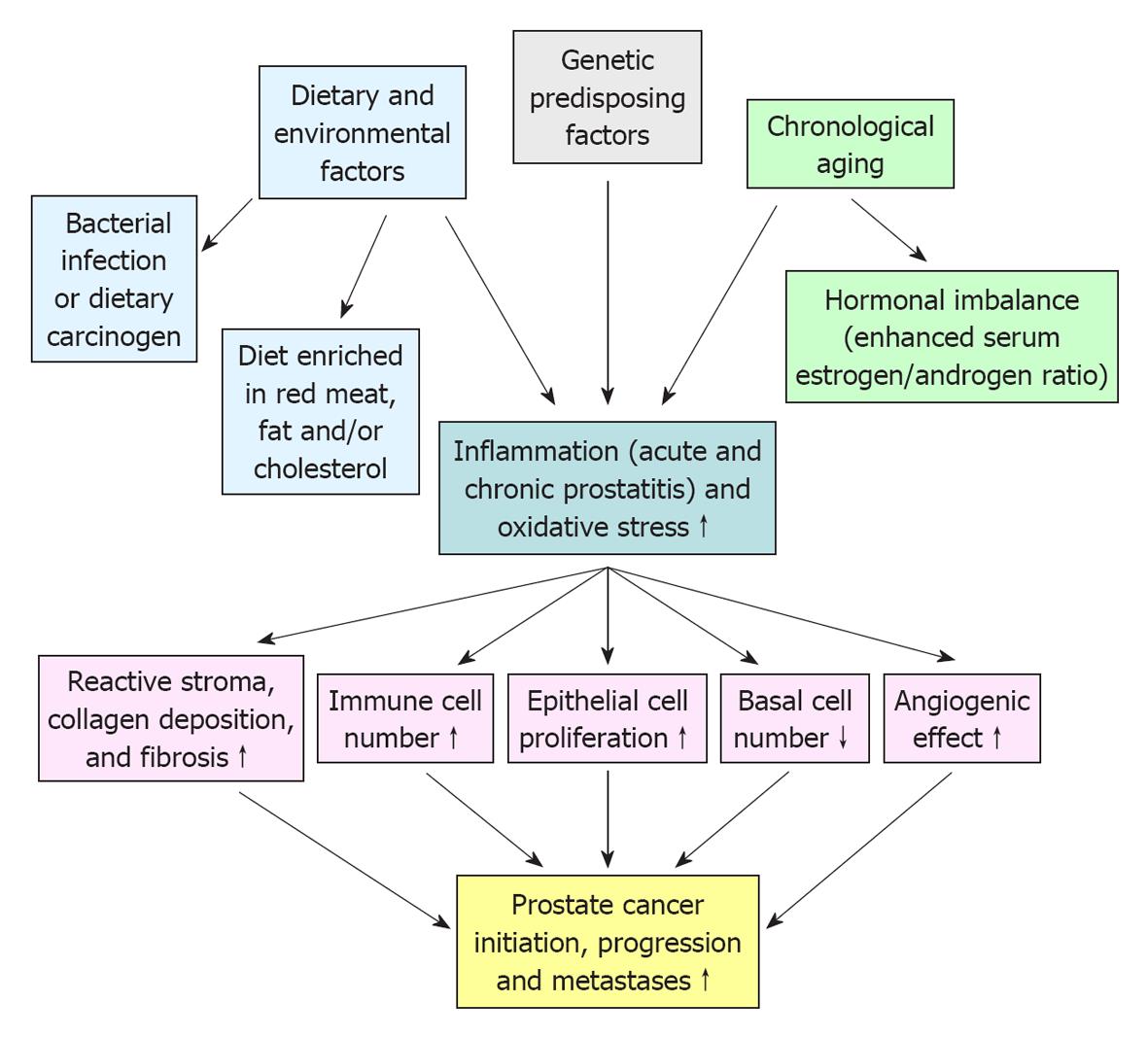
PC is a complex, heterogeneous and multifactorial disease (Figure 1). In this regard, the epidemiologic data have indicated substantial geographic and racial disparity in the PC incidence and mortality, including a higher occurrence of aggressive PCs in black American men as compared to white American and Asian men[11,12]. This suggests that the genetic and environmental factors, including the diet and behavior, may influence the PC development[12,13]. Importantly, a growing body of evidence has also revealed that the accumulation of genetic and epigenetic alterations in prostate stem/progenitor cells and their differentiated progenies concomitant with the changes in their local microenvironment, including in reactive stromal cells, may occur during severe injury, inflammation, oxidative stress and aging of the prostate gland and lead to PC development (Figures 1 and 2)[6,7,14-18]. Moreover, it has been shown that PC stem/progenitor cells and their differentiated progenies can acquire more malignant phenotypes during epithelial-mesenchymal (EMT) program and PC progression to locally invasive and metastatic CRPCs[6,17]. In this matter, we review some investigations that have been carried out with animal models in last years to establish the genetic and environmental changes that may contribute to the development of human proinflammatory lesions and their potential relationship with the prostate carcinogenesis and progression.
