Published online Dec 10, 2012. doi: 10.5306/wjco.v3.i12.155
Revised: October 29, 2012
Accepted: November 11, 2012
Published online: December 10, 2012
We discuss an extremely rare case of ganglioneuroblastoma arising within a retroperitoneal mature cystic teratoma. Radiological examinations showed a cystic tumor sandwiched between the pancreas and left kidney. Surgery was scheduled because the tumor seemed to have originated from the pancreas. En-block resection of the tumor with distal pancreatectomy, splenectomy, and left adrenalectomy was performed. In terms of macroscopic appearance, the tumor mainly consisted of a unilocular cystic mass, but the presence of a smaller, solid mass was also noted within the tumor. Histopathologic examination confirmed that the cystic mass was consistent with a mature cystic teratoma of the retroperitoneum, and in addition, a ganglioneuroblastoma was evident in the solid component. Histopathologically, the ganglioneuroblastomatous area was intimately associated with dermoid tissue of the mature cystic teratoma, thus this case was diagnosed to be a mature cystic teratoma with malignant transformation. To best of our knowledge, this is the first reported case of ganglioneuroblastoma arising in a mature cystic teratoma.
- Citation: Hayama S, Ohmi M, Yonemori A, Yamabuki T, Inomata H, Nihei K, Hirano S. Ganglioneuroblastoma arising within a retroperitoneal mature cystic teratoma. World J Clin Oncol 2012; 3(12): 155-158
- URL: https://www.wjgnet.com/2218-4333/full/v3/i12/155.htm
- DOI: https://dx.doi.org/10.5306/wjco.v3.i12.155
Malignant transformation of mature cystic teratoma (MCT) is a rare complication occurring in approximately 1%-3% of patients with MCT[1]. Although any of the constituent tissues of a teratoma has the potential to undergo malignant transformation, squamous cell carcinoma is the most commonly associated malignancy[1]. Other reported malignancies arising in MCT include carcinoid tumor, adenocarcinoma, basal cell carcinoma, adenosquamous carcinoma, thyroid carcinoma, sebaceous carcinoma, malignant melanoma, sarcoma, and neuroectodermal tumor[1]. Ganglioneuroblastoma (GNB) is a primary malignant tumor with neuroendocrine differentiation, and is rarely seen in adults[2]. We treated a retroperitoneal MCT with ganglioneuroblastomatous transformation. To the best of our knowledge, this type of malignant transformation has never been reported in the international medical literature. We therefore present herein the clinicopathological findings of this extremely rare case.
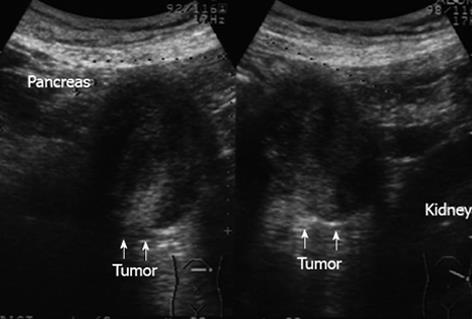
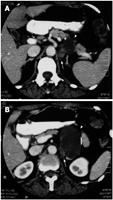
A 55-year-old woman with a history of chronic hepatitis C infection presented to another hospital complaining of abdominal pain, general fatigue, and weight loss. Periumbilical tenderness was elicited on abdominal palpation, and laboratory data revealed liver dysfunction. Abdominal ultrasonography showed a low-echoic mass between the pancreas and left kidney measuring 55 mm in its greatest diameter (Figure 1). A primary pancreatic tumor was suspected and the patient was admitted to our hospital for further investigation. Abdominal computed tomography (CT) revealed the cystic tumor to have a thin wall with calcification, and to be accompanied by a solid component (Figure 2). The tumor seemed to have originated from the pancreatic body and to be in contact with the left adrenal gland. The liver dysfunction was thought to be the result of hepatitis C and improved with conservative treatment. The serum concentrations of the tumor markers carcinoembryonic antigen, carbohydrate antigen 19-9, s-pancreas-1 antigen, and Duke pancreatic monoclonal antigen type 2 were within normal ranges. Endoscopic retrograde cholangiopancreatography demonstrated a normal main pancreatic duct which did not communicate with the cystic tumor. Celiac arteriography did not show any tumor staining, and major vessels such as the portal vein, superior mesenteric vessels, and splenic vessels were not invaded or encased. We strongly suspected a pancreatic neoplasm such as mucinous cyst neoplasm and therefore scheduled the patient for surgery.
At surgery, after the gastrocolic omentum was dissected away from the transverse colon, the tumor was palpated in the pancreatic body to tail. It seemed to be mainly situated in the posterior wall of the pancreas, and to have expanded into the retroperitoneal space, with involvement of the left adrenal gland. We therefore performed en-block R0 resection of the tumor with distal pancreatectomy, splenectomy, and left adrenalectomy.
