Published online Feb 10, 2012. doi: 10.5306/wjco.v3.i2.29
Revised: December 19, 2011
Accepted: February 6, 2012
Published online: February 10, 2012
The association of gastrointestinal malignancy with aplastic anemia has rarely been reported in the literature. Although it is not clear whether there is any direct relationship between aplastic anemia and gastrointestinal cancers, a retrospective analysis did suggest the notion that patients with aplastic anemia might have a higher incidence of colorectal cancer. Here, we report the diagnostic and therapeutic challenges in managing a patient with aplastic anemia and advanced colorectal cancer. Early diagnosis is challenging due to overlapping symptomatology and clinical features, increased risk of diagnostic procedures, and confounding complications arising from aplastic anemia and its treatment. A high index of suspicion and multidisciplinary input are essential.
- Citation: Wong H, Chan P, Yau T. Colon cancer in a patient with underlying aplastic anemia: A clinical challenge. World J Clin Oncol 2012; 3(2): 29-31
- URL: https://www.wjgnet.com/2218-4333/full/v3/i2/29.htm
- DOI: https://dx.doi.org/10.5306/wjco.v3.i2.29
The association of aplastic anemia with gastrointestinal malignancy has rarely been described in the literature, except in patients with Fanconi’s anemia. In fact, Fanconi’s anemia is a rare autosomal recessive disorder, consisting of aplastic anemia and genetic predisposition to cancers. In this congenital condition, the susceptibility to the development of cancer is accounted by the inherited chromosomal fragility and genetic defects in DNA repair mechanisms[1]. Although it is not clear whether there is any direct relationship between aplastic anemia and solid tumors, a retrospective analysis of 734 patients suggested that aplastic anemia patients did show a higher incidence of colorectal cancer (CRC)[2]. There is also evidence that solid cancers occur after treatment of aplastic anemia[3,4]. On the other hand, there has been interesting speculation that autoimmunity against hematopoietic stem cells which mediates acquired aplastic anemia, may actually be anti-neoplastic[5].
Here, we report the diagnostic and therapeutic challenges in managing a patient with aplastic anemia who subsequently developed advanced CRC.
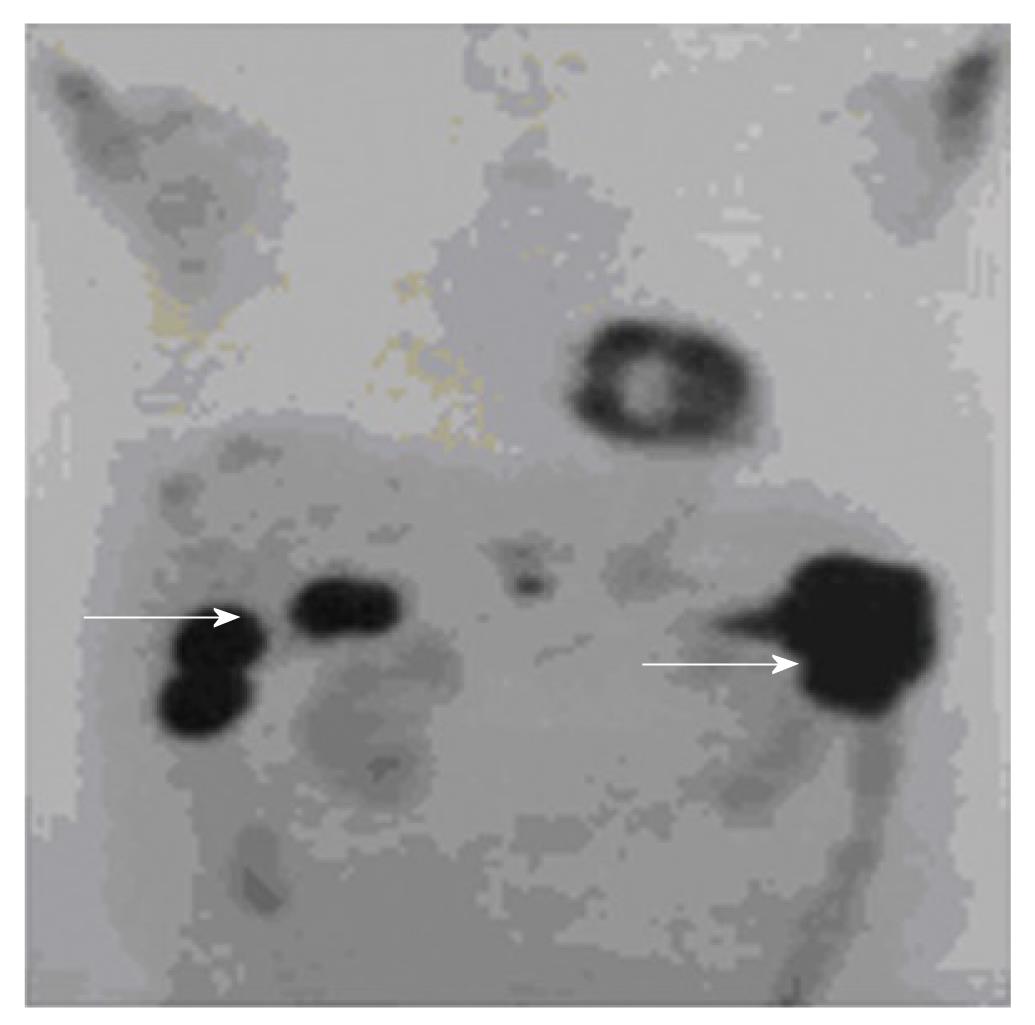
A 55-year-old photographer initially presented with the incidental finding of pancytopenia. He had otherwise good past health. The patient had prior workplace exposure to chemicals used in film development. Physical examination was unremarkable. White cell count (WCC) was 3.8 × 109/L (normal: 4 × 109/L-11 × 109/L) with a normal differential count, hemoglobin 89 g/L (normal: 135-175 g/L) and platelet count 40 × 109/L (normal: 150 × 109/L -400 × 109/L). Anemic workup, particularly vitamin B12 and folate levels, were normal. Reticulocyte count was 1%. Both Ham’s test and urine hemosiderin were negative. Finally, bone marrow examination showed hypocellular and hypoplastic marrow without increased blasts, dysplastic features or