The characterization of phenotypic features of different animal models of prostate inflammation and PCs has indicated that the genetic background of animal strains, dietary and environmental factors, such as carcinogenic substances, high-fat diet and cholesterol as well as advancing age and hormonal imbalance may influence the incidence and progression of inflammatory lesions and PCs as suggested by human epidemiologic data (Figure 1)[19-27]. For instance, it has been observed that 72% of Lewis rat developed spontaneous prostatitis with advancing age compared to only 27% for Wistar rats while the administration of 17β-estradiol or castration promoted the incidence and severity of non-bacterial prostatitis to 100% in old adult Wistar rats[20]. The up-regulation of the systemic endogenous estrogens, including serum 17β-estradiol, concomitant with a decrease in the serum testosterone level by overexpressing aromatase (AROM) in FVB/N mice has also been observed to induce a chronic inflammation at 48 wk of age[28]. The inflammatory responses in AROM+/+ mice was also associated with an enhanced number of mast cells, macrophages, neutrophiles and T-lymphocytes and culminated to the formation of prostatic intraepithelial neoplasias (PINs) at 52 wk of age[28]. In this regard, a treatment of immortalized, non-transformed and androgen-responsive rat NRP-152 prostatic epithelial cell line with 17β-estradiol at concentrations 1-3 μmol/L for a period of 2-6 wk has also been observed to induce their capacity of forming colonies in soft agar and tumors in immunodeficient nude mice[29]. The oncogenic effect of 17β-estradiol on NRP-152 cells was accompanied by an increase of expression levels of estrogen receptor-α (ER-α) and PC stem cell-like markers (integrins α2β1, CD44, CD133, ABCG2 and CXCR4) but a decrease of ER-β and androgen receptor (AR) expression levels[29]. Moreover, it has been reported that the androgen replacement therapy with 4-dihydrotestosterone (4-DHT) or testosterone may prevent the 17β-estradiol-induced inflammatory reaction and proliferative epithelial response in the rat prostate of castrated Noble rats in a dose-dependent manner[30]. Altogether, these data suggest that the hormone imbalance, including the decrease of serum testosterone level in men with advancing age, which may promote the development of different prostate disorders, inflammation and pre-neoplastic lesions could be attenuated by a treatment aiming to increase the androgen-to-17β-estradiol ratio in serum.
In addition, a non-bacterial mouse model of acute prostatitis has also been developed which consists to induce the inflammation in the anterior, dorsolateral and ventral prostate in prostate ovalbumin expressing transgenic mice (POEAT-3) or POEAT-3/Luc/tensin deleted on chromosome 10 (PTEN-/+) mice by an adoptive transfer of ovalbumin-specific CD8+ T cells[31]. The acute prostatitis in these mice was characterized by the leukocyte infiltration, enhanced levels of pro-inflammatory cytokines and chemokines, marked epithelial cell proliferation, activated stromal cells and increase of collagen deposition that was maintained for up to 80 d after the adoptive transfer of CD8+ T cells[31]. Hence, future investigations by crossing these POEAT-3 mice with transgenic mouse models of PCs should help to shed the light on the molecular events involved in the prostatitis and their potential implications in PC development. In this regard, it has been reported that the induction of chronic bacterial prostatitis in C3H/HeOuJ mice by performing an infection with Escherichia coli (E. coli) bacteria led to intense inflammatory infiltrates in the stroma, genotoxic stress and focal atypical hyperplasia in prostatic epithelium[32]. These molecular events were associated with a loss of the expression levels of AR, glutathione-S-transferase, p27Kip1 and PTEN tumor suppressor proteins as compared to control mice[32]. In the same way, the induction of bacterial prostatitis in C3H/HeOuJ mice by intraurethral inoculation of E. coli has also been associated with a marked decrease of the expression level of Nkx3.1 tumor suppressor protein in infected prostate lobes and development of chronic inflammatory response within 14 d postinoculation[33]. The down-regulation of Nkx3.1 also correlated with an increased expression of a proliferation marker, reduction of AR level and a marked increase in the basal cell marker p63[33]. Hence, the decrease expression of key tumor suppressor products, including p27Kip1, PTEN and Nkx3.1 in these animal models of bacterial prostatitis that are frequently down-regulated during PC development provide a potential link between the persistence of prostate inflammation and carcinogenesis.
In addition, a treatment with a dietary charred meat carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) of humanized mice engineered for expressing cytochrome P450 1A enzyme (CYP1A) also induced prostate inflammation and development of atrophy of acini, low-grade PINs and high-grade PINs after 30-50 wk in the prostate gland of these rodents[22]. The high-grade PINs observed in these CYP1A humanized mice treated with PhIP expressed AR but exhibited a loss of expression levels of basal cell marker p63, PTEN and E-cadherin[22]. Importantly, it has also been observed that the administration of high-fat stress diet to PhIP-treated CYP1A humanized mice promoted prostate carcinogenesis and formation of carcinoma in situ[22]. In the same pathway, the treatment of male Fischer rats with mutagenic heterocyclic amine PhIP in the diet for 20 wk also induced inflammation, post-inflammatory proliferative or glandular atrophy and PIN lesions in the ventral prostate that culminated to invasive carcinomas after a subsequent treatment of rats with testosterone propionate[23,34]. Hence, these in vivo data suggest a potential relationship between the induction of a persistent prostate inflammatory response and PC development.
Different transgenic mouse and rat models of prostate inflammation and PC have also been generated by performing prostate-specific gene alterations using probasin (PB) promoter constructs[7,35-48]. It has been shown that the down-regulation of NKX3.1, PTEN, p53 and/or p27Kip1 tumor suppressor proteins and up-regulation of different oncogenic growth factor receptors and intracellular signaling elements such as Myc and Akt, and altered metabolism in prostate epithelial cells may cooperate to their malignant transformation, PC development, metastases and treatment resistance[7,35-49]. For instance, it has been observed that the PTEN knockout transgenic mouse models mimic the continuum of genetic and histopathological changes that are frequently associated with human PC initiation, progression and metastases and development of androgen-independent (AI) PCs after castration[7,35-38,49]. More specifically, the characterization of PB-Cre4 PTEN-/- transgenic mice with prostate-specific PTEN deletion has indicated that the enhanced pAkt in Sca+ prostatic stem/progenitor cells localized in the basal compartment of prostate led to their malignant transformation into PC stem/progenitor cells and culminated to the development of PINs that progressed to invasive PCs in 100% of transgenic mice at about 9-12 wk of the age[37,38]. The metastases or micrometastases have been detected in some PB-Cre4 PTEN null transgenic mice at 12-29 wk at lymph nodes and lungs as well as an intense bone remodeling activity[37]. Moreover, it has also been shown that the compound PB-Cre4 PTEN-/-;p53-/-, PB-Cre4 PTEN-/-;Smad4-/- and Nkx3.1CE2/+;PTEN-/-;BrafCA/+ transgenic mice developed the prostatic tumors that progressed more rapidly to invasive and metastatic states and which were invariable lethal as compared to PTEN knockout mice[39-41,49]. In the same way, the crossing of transgenic mice overexpressing constitutively activated human Akt-1 (MPAkt) and human MYC (Hi-Myc) in the prostate also resulted in MPAkt/Hi-Myc bigenic mice that exhibited an accelerated progression of PIN lesions to microinvasive tumors as compared to littermate control mice[50]. A stromal remodeling and infiltration of macrophages and B- and T-lymphocytes resembling to the inflammation observed in human prostate tumors were also seen in MPAkt/Hi-Myc mice in the early stage during the development of PINs and persisted along the PC progression[50].
Among other largely investigated animal models of PC, the prostate-specific expression of the simian virus 40 (SV40) large antigen (Tag) in prostate epithelial cells under the control of PB promoter has also led to transgenic adenocarcinoma of mouse prostate (TRAMP) mice that developed epithelial hyperplasia, high-grade PIN lesions at about 8 wk that progressed to differentiated adenocarcinomas at about 18 wk of age[42,43]. A loss of E-cadherin was also observed during the progression of primary tumors to a less differentiated state in TRAMP mice and associated with distant metastases to the lymph nodes or lungs in 100% of transgenic mice at 28 wk of age[42]. It has also been shown that the castration in TRAMP mice at 12 wk of age led to a reduction of prostatic tumor burden while the progression to poorly differentiated tumors and metastases were not delayed in castrated TRAMP mice relative to non-castrated TRAMP mice[44]. Importantly, the treatment of TRAMP mice with a diet enriched in fat and cholesterol [Western-type diet containing 21.2% fat and 0.2% cholesterol (wt/wt)] accelerated the incidence, burden and histological grade of the prostate tumors, angiogenesis and lung metastases as compared to control mice fed with a regular diet (chow diet containing 4.5% fat and 0.002% cholesterol (wt/wt))[51]. The expression of SV40 Tag driving by rat PB promoter using Sprague Dawley or Lewis strain of rats has also led to the generation of transgenic animals designated as transgenic rat adenocarcinoma of the prostate (TRAP)[45,46]. TRAP displayed atypical epithelial cell proliferation in prostate at about 4 wk and formed well-differentiated adenocarcinomas with 100% incidence before 15 wk of age, respectively[45,46]. In contrast to TRAMP mice, the castration in TRAP rats at 5 or 20 wk of age completely prevented or induced a complete involution of androgen-dependent prostate tumors in the most of transgenic rats[45,46]. Of therapeutic interest, since the prostate-specific antigen and prostate acid phosphatase (PAP) are expressed in rat prostate as observed in human prostate but not in mouse prostate, TRAP rats may constitute a good animal model to test novel vaccine strategies targeting these antigens[46,52]. For instance, it has been observed that the immunization of Lewis TRAP rats with a DNA vaccine encoding PAP triggered an autologous PAP-specific T-cell responses in transgenic rats[46]. Nevertheless, the major disadvantage of these SV40 Tag transgenic mouse and rat models of PC is that they frequently give arise to tumors that are characterized by a high level of neuroendocrine differentiation and which are observed only in a small subset of PC patients limiting thereby their clinical relevance.
Recent studies have also revealed that enhanced levels of transforming growth factor-β1 (TGF-β1) and TGF-β3, macrophage-inhibitory cytokine-1/growth differentiation factor-15 (MIC-1/GDF-15), interleukin-8 (IL-8)/CXCL8 and its receptors CXC chemokine receptors (CXCR1 and CXCR2), toll-like receptor 4, IL-17 and nuclear factor-κB (NF-κB) may play critical functions for the development of prostate inflammatory lesions, PC progression and metastases[53-60]. These inflammation-associated factors may contribute to the prostate stromal remodeling, fibrosis, host immune cell modulation and induction of the EMT process in PC cells and angiogenic switch under normoxic and hypoxic conditions, which in turn may promote the invasion and metastatic spread of PC cells at distant sites including bones (Figure 2)[55-57]. For instance, transgenic mice engineered for overexpressing epitope tagged TGF-β1 in prostate epithelial cells developed severe focal attenuation of epithelium, discontinuous basal lamina, intense fibrosis, collagenous micronodules in collapsed acini concurrent with inflammation in nerve ganglia and small vessels in an age-dependent manner as observed during human PC development[53,61]. It has also been shown that the graft of a tissue recombinant prepared of immortalized and non-tumorigenic benign prostatic hyperplasia (BPH)-1 human prostatic epithelial cells plus human prostatic cancer-associated fibroblasts (CAFs) expressing high elevated levels of both TGF-β1 and stromal cell-derived factor-1 (SDF-1)/CXCL12 under the renal capsule of severe combined immune deficient (SCID) mice resulted in the development of fibrous stroma and rapidly growing and poorly differentiated prostate tumors[54]. The tumorigenic effects of TGF-β1 appear to be mediated in part through the up-regulation of CXCR4 expression in BPH-1 cells, stimulation of CXCR4 by SDF-1 released by CAFs and activation of Akt in BPH-1 cells[54]. In addition, it has also been noted that the expression of dominant negative TGF-βRII construct in BPH-1 cells or a treatment of mice with an antibody (2G7) directed against TGF-β ligand significantly suppressed the tumor volume and invasion in this tissue recombinant xenograft model of PC[54]. The blockade of TGF-β1 signaling pathway by using TGF-β1 latency-associated peptide or neutralizing antibody also inhibited the angiogenesis and tumor formation by LNCaP PC cells xenografted in nude mice[62]. Importantly, systemic delivery of oncolytic adenovirus targeting TGF-βRII or a treatment with a selective TGF-βRI kinase inhibitor, LY2109761 was also effective at suppressing the growth of MDA PCa-2b or PC-3 cells in the bone of SCID mice as compared to the untreated mice[63,64]. In the same way, the overexpressing of MIC-1, a divergent member of the TGF-β superfamily, in PC cells has also been associated with an enhanced rate of metastases at distance sites, including bones, treatment resistance and poor outcome of PC patients[18,65-71]. Moreover, a significant increase of MIC-1 expression, at both the mRNA and mature protein level, has been detected in prostatic hyperplasias and PINs formed in LBT Tag 12T-7s transgenic mouse model, a modified SV40 early region driven by the prostate-specific rat PB promoter, and associated with a stimulation of prostatic epithelial cell proliferation[35,72]. These data suggest that an enhanced expression of mature MIC-1/GDF-15 form in prostate epithelial cells may constitute an early transforming event during prostate carcinogenesis. Although the development of locally invasive PCs was observed in this transgenic 12T-7s mouse model, no metastasis at distant organs has however been detected in these mice[72]. It has also been reported that the crossing of TRAMP mice with syngeneic mice overexpressing MIC-1 in myeloid cells under control of the myeloid cell specific c-fms promoter (MIC-1fms) produced syngeneic TRAMPfmsmic-1 mice that exhibited smaller prostate tumor size but marked increase of metastases at distant sites as compared to wild-type TRAMP mice[73]. The number of lung tumor colonies formed by TC1-T5 PC cell line derived from TRAMP mice intravenously injected in MIC-1fms mice was also superior to the number of lung colonies detected in control C57BL/6 mice[73]. Similarly, the overexpression of MIC-1 in human and AI PC3 cells was also effective at promoting their metastases at distant sites in an orthotopic tumor model in mice[74,75]. Hence, in considering the fact that MIC-1 is typically overexpressed in the majority of PCs and can contribute to the metastases of PC cells and to their resistance to current docetaxel-based chemotherapeutic treatments, which are the major causes of the death of CRPC patients, it will be of interest to develop novel transgenic mice with prostate-specific MIC-1 expression[18,65-71].
On the other hand, it has also been reported that the prostate-specific expression of a constitutively activated IκB kinase 2 (IKK2) form, which can stimulate the pro-inflammatory factor NF-κB, was insufficient for inducing prostate tumorigenesis in transgenic mice[76]. However, the crossing of PTEN+/- mice with IKK2 mice generated compound PTEN+/-;IKK2+/+ transgenic mice that exhibited all prostate lobes enlarged at 8 mo and older, formation of cribriform structures, and increase in fiber in the fibroblastic stroma associated with inflammation as compared to littermate PTEN+/- mice which had only some hyperplasia and PINs[76,77]. The malignant transformation of prostate epithelial cells in PTEN+/-;IKK2+/+ transgenic mice was also associated with a persistent inflammation as revealed by the infiltration of granulocytes and macrophages and up-regulated expression levels of pro-inflammatory chemokines (CXCL5, CXCL15, CCL3, CXCL10, and CXCL2) and cytokines [tumor necrosis factor (TNF) and IL-1b] in the prostate epithelium and stroma[76]. Moreover, it has also been observed that the Vav3+/+ transgenic mice generated by overexpressing a constitutive active form of guanine nucleotide exchange factors for Rho family GTPases, Vav3 under the control of ARR2-PB promoter in the prostatic epithelium exhibited a marked activation of AR, NF-κB and phosphatidylinositol 3-kinase-Akt signaling elements[78]. These molecular events led to the development of nonbacterial chronic prostatitis in the prostate gland which was associated with the infiltration of monocytes, lymphocytes, and plasma cells as well as the formation of PIN lesions and invasive PCs at the age as early as 3 mo[78]. In addition, it has also been reported that fibroblast growth factor-8b (FGF-8b)+/+ transgenic mice overexpressing FGF-8b in the prostate epithelium exhibited activated stroma containing increased proportion of fibroblastic cells, collagen deposition, and aggregates of inflammatory cells, including T cells, B cells and macrophages and intensive neoangiogenesis[79]. The intensive stromal changes and inflammation in FGF-8b+/+ transgenic mice preceded the development of PIN lesions that culminated to the tumor formation with phenotypical features of adenocarcinoma and sarcoma[79]. These data suggest that the overexpression and secretion of FGF-8b by prostate epithelial cells can promote prostate carcinogenesis in part via the stromal activation and induction of an inflammatory response. On the other hand, several investigations have also revealed the major contribution of the activation of AR in the stromal cells and recruitment of bone marrow (BM)-derived cells in the induction of inflammatory response and PC progression.
