INTRODUCTION
CO2 is a fundamental chemical species in many biological systems, injorgqanisms all over the phylogenetic tree. Its reaction with watyer is a slow process at the physiological pH, and it needs the presence of a catalysts to become effective. These catalysts are the carbonic anhydrases (CAs, EC 4.2.1.1), a superfamily of metalloenzymes which evolved at least 5 times independently in diufferent organisms[1,2]. In mammals, including humns, only α-CAs are present[1,2], but 16 different isozymes have been characterized to date, which differ in their subcellular localization, catalytic activity, and susceptibility to different classes of inhibitors. There are cytosolic isozymes (CA I, CA II, CA III, CA VII and CA XIII), membrane bound ones (CA IV, CA IX, CA XII and CA XIV), mitochondrial (CA VA and CA VB) and secreted (CA VI) isoforms. Three acatalytic forms, called CA-related proteins (CARPs), CARP VIII, CARP X and CARP XI, are also known[1]. In humans, CAs are present in a large variety of tissues such as the gastrointestinal tract, the reproductive tract, the nervous system, kidneys, lungs, skin and eyes[1,2]. Most CAs are very efficient catalysts for the reversible hydration of carbon dioxide to bicarbonate and protons (CO2 + H2O ↔ HCO3- + H+), which is the only physiological reaction in which they are involved[1].
Many CA isoforms are involved in critical physiologic processes such as respiration and acid-base regulation, electrolyte secretion, bone resorption, calcification and biosynthetic reactions which require bicarbonate as a substrate (lipogenesis, gluconeogenesis, and ureagenesis)[1]. Two CA isozymes (CA IX and CA XII) are predominantly associated with and overexpressed in many tumors, being involved in critical processes connected with cancer progression and response to therapy[1-3]. CA IX is confined to few normal tissues (stomach and body cavity lining), but it is ectopically induced and highly overexpressed in many solid tumor types, through the strong transcriptional activation by hypoxia, accomplished via the hypoxia inducible factor-1 (HIF-1) transcription factor[1,3,4]. The detailed mechanism by which HIF-1α leads to the potent overexpression of CA IX in hypoxia was discussed in an earlier review[1]. In contrast to other α-CAs, CA IX is a multidomain protein formed of a short intracytosolic tail, one transmembrane segment, an extracellular CA domain and a proteoglycan (PG)-like domain composed of 68 amino acid residues[4-12]. Expression of CA IX is strongly increased in many types of tumors, such as gliomas/ependymomas, mesotheliomas, papillary/follicular carcinomas, as well as carcinomas of the bladder, uterine cervix, kidneys, esophagus, lungs, head and neck, breast, brain, vulva, and squamous/basal cell carcinomas, among others. In some cancer cells, the VHL gene is mutated leading to the strong upregulation of CA IX (up to 150-fold) as a consequence of constitutive HIF activation[13-15]. On the other hand, as this protein is present in extremely low amounts only in few normal tissues such as the gastric mucosa (whereit seems to be in a catalytically inactive state) inhibitors of CA IX may show less side effects compared to other anticancer druigs which interact with their target both in the normal and cancerous tissues[6]. As the role of CA XII in cancer is less understood at this moment, in this review only CA IX will be discussed.
CATALYTIC ACTIVITY OF CA IX PLAYS A ROLE IN TUMOR ACIDIFICATION
The expression of CA IX is strongly up-regulated by hypoxia and is down-regulated by the wild-type von Hippel-Lindau tumor suppressor protein (pVHL)[2-4,11-13]. The transcription factor HIF-1 is a heterodimer consisting of an inducible subunit (HIF-1α) and a constitutively expressed subunit (HIF-1β)[1,2,12,13]. HIF-1 activation under hypoxia is achieved by stabilization and/or expression of the α-subunit. Oxygen-dependent prolyl-4-hydroxylase domains (PHD) covalently modify a HIF-1α domain known as the oxygen-dependent degradation domain, by hydroxylating proline residues. Hypoxia attenuates proline hydroxylation due to inactivity of PHD in the absence of oxygen, resulting in HIF-1α stabilization and non-recognition by pVHL. The association of HIF-1α with the β-subunit leads to the formation of HIF-1 and expression of target genes that contain HRE (hypoxia responsive element) sites, including glucose transporters (GLUT-1 and GLUT-3), vascular endothelial growth factor, which triggers neoangiogenesis, and, last but not least, CA IX, which is involved in pH regulation and cell adhesion[13-15].