other abnormal cellular infiltration. Thus, the diagnosis of acquired aplastic anemia was made. The patient was started on cyclosporine 50 mg twice daily thereafter with stable blood counts, until two years later, when he was found to have reducedhemoglobulin level to 5.1g/dL necessitating repeated red cell transfusions. At that time, the total WCC was 2.7 × 109/L with neutropenia of 1.3 × 109/L, platelet count of 11 × 109, and reticulocyte count less than 1%. Repeated bone marrow examination showed particularly severe erythroid and megakaryocytic hypoplasia, while excluding hypoplastic leukemia andmyelodysplastic syndrome. He was thus diagnosed as having severe aplastic anemia (SAA) and was treated with a course of intravenous horse anti-thymocyte globulin at a dose of 2720 mg daily (40 mg/kg per day) for 4 d. He subsequently developed severe gastrointestinal bleeding. Colonoscopy was not performed at that juncture due to low platelet count. Moreover, the patient had repeated episodes of septicemia respectively caused by Escherichia coli, Enterococcus gallinarium and cytomegalovirus, which were treated with prolonged courses of broad-spectrum antibiotics including ceftazidime, ertapenem and meropenem, and foscarnet. Plain computed tomography (CT) of the abdomen and gallium scintigraphy were arranged to identify possible occult sources of recurrent bacteremia. A contrast CT scan was not performed due to impaired renal function. The plain CT images revealed four hypodense lesions in the liver. Gallium scintigraphy showed no corresponding uptake in the liver lesions but increased uptake at the proximal descending colon instead. Subsequent colonoscopy showed a fungating tumor at the proximal descending colon with biopsy confirmed adenocarcinoma. Positron emission tomography (PET) demonstrated a primary colon cancer with multiple liver metastases (Figure 1). Carcinoembryonic antigen was also grossly raised (460 ng/mL). He was referred to the medical oncologist for consideration of systemic therapy for metastatic CRC. Systemic chemotherapy was not recommended due to his underlying aplastic anemia and cytopenia. Single agent cetuximab was suggested but he could not afford the treatment. He was thus treated with best supportive care only and died of advanced CRC approximately 8 mo after the diagnosis.
Despite the speculated association between aplastic anemia and gastrointestinal malignancy as summarized in the Introduction, we cannot ascertain such a causal relationship between aplastic anemia and CRC in our patient. In fact, the co-existence of these two diseases may be coincidental as CRC is a commonly encountered malignancy nowadays with a rising incidence in most countries. In the presented patient, there could have been occupational exposure to benzene and its metabolite hydroquinone, which are present in radiographic developer solutions, and have been reported to be both toxic to the bone marrow and possibly carcinogenic[6,7]. Nonetheless, a few interesting diagnostic and therapeutic challenges in managing this case are worth highlighting.