The sustained activation of AR expressed by epithelial and adjacent stromal fibromuscular cells in the prostate gland, which plays critical functions in the modulation of stromal-epithelial interactions for the maintaining of normal prostate homeostasis, also can promote the development of prostatic inflammatory lesions, including inflammation-associated BPH and PCs[80-84]. For instance, it has been observed using an in vitro cell co-culture system that immortalized and non-tumorigenic BPH-1 human prostate epithelial cells significantly increased the migration of THP-1 macrophages which, in turn, induced the EMT marker expression, such as N-cadherin, snail and TGF-β2, in BPH-1 cells as well as their sphere-forming ability[80]. Moreover, the exogenous expression of AR in BPH-1-AR also promoted the THP-1 cell migration and enhanced EMT marker expression and sphere-forming capacity of BPH-1-AR cells relative to BPH-1-vector cells used as control[80]. Conversely, the BPH-1/THP-1 co-culture in the presence of an anti-TGF-β2 antibody or silencing of AR function in BHP-1-AR cells using AR degradation enhancer, ASC-J9, has also been observed to decrease the THP-1 macrophage migration and suppress the induction of EMT marker expression in BPH-1 cells[80]. Moreover, the results from in vivo tissue recombination studies have indicated that the combination of BPH-1 cells with human PC-associated fibroblasts from PC surgical specimens generated large tumors while no tumor was formed by BPH-1 cells in the presence of normal prostatic fibroblasts[84]. The data from investigations performed with tissue recombinants composed of mouse or rat urogenital sinus mesenchyme expressing ARs and ERs and BPH-1 cells showing undetectable levels of ARs and ERs grown under the kidney capsule of male athymic nude mice have also revealed that a treatment with 17β-estradiol plus testosterone induced only invasive PC development in the presence of functional mesenchymal AR[80-83]. It has also been noted that the tumore derived from rat UGM plus BPH-1 cells metastasized to lymph nodes, liver and lungs[81]. Additionally, the selective AR knockout in fibroblasts and smooth muscle cells in the dARKO/PTEN+/- mouse model of PC has also been observed to inhibit the prostate epithelial cell proliferation concomitant with a decrease of the development of low- and high-grade PIN lesions and low-grade PIN progression as compared to wild-type AR/Pten+/- mice[85]. The AR deletion in fibromuscular cells of dARKO/PTEN+/- mice was also accompanied by a reduction of the extracellular matrix remodelling, collagen deposition and number of infiltrating immune cells, including T cells, B cells and macrophages and neovasculature formation in the stromal compartment of prostate gland[85]. Moreover, it has also been shown that the AR activation by 4-DHT in prostate stromal cells isolated from Pten+/- mouse prostates up-regulated the expression of pro-inflammatory cytokines and chemokines such as macrophage inflammatory protein-1α (MIP-1α), MIP-1β, MIP-2 and IL-10[85]. These pro-inflammatory factors, in turn, induced an immune cell recruitment and inflammatory response that promoted the PIN development in Pten+/- mouse prostate[85]. Of therapeutic interest, the down-regulation of AR in stromal fibromuscular cells and prostate epithelial cells with the AR degradation enhancer, ASC-J9, has also been observed to be effective at reducing the stromal remodeling and PIN development and progression in Pten+/- mice[85]. Hence, together these data supports the benefit to target AR in stromal and epithelial cells of the prostate to suppress the inflammatory response and prevent PC development.
Several investigations have revealed that circulating BM-derived adult stem/progenitor cells, including hematopoietic stem/progenitor cells, mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs) and/or myeloid cells may be recruited at prostatic inflammatory lesions and contribute to the PC development and metastases[86-93]. In fact, although BM-derived adult stem/progenitor cells appear to play only minimal roles in prostate epithelial regeneration after severe prostate inflammation and glandular disruption via cell fusion with prostate epithelial cells or transdifferentiation into prostate epithelial-like cells, they can induce immunosuppressive and angiogenic effects that promote prostate carcinogenesis[86-93]. More specifically, the release of soluble pro-inflammatory chemokines and cytokines acting as chemoattractant factors, such as SDF-1 (CXCL12), chemokine (C-C motif) ligand 5 (CCL5, RANTES) and monocyte chemotactic protein-1 (CCL2/MCP-1) by prostate epithelial cells, activated stromal cells and/or immune cells may recruit BM-derived adult stem/progenitor cells expressing their cognate receptors at injured prostate site and PC[88-90]. More specifically, it has been observed that PC-derived stromal cells, which express fibroblast activation protein-α, CD90-, CD73- and CD105 but undetectable levels of CD14-, CD20-, CD34-, CD45- and human leukocyte antigen DR exhibited morphological and phenotypic features comparable to BM-derived MSCs[94]. It has also been noted that PC-derived stromal cells represented about 0.01%-1.1% of the total cells present in core biopsies from primary human PC specimens[94]. Moreover, the stimulation of murine RM-1 PC cells by inflammatory cytokines, such as interferon-γ and tumor necrosis factor-α has been shown to be accompanied by the production of platelet-derived growth factor-BB that in turn promoted the proliferation of MSCs in vivo and in vitro[88]. The exogenous and endogenous MSCs recruited into the tumor microenvironment was also able to promote the tumor growth of RM-1 cells subcutaneously implanted in mice[88]. These data suggest the potential implication of the recruitment of MSCs in prostate inflammatory microenvironment induced via the proinflammatory mediators in the induction of immunosuppressive effects that can allow PC cells to escape the immune surveillance and favor PC development.
In addition, it has been observed that CXCR4+/sca-1+, vascular endothelial growth factor receptor-2 (VEGFR-2+)/CD34+ and VEGFR-2+/CD117+ BM-derived cell subpopulations were increased in the peripheral blood of SCID mice bearing PC cell xenografts and contributed to the tumor growth by promoting neoangiogenesis[91-93]. It has also been noticed that a treatment of tumor-bearing mice for 5 d with doxorubicin or daunorubicin was effective at reducing the tumor vascularization at least in part by inhibiting the recruitment of BM-derived cells at tumor via the inhibition of hypoxia-inducible factor-α[92]. Moreover, the data from BM transplantation/reconstitution and genetic lineage-tracing experiments have also revealed that BM-derived myelomonocytic cells can transform into lymphatic endothelial cells and integrated into PC-associated lymphatic vessels in the TRAMP-C1 cell transplantation model and thereby contribute to lymphangiogenesis[93].
Of therapeutic interest, it has also been shown that parental or genetically-engineered MSCs and EPCs, which show an innate tropism for damaged epithelial tissues, including PCs may be exploited as vehicles for targeted-delivery of anti-inflammatory, cytotoxic and/or anti-angiogenic agents at injured prostatic sites. For instance, it has been observed that MSCs engineered for expressing secreted frizzled related protein-2 suppressed the tumor growth and increased apoptosis and necrosis within tumors formed by C4-2B human CRPC cells orthotopically implanted into the prostates of castrated host SCID mice[89].
The generation of different animal models of PC has led to a better understanding of the roles of specific genetic and environmental factors that may contribute to trigger molecular events occurring in the prostate epithelium and stroma during the pathogenesis of prostate inflammatory lesions and PCs. Future investigations to develop novel compound transgenic mouse and rat models of PC and metastases with prostate-specific gene alterations relevant to the molecular events that frequently occur during PC etiology and progression, metastases at distant sites including bones and treatment resistance is of great interest. More particularly, additional studies are necessary to further establish the molecular mechanisms by which the dietary factors, prostate inflammatory process and chronological aging of prostatic stem/progenitor cells and their differentiated progenies may lead to the damages to epithelium and reactive stroma, and thereby promote prostate carcinogenesis. It will be important to establish the potential promoting effect of crossing POEAT-3 mice or transgenic mice engineered for overexpressing TGF-β1 or MIC-1 in prostate epithelial cells with PB-Cre4 PTEN-/- or compound PB-Cre4 PTEN-/-;p53-/- transgenic mice on inflammatory response and PC etiology, progression and metastases. The determination of anti-carcinogenic effects induced by different anti-inflammatory drugs, antioxidants and immune-based vaccines, alone or in combination with current anti-hormonal and chemotherapeutic treatments on transgenic mouse and rat models of PC is also important to develop novel effective combination therapies for treating PC patients diagnosed at early and late stages of the disease.
P-Reviewers Ke YQ, Ku JH S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Winquist E, Waldron T, Berry S, Ernst DS, Hotte S, Lukka H. Non-hormonal systemic therapy in men with hormone-refractory prostate cancer and metastases: a systematic review from the Cancer Care Ontario Program in Evidence-based Care’s Genitourinary Cancer Disease Site Group. BMC Cancer. 2006;6:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4284] [Cited by in RCA: 4355] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 3. | Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2824] [Cited by in RCA: 2815] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 4. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 5. | Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006;27:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Mimeault M, Batra SK. Frequent gene products and molecular pathways altered in prostate cancer- and metastasis-initiating cells and their progenies and novel promising multitargeted therapies. Mol Med. 2011;17:949-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Mimeault M, Batra SK. Animal models relevant to human prostate carcinogenesis underlining the critical implication of prostatic stem/progenitor cells. Biochim Biophys Acta. 2011;1816:25-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Freedland SJ. Screening, risk assessment, and the approach to therapy in patients with prostate cancer. Cancer. 2011;117:1123-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1737] [Cited by in RCA: 1734] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 10. | Ye XC, Choueiri M, Tu SM, Lin SH. Biology and clinical management of prostate cancer bone metastasis. Front Biosci. 2007;12:3273-3286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Klassen AC, Platz EA. What can geography tell us about prostate cancer. Am J Prev Med. 2006;30:S7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Hatcher D, Daniels G, Osman I, Lee P. Molecular mechanisms involving prostate cancer racial disparity. Am J Transl Res. 2009;1:235-248. [PubMed] |
| 13. | Gerber M. Background review paper on total fat, fatty acid intake and cancers. Ann Nutr Metab. 2009;55:140-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Zenzmaier C, Untergasser G, Berger P. Aging of the prostate epithelial stem/progenitor cell. Exp Gerontol. 2008;43:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wang W, Bergh A, Damber JE. Morphological transition of proliferative inflammatory atrophy to high-grade intraepithelial neoplasia and cancer in human prostate. Prostate. 2009;69:1378-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Hu WY, Shi GB, Hu DP, Nelles JL, Prins GS. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol Cell Endocrinol. 2012;354:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18:1605-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Mimeault M, Johansson SL, Batra SK. Pathobiological implications of the expression of EGFR, pAkt, NF-κB and MIC-1 in prostate cancer stem cells and their progenies. PLoS One. 2012;7:e31919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Svensson RU, Haverkamp JM, Thedens DR, Cohen MB, Ratliff TL, Henry MD. Slow disease progression in a C57BL/6 pten-deficient mouse model of prostate cancer. Am J Pathol. 2011;179:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049-1053. [PubMed] |
| 21. | Asamoto M, Hokaiwado N, Cho YM, Shirai T. Effects of genetic background on prostate and taste bud carcinogenesis due to SV40 T antigen expression under probasin gene promoter control. Carcinogenesis. 2002;23:463-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Endo F. Re: Holmium laser enucleation of the prostate: a modified enucleation technique and initial results: Y. G. Gong, D. L. He, M. Z. Wang, X. D. Li, G. D. Zhu, Z. H. Zheng, Y. F. Du, L. S. Chang and X. Y. Nan J Urol 2012; 187: 1336-1340. J Urol. 2013;189:395-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Shirai T, Cui L, Takahashi S, Futakuchi M, Asamoto M, Kato K, Ito N. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) in the rat prostate and induction of invasive carcinomas by subsequent treatment with testosterone propionate. Cancer Lett. 1999;143:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Borowsky AD, Dingley KH, Ubick E, Turteltaub KW, Cardiff RD, Devere-White R. Inflammation and atrophy precede prostatic neoplasia in a PhIP-induced rat model. Neoplasia. 2006;8:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Michaud DS, Augustsson K, Rimm EB, Stampfer MJ, Willet WC, Giovannucci E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control. 2001;12:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 26. | Norrish AE, Ferguson LR, Knize MG, Felton JS, Sharpe SJ, Jackson RT. Heterocyclic amine content of cooked meat and risk of prostate cancer. J Natl Cancer Inst. 1999;91:2038-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Das A, Bortner JD, Aliaga CA, Baker A, Stanley A, Stanley BA, Kaag M, Richie JP, El-Bayoumy K. Changes in proteomic profiles in different prostate lobes of male rats throughout growth and development and aging stages of the life span. Prostate. 2013;73:363-375. [PubMed] |
| 28. | Ellem SJ, Wang H, Poutanen M, Risbridger GP. Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic pre-malignancy. Am J Pathol. 2009;175:1187-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Dwarakanath AD, Michael J, Allan RN. Sulphasalazine induced renal failure. Gut. 1992;33:1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Yatkin E, Bernoulli J, Talvitie EM, Santti R. Inflammation and epithelial alterations in rat prostate: impact of the androgen to oestrogen ratio. Int J Androl. 2009;32:399-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Haverkamp JM, Charbonneau B, Crist SA, Meyerholz DK, Cohen MB, Snyder PW, Svensson RU, Henry MD, Wang HH, Ratliff TL. An inducible model of abacterial prostatitis induces antigen specific inflammatory and proliferative changes in the murine prostate. Prostate. 2011;71:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Elkahwaji JE, Hauke RJ, Brawner CM. Chronic bacterial inflammation induces prostatic intraepithelial neoplasia in mouse prostate. Br J Cancer. 2009;101:1740-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Khalili M, Mutton LN, Gurel B, Hicks JL, De Marzo AM, Bieberich CJ. Loss of Nkx3.1 expression in bacterial prostatitis: a potential link between inflammation and neoplasia. Am J Pathol. 2010;176:2259-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Inaguma S, Takahashi S, Ohnishi H, Suzuki S, Cho YM, Shirai T. High susceptibility of the ACI and spontaneously hypertensive rat (SHR) strains to 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) prostate carcinogenesis. Cancer Sci. 2003;94:974-979. [PubMed] |
| 35. | Noorali S, Kurita T, Woolcock B, de Algara TR, Lo M, Paralkar V, Hoodless P, Vielkind J. Dynamics of expression of growth differentiation factor 15 in normal and PIN development in the mouse. Differentiation. 2007;75:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Luchman HA, Benediktsson H, Villemaire ML, Peterson AC, Jirik FR. The pace of prostatic intraepithelial neoplasia development is determined by the timing of Pten tumor suppressor gene excision. PLoS One. 2008;3:e3940. [PubMed] |
| 37. | Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 842] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 38. | Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA. 2006;103:1480-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 39. | Berquin IM, Min Y, Wu R, Wu H, Chen YQ. Expression signature of the mouse prostate. J Biol Chem. 2005;280:36442-36451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, Perry SR, Labrot ES, Wu X, Lis R. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 41. | Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1572] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 42. | Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096-4102. [PubMed] |
| 43. | Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687-4691. [PubMed] |
| 45. | Asamoto M, Hokaiwado N, Cho YM, Takahashi S, Ikeda Y, Imaida K, Shirai T. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res. 2001;61:4693-4700. [PubMed] |
| 46. | Johnson LE, Becker JT, Dubovsky JA, Olson BM, McNeel DG. Prostate carcinoma in transgenic Lewis rats - a tumor model for evaluation of immunological treatments. Chin Clin Oncol. 2012;1:1-9. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci USA. 2003;100:7841-7846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 767] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 49. | Wang J, Kobayashi T, Floc’h N, Kinkade CW, Aytes A, Dankort D, Lefebvre C, Mitrofanova A, Cardiff RD, McMahon M. B-Raf activation cooperates with PTEN loss to drive c-Myc expression in advanced prostate cancer. Cancer Res. 2012;72:4765-4776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Clegg NJ, Couto SS, Wongvipat J, Hieronymus H, Carver BS, Taylor BS, Ellwood-Yen K, Gerald WL, Sander C, Sawyers CL. MYC cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors. PLoS One. 2011;6:e17449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Llaverias G, Danilo C, Wang Y, Witkiewicz AK, Daumer K, Lisanti MP, Frank PG. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am J Pathol. 2010;177:3180-3191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, McNeel DG. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33:639-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Barron DA, Strand DW, Ressler SJ, Dang TD, Hayward SW, Yang F, Ayala GE, Ittmann M, Rowley DR. TGF-β1 induces an age-dependent inflammation of nerve ganglia and fibroplasia in the prostate gland stroma of a novel transgenic mouse. PLoS One. 2010;5:e13751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 55. | Amatangelo MD, Goodyear S, Varma D, Stearns ME. c-Myc expression and MEK1-induced Erk2 nuclear localization are required for TGF-beta induced epithelial-mesenchymal transition and invasion in prostate cancer. Carcinogenesis. 2012;33:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Darrington E, Zhong M, Vo BH, Khan SA. Vascular endothelial growth factor A, secreted in response to transforming growth factor-β1 under hypoxic conditions, induces autocrine effects on migration of prostate cancer cells. Asian J Androl. 2012;14:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Walker L, Millena AC, Strong N, Khan SA. Expression of TGFβ3 and its effects on migratory and invasive behavior of prostate cancer cells: involvement of PI3-kinase/AKT signaling pathway. Clin Exp Metastasis. 2013;30:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Garlick DS, Li J, Sansoucy B, Wang T, Griffith L, Fitzgerald T, Butterfield J, Charbonneau B, Violette SM, Weinreb PH. α(V)β(6) integrin expression is induced in the POET and Pten(pc-/-) mouse models of prostatic inflammation and prostatic adenocarcinoma. Am J Transl Res. 2012;4:165-174. [PubMed] |
| 59. | Dubey S, Vanveldhuizen P, Holzbeierlein J, Tawfik O, Thrasher JB, Karan D. Inflammation-associated regulation of the macrophage inhibitory cytokine (MIC-1) gene in prostate cancer. Oncol Lett. 2012;3:1166-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Maxwell PJ, Coulter J, Walker SM, McKechnie M, Neisen J, McCabe N, Kennedy RD, Salto-Tellez M, Albanese C, Waugh DJ. Potentiation of Inflammatory CXCL8 Signalling Sustains Cell Survival in PTEN-deficient Prostate Carcinoma. Eur Urol. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912-2923. [PubMed] |
| 62. | Tuxhorn JA, McAlhany SJ, Yang F, Dang TD, Rowley DR. Inhibition of transforming growth factor-beta activity decreases angiogenesis in a human prostate cancer-reactive stroma xenograft model. Cancer Res. 2002;62:6021-6025. [PubMed] |