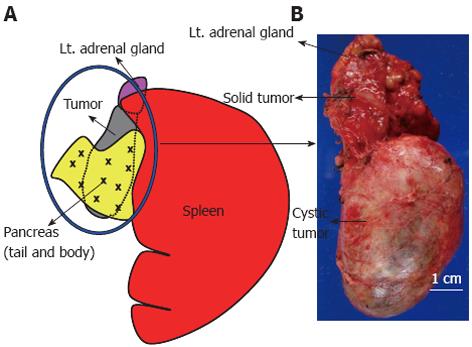
Although intraoperative findings suggested that the pancreatic tumor involved the left adrenal gland, macroscopic examination of the surgical specimen revealed that the tumor was, in fact, separated from the pancreas by a fibrous tumor membrane. The tumor was a unilocular mass with an intact smooth capsule and measured 10 cm × 4 cm in diameter. Examination of the cut surface of the tumor indicated that this unilocular cyst was filled with homogenous, grayish, creamy fluid and contained hair and cheesy sebaceous material. On further examination, part of the tumor consisted of a solid mass measuring 3 cm × 2.5 cm in diameter, which was in contact with the left adrenal gland (Figure 3A and B).
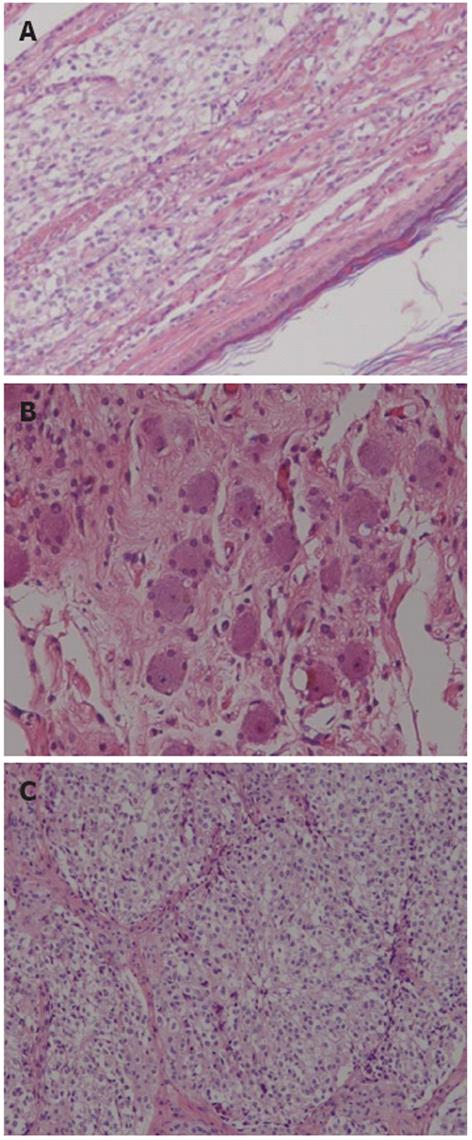
Microscopically, sections from areas of the cyst wall contained squamous epithelium, hair follicle, and sebaceous glands. No immature elements were identified. These findings were consistent with a mature cystic teratoma. The solid component noted macroscopically, however, was histologically diagnosed as ganglioneuroblastoma. Positive immunohistochemical staining in this solid area for S-100 protein, neurofilament protein, and synaptophysin also supported this diagnosis. The area of ganglioneuroblastoma was intimately associated with the mature cystic teratoma. Thus, the final diagnosis was ganglioneuroblastoma arising in retroperitoneal mature cystic teratoma. And en-block R0 resection of the tumor was obvious histopathologically (R0 resection indicates a microscopically margin-negative resection, in which no gross or microscopic tumor remains in the primary tumor bed[3]) (Figure 4).
The patient made an uneventful postoperative recovery and was discharged from hospital on the 19th postoperative day. She received neither adjuvant chemotherapy nor radiation therapy. Three years and ten months after the operation, she was in good health with no signs of recurrence.
Teratomas are uncommon neoplasms comprised of mixed dermal elements derived from the three germ cell layers. While the majority of teratomas are congenital and present in the ovaries of adolescent females and in the testes of young men, 1%-5% are found in extragonadal sites[4]. At week 4 of gestation, germ cells capable of differentiating to form tissue components of the three germ layers migrate down the fetal midline to reach the gonads. During this embryological migration, some of the cells can arrest and survive in an extragonadal location, mainly at mid-line sites[4], explaining why a few teratomas are found at these sites. The most frequent extragonadal site is the anterior mediastinum, followed by the retroperitoneum, sacrococcygeal, and intracranial regions[4]. Of the extragonadal teratomas, primary retroperitoneal teratomas are most commonly found in children and young adults[5], and the male to female ratio for this tumor is 1:2[4]. In many cases, retroperitoneal teratomas are metastases from the testes or ovaries, so it is important to distinguish true primary retroperitoneal teratomas from such metastatic lesions[5].
Teratomas are classified on the basis of their histopathologic findings and can be divided into two categories-mature and immature-which contain adult and embryonic type tissues, respectively[4]. Microscopically, MCTs are usually unilocular cysts containing tissue derived from all three germ cells, and may contain teeth, bone, and neural tissue[6]. The term teratoma with malignant transformation (TMT) refers to an existing MCT that gives rise to a malignant tumor; this is a rare event, occurring in approximately 1%-3% of all such lesions[1]. Primary retroperitoneal TMT is even rarer, with fewer than 20 cases reported in the literature[7]. Risk factors for malignancy in MCT include age over 45 years, tumor diameter greater than 10 cm, and rapid growth[6]. The most common malignancy has been reported as squamous cell carcinoma because many MCTs contain large amounts of squamous epithelium. However, although rare, other types of malignancies can occur within MCT: these include carcinoid tumor, adenocarcinoma, basal cell carcinoma, adenosquamous carcinoma, thyroid carcinoma, sebaceous carcinoma, malignant melanoma, sarcoma, and neuroectodermal tumor[1].
The preoperative diagnosis of retroperitoneal MCT may be aided by visualization of fluid, fat, soft tissue, and calcification in the tumor with both cystic and solid components on CT[7]. However, retroperitoneal MCT can be confused with various neoplasms as in the present case. Moreover, preoperative diagnosis of TMT is virtually impossible. Definitive diagnosis is made following surgical resection and histopathologic evaluation[5].
GNB is a primary malignant tumor of the sympathetic nervous system and generally considered a disease of early childhood that rarely occurs in adulthood[8]. GNB generally has a favorable prognosis, with no deaths from recurrence having been reported after complete resection. Nonetheless, this neoplasm is undoubtedly malignant, because most cases with distant metastasis die within 1 year[2]. This tumor, being of sympathetic-cell origin, can arise in various locations: in cervical, mediastinal, adrenal, and retroperitoneal locations in ascending order of frequency[8]. Similarly, GNB may arise when the sympathetic-cell component of MCT undergoes malignant transformation. In the present patient, the MCT most likely underwent malignant transformation toward the formation of GNB, although the pathogenic mechanism of TMT is not clearly understood. To the best of our knowledge, GNB arising within a MCT has never been reported. This unique case report suggests that sectioning of the entire MCT for histologic evaluation is indispensable to avoid missing a malignant tumor within the lesion. Because of the rarity of malignancies arising from MCT, there is no general agreement about the optimal treatment or prognostic factors of such tumors. In the present case, we assumed that, as for any GNB, complete excision would be associated with better survival.
In this case, the distinction between MCT with ganglioneuroblastomatous transformation versus collision tumors is of academic interest. Collision tumors occur when two different malignancies arise metachronously in the same organ. Although they are in contact at one point, for the most part the two lesions are separate from each other. In this reported case, however, the area of GNB was intimately associated with the MCT, which may rule out the diagnosis of collision tumor.
In conclusion, we reported an extremely rare case of ganglioneuroblastoma arising in a mature cystic teratoma. GNB without distant metastasis and for which radical resection is performed is reported to have a good prognosis. In the present case, we anticipated a favorable prognosis because complete resection was accomplished. However, because the nature of GNB arising within MCT remains unclear, further careful follow-up is warranted.
Peer reviewer: Piura Benjamin, Ben Gurion University Negev, Gynaecology Oncology Unit, Department of obstetrics and Gynaecology, Soroka Medical Centre, POB 151, IL-84101 Beer Sheva, Israel
S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Park JH, Whang SO, Song ES, Choi SJ, Lee WY. An ovarian mucinous cystadenocarcinoma arising from mature cystic teratoma with para-aortic lymph node metastasis: a case report. J Gynecol Oncol. 2008;19:275-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Yamanaka M, Saitoh F, Saitoh H, Nisimura S, Sawada Y, Tsukui A, Kaimori M, Takahashi N. Primary retroperitoneal ganglioneuroblastoma in an adult. Int J Urol. 2001;8:130-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Hermanek P, Wittekind C. Residual tumor (R) classification and prognosis. Semin Surg Oncol. 1994;10:12-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Scott AL, Abbassi-Ghadi N, Archer CM, Swamy R, Gupta S. Neuroendocrine carcinoma arising within a retroperitoneal mature teratoma. Ann R Coll Surg Engl. 2010;92:W5-W8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Gatcombe HG, Assikis V, Kooby D, Johnstone PA. Primary retroperitoneal teratomas: a review of the literature. J Surg Oncol. 2004;86:107-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Allam-Nandyala P, Bui MM, Caracciolo JT, Hakam A. Squamous cell carcinoma and osteosarcoma arising from a dermoid cyst--a case report and review of literature. Int J Clin Exp Pathol. 2010;3:313-318. [PubMed] [Cited in This Article: ] |
| 7. | Terado Y, Kurata A, Ishida T, Imamura T, Sakamoto A. Adenocarcinoma of small intestinal type in retroperitoneal mature teratoma. Pathol Int. 2010;60:701-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Peycru T, Guiramand J, Tardat E, Savoie PH, Avaro JP, Balandraud P. Nodular ganglioneuroblastoma in adults. Can J Surg. 2009;52:E111-E113. [PubMed] [Cited in This Article: ] |