The overall consequence of the strong CA IX over-expression is the pH imbalance of the tumor tissue, with most hypoxic tumors having acidic extracellular pH (pHe) values around 6.5, in contrast to normal tissue which has characteristic pHe values around 7.4. The role played by CA IX in such acidification processes of hypoxic tumors was recently demonstrated by our and Pastorekova’s groups[13]. Using Madin-Darby canine kidney epithelial cells, Svastova and colleagues proved that CA IX is able to decrease the pHe of these cultivated cells. CA IX selective sulfonamide inhibitors (of type 1 and 2) reduced the medium acidity by inhibiting the catalytic activity of the enzyme, and thus the generation of H+ ions, binding specifically only to hypoxic cells expressing CA IX. Deletion of the CA active site was also shown to reduce the medium acidity, but a sulfonamide inhibitor did not bind to the active site of such mutant proteins[13]. Therefore, tumor cells decrease their pHe both by production of lactic acid (due to the high glycolysis rates), and by CO2 hydration catalyzed by the tumor-associated CA IX, possessing an extracellular catalytic domain. Low pHe has been associated with tumorigenic transformation, chromosomal rearrangements, extracellular matrix breakdown, migration and invasion, induction of the expression of cell growth factors and protease activation[12,13]. CA IX probably also plays a role in providing bicarbonate to be used as a substrate for cell growth, whilst it is established that bicarbonate is required in the synthesis of pyrimidine nucleotides[14-16].
The crystal structure of the catalytic domain of human CA IX, was recently reported by this group[17]. As for other α-CAs, the CA IX catalytic domain appeared as a compact globular domain, with an ovoid shape of 47 × 35 × 42 Å3 in size. CA IX has a 3D fold characteristic of other α-CAs, for which the structure has been solved earlier[17], in which a ten-stranded antiparallel-sheet forms the core of the molecule. An intramolecular disulfide bond, which is common to the other membrane-associated α-CAs (CA IV, CA XII and CA XIV), was observed between Cys23 and Cys203[1,17]. The active site details, including the Zn(II) coordination (by His 94, 96, 119 and a water molecule), proton shuttle residue (His64) as well as amino acid residues involved in binding of inhibitors are rather similar with those of other α-CAs[1,17]. Thus, Leu91, Val121, Val131, Leu135, Leu141, Val143, Leu198 and Pro202 define the hydrophobic region of ther active site, whereas Arg58, Arg60, Asn62, His64, Ser65, Gln67, Thr69, and Gln92 identify the hydrophilic one. The crystallographic data showed the dimeric nature of the enzyme, which has been inferred from previous experiments reported by Hilvo et al[8]. Indeed, two identical dimers, resulting from a Cys41-mediated intermolecular disulfide bond between two adjacent monomers, were observed in the asymmetric unit of the crystals[17]. The dimer assembly, by means of an intermolecular disulfide bond, is consistent with the proposed function of the enzyme in tissues where its expression has been reported, as both active sites of the dimer are clearly exposed to the extracellular medium, being thus able to efficiently hydrate CO2. In addition, the N-terminal regions of both monomers are located on the same face of the dimer, while both the C-termini are situated on the opposite face. This structural organization allows for concomitant positioning of both PG domains, at the entrance to the active site clefts, oriented toward the extracellular milieu to mediate cell interaction, and of both C-terminal transmembrane portions for proper CA IX anchoring to the cell membrane. Furthermore, the position of the PG portion, at the border of the active site, suggests a further role of this domain in assisting CA domain-mediated catalysis. Indeed, as shown recently by our group[18] the CO2 hydrase activity of the CA IX full length has an optimum at a pH of 6.5 (typical of hypoxic solid tumors) whereas that of CA IX catalytic domain (similarly to that of CA I or CA II) has an optimum at pH around 7[17,18]. Thus, the PG domain, which is rich in acidic amino acid residues (26 dicarboxylic amino acids, Asp and Glu, on a total of 58 amino acid residues forming the PG domain) was postulated to act as an intrinsic buffer of this enzyme, which facilitates the CO2 hydration reaction at acidic pH values which are one of the main features of hypoxic tumors[17,18].
CA IX INHIBITORS ACCUMULATE IN HYPOXIC TUMORS AND IMPAIR THEİR GROWTH AND METASTASİS GROWTH
Many sulfonamide/sulfamate/sulfamide and coumarin CA inhibitors (CAIs) were reported to efficiently target CA IX in recent years[19-39]. The compounds specifically designed for targeting CA IX, which were important to understand its role in tumorigenesis were, among others: (1) fluorescent sulfonamides, used for imaging purposes and for determining the role of CA IX in tumor acidification[13,16,22,24,30]; (2) positively or negatively-charged compounds, which cannot cross plasma membranes due to their charged character and thus inhibit selectively only extracellular CAs, among which CA IX[1,13,23,25]; (3) ureido-substituted benzenesulfonamides with potent antitumor effects both for the primary tumor and metastases (in animal models)[31,36]; and (4) diverse chemotypes than the sulfonamides and their bioisosteres, such as the coumarins[32,33], which showed notable inhibition for the growth of the primary tumors and impair metastases formation in animal models of hypoxic tumors[36].
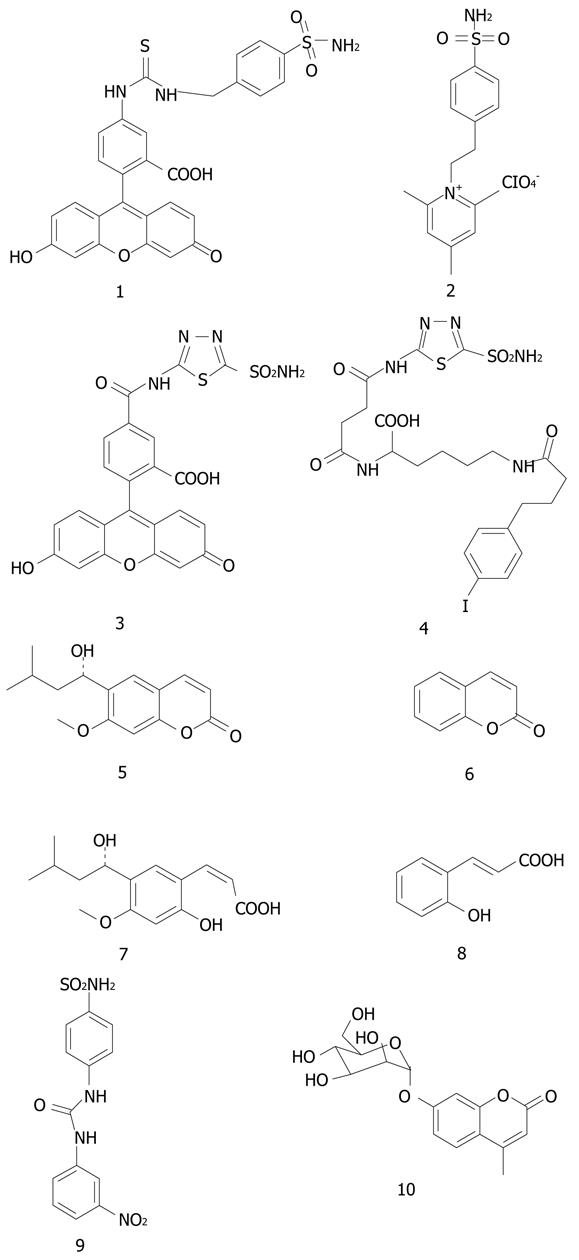
Some of the most interesting CA IX inhibitors available initially, were the compounds investigated by Svastova et al[13] (possessing structures 1 and 2) for their in vivo role in tumor acidification. These compounds present a special interest because derivative 1 is a fluorescent sulfonamide with high affinity for CA IX (KI of 24 nmol/L)[1,13], which was shown to be useful as a fluorescent probe for hypoxic tumors[1,13,16,22,24]. This inhibitor binds to CA IX only under hypoxia in vivo, in cell cultures or animals with transplanted tumors[13,16,24,30,36]. Although the biochemical rationale for this phenomenon is not understood in details, these properties may be exploited for designing diagnostic tools for the imaging of hypoxic tumors[16,24]. Indeed, Dubois et al[16,24] showed the accumulation of 1 only in the hypoxic regions of animals with transplanted hypoxic colorectal tumors (see discussion later in the text).
Compound 2 belongs to type II mentioned above, of permanently charged, membrane-impermeant derivatives, and is also a very strong CA IX inhibitor (KI of 14 nmol/L)[1,29]. It belongs to the class of positively charged, membrane-impermeant compounds previously reported by our group[1,29], which are highly attractive for targeting CA IX with its extracellular active site, since such compounds do not inhibit intracellular CAs, and may thus lead to drugs with less side effects as compared to the presently available compounds which indiscriminately inhibit all CAs[1].
The in vivo proof of concept that sulfonamide CA IX inhibitors may indeed show antitumor effects, has been first published by Neri’s group[34]. By using membrane-impermeant derivatives of types 3 and 4, based on the acetazolamide scaffold to which either fluorescein-carboxylic acid or albumin-binding moieties were attached, this group demonstrated the strong tumor retardation (in mice with xenografts of a renal clear cell carcinoma line, SK-RC-52) in animals treated for one month with these CA inhibitors[34].
The same group[35] also reported the proof-of-concept study showing that human monoclonal antibodies targeting CA IX can also be used for imaging of hypoxic tumors. The generation of high-affinity human monoclonal antibodies (A3 and CC7) specific to hCA IX, using phage technology has been reported[35]. These antibodies were able to stain CA IX ex vivo and to target the cognate antigen in vivo. In one animal model of colorectal cancer studied (LS174T), CA IX imaging closely matched pimonidazole staining, with a preferential staining of tumour areas characterised by little vascularity and low perfusion. These new human anti-CA IX antibodies are expected thus to be non-immunogenic in patients with cancer and might serve as broadly applicable reagents for the non-invasive imaging of hypoxia and for pharmacodelivery applications[35] (Figure 1).
Figure 1 The strctures of 1-10.
1: KI (CA IX) = 24 nmol/L; 2: KI (CA IX) = 14 nmol/L.
The same conclusion has been reached by our and Lambin’s groups by using small molecule CA IX-selective inhibitors of the type 1[16]. Fluorescent sulfonamides 1 with a high affinity for CA IX have been developed and shown to bind to cells only when CA IX protein was expressed and while cells were hypoxic[16]. NMRI-nu mice subcutaneously transplanted with HT-29 colorectal tumours were treated with 7% oxygen or with nicotinamide and carbogen and were compared with control animals. Accumulation of sulfonamide 1 was monitored by non-invasive fluorescent imaging. Specific accumulation of 1 could be observed in delineated tumour areas as compared with a structurally similar non-sulfonamide analogue incorporating the same scaffold (i.e., a derivative with the same structure as compound 1 but without the SO2NH2 moiety). Administration of nicotinamide and carbogen, decreasing acute and chronic hypoxia, respectively, and prevented accumulation of 1 in the tumor. When treated with 7% oxygen breathing, a 3-fold higher accumulation of 1 was observed. Furthermore, the bound inhibitor fraction was rapidly reduced upon tumour reoxygenation. Such in vivo imaging results confirm previous in vitro data demonstrating that CAI binding and retention require exposure to hypoxia. Fluorescent labelled sulfonamides may thus provide a powerful tool to visualize hypoxia response in solid tumors. An important step was thus made towards clinical applicability, indicating the potential of patient selection for CA IX-directed therapies[16].
Dubois et al[39] also recently showed that combining sulfonamide CA IX inhibitors with tumor irradiations hase an enhanced antitumor effect in mice bearing HT29 colorectal transplanted tumors.
More recently, sulfonamide 1 has also been shown to significantly decrease the growth of primary tumors in the 4T1 mouse metastatic breast cancer animal model by Lou et al[36]. However, an even stronger effect has been observed with an ureido sulfonamide (compound 9) which at pharmacological doses of 15-30 mg/kg strongly inhiibted both the growth of the primary 4T1 tumor, as well as the formation of lung metastases[31,36]. It is interesting to note that the 4T1 model tumors overexpress a very high amount of CA IX. The same study also used another mouse breast tumor cell line, the 67R1 line, which does not express at all CA IX. Indeed, the animals harboring these tumors were treated with sulfonamide or coumarin CA IX inhibitors but no influence of the tumor growth has been observed, which represents a clear-cut proof of concept that inhibition of CA IX is indeed responsible for the tumor/metastases growth inhibition with these compounds[36].
Coumarin and thiocoumarins were only recently discovered to act as CAIs, and their inhibition mechanism deciphered in detail by one of our groups[32,33]. We demonstrated recently that the natural product 6-(1S-hydroxy-3-methylbutyl)-7-methoxy-2H-chromen-2-one 5 as well as the simple, unsubstituted coumarin 6 are hydrolyzed within the CA active site with formation of the 2-hydroxy-cinnamic acids 7 and 8, respectively, which represent the de facto enzyme inhibitors[32,33]. Some other interesting facts emerged during such studies: (1) this new class of CAIs, the coumarins/thiocoumarins, binds in hydrolyzed form at the entrance of the CA active site and does not interact with the metal ion, constituting thus an entirely new category of mechanism-based inhibitors; and (2) it is possible to obtain highly isoforms-selective CAIs belonging to the coumarin/thiocoumarin class. Indeed, we reported coumarins which selectively inhibit CA IX and XII, without inhibition of CA I and II (the main offtarget isoforms)[32,36-38].
One of these derivatives, a glycosyl coumarin (compound 10) strongly inhibited the growth of the primary tumor and the formation of metastases in the same 4T1 animal model of hypoxic tumor overexpressing high amounts of CA IX, whereas in a breast cancer cell line with no CA IX expression (67R1) no such effects have been observed[39].
There are ongoing clinical trials with a monoclonal antibody targeting specifically CA IX-girentuximab (which is in Phase III cliniacl trials for the tretment of renal carcinomas) and several sulfonamide/coumarin CA IX ihiibtors are in advanced preclinical evaluation[40].
CONCLUSION
With its overexpression in many cancer tissues and not in their normal counterparts, CA IX constitutes an interesting target for novel approaches in the design of anticancer therapies. CA IX is crucial for tumor pH regulation contributing both to the acquisition of metastasic phenotypes and to chemoresistance. Consequently, further research needs to be done in the field of the tumor-associated CA IX in order to better understand its exact role in cancer. CA IX selective inhibitors are now available and they constitute interesting tools for studying the physiological and/or pathological effects of this enzyme. The design of CA IX selective inhibitors containing a variety of scaffolds and with interesting physico-chemical properties has been achieved. New sulfonamides and coumarins have been synthesized with some of these strongly and selectively inhibiting CA IX (over the offtarget isoforms CA I and Ii), with inhibition constants in the low nanomolar. Thus, many biochemical, physiological and pharmacological novel data point to the use of CA IX inhibition in the management of hypoxic tumors, which do not respond to the classical chemo- and radiotherapy. There are possibilities of developing both diagnostic tools for the non-invasive imaging of these tumors and therapeutic agents, that probably perturb the extratumoral acidification in which CA IX is involved. Much pharmacologic work is however warranted in order to understand whether a successful new class of antitumor drugs may be developed starting from these preliminary but highly encouraging observations, but girentuximab, a CA IX monoclonal antibody is alreday in Phase III clinical trials and several small molecule inhibitors are in advanced preclinical evaluation
ACKNOWLEDGMENTS
We are very grateful to our close friends and collaborators, Dr. Silvia Pastorekova and Professor Jaromir Pastorek (Slovak Academy of Sciences, Bratislava, Slovakia) for having discovered this fascinating protein and for the many discussion along the years regarding the relevant phases of the drug design of inhibitors targeting it.
Peer reviewers: Dr. Melanie H Kucherlapati, Harvard Medical School, 77 Avenue Louis Pasteur, Boston, MA 02115, United States; Dr. Rafael Moreno-Sánchez, Department of Biochemistry, Instituto Nacional de Cardiologia, Juan Badiano No. 1, Seccion XVI, Mexico City 14080, Mexico
S- Editor Yang XC L- Editor A E- Editor Yang XC