| 63. | Hu Z, Gupta J, Zhang Z, Gerseny H, Berg A, Chen YJ, Zhang Z, Du H, Brendler CB, Xiao X. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-β inhibits established bone metastasis in a prostate cancer mouse model. Hum Gene Ther. 2012;23:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Wan X, Li ZG, Yingling JM, Yang J, Starbuck MW, Ravoori MK, Kundra V, Vazquez E, Navone NM. Effect of transforming growth factor beta (TGF-β) receptor I kinase inhibitor on prostate cancer bone growth. Bone. 2012;50:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Rasiah KK, Kench JG, Gardiner-Garden M, Biankin AV, Golovsky D, Brenner PC, Kooner R, O’neill GF, Turner JJ, Delprado W. Aberrant neuropeptide Y and macrophage inhibitory cytokine-1 expression are early events in prostate cancer development and are associated with poor prognosis. Cancer Epidemiol Biomarkers Prev. 2006;15:711-716. [PubMed] |
| 66. | Mimeault M, Johansson SL, Batra SK. Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1. Br J Cancer. 2013;Epub ahead of print. [PubMed] |
| 67. | Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Physiol. 2010;224:626-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 68. | Zhao L, Lee BY, Brown DA, Molloy MP, Marx GM, Pavlakis N, Boyer MJ, Stockler MR, Kaplan W, Breit SN. Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling. Cancer Res. 2009;69:7696-7703. [PubMed] |
| 69. | Brown DA, Stephan C, Ward RL, Law M, Hunter M, Bauskin AR, Amin J, Jung K, Diamandis EP, Hampton GM. Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin Cancer Res. 2006;12:89-96. [PubMed] |
| 70. | Brown DA, Lindmark F, Stattin P, Bälter K, Adami HO, Zheng SL, Xu J, Isaacs WB, Grönberg H, Breit SN. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15:6658-6664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 71. | Selander KS, Brown DA, Sequeiros GB, Hunter M, Desmond R, Parpala T, Risteli J, Breit SN, Jukkola-Vuorinen A. Serum macrophage inhibitory cytokine-1 concentrations correlate with the presence of prostate cancer bone metastases. Cancer Epidemiol Biomarkers Prev. 2007;16:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, Dodd JG, Duckworth ML, Matusik RJ. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78:i-xv. [PubMed] |
| 73. | Husaini Y, Qiu MR, Lockwood GP, Luo XW, Shang P, Kuffner T, Tsai VW, Jiang L, Russell PJ, Brown DA. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) slows cancer development but increases metastases in TRAMP prostate cancer prone mice. PLoS One. 2012;7:e43833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK. Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene. 2010;29:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Tsui KH, Chang YL, Feng TH, Chung LC, Lee TY, Chang PL, Juang HH. Growth differentiation factor-15 upregulates interleukin-6 to promote tumorigenesis of prostate carcinoma PC-3 cells. J Mol Endocrinol. 2012;49:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Birbach A, Eisenbarth D, Kozakowski N, Ladenhauf E, Schmidt-Supprian M, Schmid JA. Persistent inflammation leads to proliferative neoplasia and loss of smooth muscle cells in a prostate tumor model. Neoplasia. 2011;13:692-703. [PubMed] |
| 77. | Sokoloff MH, Tso CL, Kaboo R, Taneja S, Pang S, deKernion JB, Belldegrun AS. In vitro modulation of tumor progression-associated properties of hormone refractory prostate carcinoma cell lines by cytokines. Cancer. 1996;77:1862-1872. [PubMed] |
| 78. | Liu Y, Mo JQ, Hu Q, Boivin G, Levin L, Lu S, Yang D, Dong Z, Lu S. Targeted overexpression of vav3 oncogene in prostatic epithelium induces nonbacterial prostatitis and prostate cancer. Cancer Res. 2008;68:6396-6406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Elo TD, Valve EM, Seppänen JA, Vuorikoski HJ, Mäkelä SI, Poutanen M, Kujala PM, Härkönen PL. Stromal activation associated with development of prostate cancer in prostate-targeted fibroblast growth factor 8b transgenic mice. Neoplasia. 2010;12:915-927. [PubMed] |
| 80. | Lu T, Lin WJ, Izumi K, Wang X, Xu D, Fang LY, Li L, Jiang Q, Jin J, Chang C. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol. 2012;26:1707-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118:2123-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 83. | Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol. 2004;92:221-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 84. | Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002-5011. [PubMed] |
| 85. | Lai KP, Yamashita S, Huang CK, Yeh S, Chang C. Loss of stromal androgen receptor leads to suppressed prostate tumourigenesis via modulation of pro-inflammatory cytokines/chemokines. EMBO Mol Med. 2012;4:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 86. | Ikehara A, Maeda H, Kimura T, Saito S, Ochiai A. Bone marrow-derived macrophages are associated with androgen modulated prostate regeneration. Prostate. 2012;72:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Palapattu GS, Meeker A, Harris T, Collector MI, Sharkis SJ, DeMarzo AM, Warlick C, Drake CG, Nelson WG. Epithelial architectural destruction is necessary for bone marrow derived cell contribution to regenerating prostate epithelium. J Urol. 2006;176:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Cheng J, Li L, Liu Y, Wang Z, Zhu X, Bai X. Interleukin-1α induces immunosuppression by mesenchymal stem cells promoting the growth of prostate cancer cells. Mol Med Rep. 2012;6:955-960. [PubMed] |
| 89. | Placencio VR, Li X, Sherrill TP, Fritz G, Bhowmick NA. Bone marrow derived mesenchymal stem cells incorporate into the prostate during regrowth. PLoS One. 2010;5:e12920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Giannoni E, Taddei ML, Parri M, Bianchini F, Santosuosso M, Grifantini R, Fibbi G, Mazzanti B, Calorini L, Chiarugi P. EphA2-mediated mesenchymal-amoeboid transition induced by endothelial progenitor cells enhances metastatic spread due to cancer-associated fibroblasts. J Mol Med (Berl). 2013;91:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Li H, Gerald WL, Benezra R. Utilization of bone marrow-derived endothelial cell precursors in spontaneous prostate tumors varies with tumor grade. Cancer Res. 2004;64:6137-6143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci USA. 2009;106:2353-2358. [PubMed] |
| 93. | Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Rüegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One. 2009;4:e7067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Brennen WN, Chen S, Denmeade SR, Isaacs JT. Quantification of Mesenchymal Stem Cells (MSCs) at Sites of Human Prostate Cancer. Oncotarget. 2013;4:106-117. [PubMed] |