First, colon cancer and aplastic anemia share many similar presenting symptoms and clinical features, leading to difficulties in the diagnosis of one condition when the other is present. Gastrointestinal bleeding is a well-known complication and presenting symptom in colorectal cancer[8], and also, albeit less commonly, in aplastic anemia. A recent study showed that up to 12% of patients with SAA developed overt gastrointestinal bleeding, especially lower gastrointestinal bleeding, due to neutropenicenterocolitis or solitary ulcer[9]. Chronic thrombocytopenia frequently present in bone marrow failure states, including aplastic anemia, can also be associated with active gastrointestinal bleeding[10]. Anemia is another common clinical feature encountered in both diseases. Notably, patients with colorectal cancer can present with anemia alone, related to iron deficiency and chronic blood loss[8], while anemia is disease-defining in aplastic anemia. Moreover, recurrent infections may occur in both CRC and aplastic anemia. Streptoccocusbovis septicemia is classically associated with colorectal cancer[11]; other organisms include Enterobacter[12] and Clostridium species[13]. Infections that are seen in aplastic anemia also include Streptoccocus and Clostridium species, in addition to Escherichia coli and others[14]. In the present case where an initial diagnosis of aplastic anemia had been established, new symptoms of gastrointestinal bleeding, worsening anemia and recurrent bacterial septicemia were thought to be related to the complications or progression of aplastic anemia, thus delaying investigations in search of other important causes including colorectal malignancy.
Second, there is a higher risk of performing invasive diagnostic procedures in patients with aplastic anemia. Neutropenia and thrombocytopenia increase rates of infection and bleeding with colonoscopy and biopsy. Due to abnormal renal function caused by a combination of sepsis and administration of renal-toxic drugs including cyclosporine and foscarnet, contrast-enhanced CT could not be performed to delineate the underlying pathology in order to avoid contrast nephropathy.At the time of reporting, the PET imaging modality was not readily accessible especially as the patient was financially challenged, although this could potentially serve as a less invasive diagnostic means in patients with limiting co-morbidities suspected of colon cancer.
Last but not least, the management of either aplastic anemia or colon cancer is highly dependent on the treatment response, toxicities and complications of the other condition among other factors, often presenting a clinical dilemma.In view of poor marrow tolerance with low blood counts and recurrent septicemia caused by prolonged neutropenia and the administration of immunosuppressive agents, systemic chemotherapy as treatment of metastatic CRC was decided against in the present case as there was a high probability of treatment-related morbidity and mortality. Unfortunately, the patient could not afford targeted therapy as he might potentially have benefited from it without a significant drop in blood counts.
Peer reviewer: Hang Xiao, PhD, Assistant Professor, De-partment Food Science, University of Massachusetts, 100 Holdsworth Way, Amherst, MA01003, United States
S- Editor Yang XC L- Editor Webster JR E- Editor Li JY
| 1. | Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006;25:5875-5884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Kishida T, Yonezawa M, Shibata Y, Tanaka S, Shinozawa I, Hoshino T, Tatsuguchi A, Feng L, Sato J, Fujimori S. Risk of colorectal cancer in patients with hematologic disease. J Gastroenterol Hepatol. 2000;15:1272-1276. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Socié G, Henry-Amar M, Bacigalupo A, Hows J, Tichelli A, Ljungman P, McCann SR, Frickhofen N, Van't Veer-Korthof E, Gluckman E. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. N Engl J Med. 1993;329:1152-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 312] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Witherspoon RP, Storb R, Pepe M, Longton G, Sullivan KM. Cumulative incidence of secondary solid malignant tumors in aplastic anemia patients given marrow grafts after conditioning with chemotherapy alone. Blood. 1992;79:289-291. [PubMed] [Cited in This Article: ] |
| 5. | Nissen C, Stern M. Acquired immune mediated aplastic anemia: is it antineoplastic? Autoimmun Rev. 2009;9:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Regev L, Wu M, Zlotolow R, Brautbar N. Hydroquinone, a benzene metabolite, and leukemia: A case report and review of the literature. Toxicol Ind Health. 2012;28:64-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | US Environmental Protection Agency. Technology transfer network air toxics website. Hydroquinone. Available from: http: //www.epa.gov/ttn/atw/hlthef/hydroqui.html. Last accessed: 6 Nov 2007. [Cited in This Article: ] |
| 8. | De Vita, Hellman , and Rosenberg’s Cancer: principles and practice of oncology; De Vita VT, Lawrence TS, Rosenberg SA (Editors). Colon Cancer. 8th Ed. Philadelphia: Lippincott Williams and Wilkins 2008; pp 1232-1284. [Cited in This Article: ] |
| 9. | Park YB, Lee JW, Cho BS, Min WS, Cheung DY, Kim JI, Cho SH, Park SH, Kim JK, Han SW. Incidence and etiology of overt gastrointestinal bleeding in adult patients with aplastic anemia. Dig Dis Sci. 2010;55:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Salacz ME, Lankiewicz MW, Weissman DE. Management of thrombocytopenia in bone marrow failure: a review. J Palliat Med. 2007;10:236-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Gupta A, Madani R, Mukhtar H. Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis. 2010;12:164-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Odeh B, Bareford D. Colonic carcinoma presenting as repeated episodes of enterobacter septicaemia during induction of remission in acute myeloblastic leukaemia. BMJ. 2009;339:b4027. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Mirza NN, McCloud JM, Cheetham MJ. Clostridium septicum sepsis and colorectal cancer - a reminder. World J Surg Oncol. 2009;7:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |