Published online Feb 10, 2011. doi: 10.5306/wjco.v2.i2.125
Revised: September 26, 2010
Accepted: October 2, 2010
Published online: February 10, 2011
The results of clinical trials conducted in Europe and North America have been incorporated into treatment strategies for breast cancer in Japan. Despite the use of similar treatment regimens, why has mortality from breast cancer been increasing in Japan? Procedures for surgical treatment and sentinel lymph node biopsy in breast cancer do not differ between Japan and Western countries, but the strategies for radiotherapy differ slightly. Hormonal therapy is now selected on the basis of scientific evidence, and similar regimens are used in Japan and Western countries. As for postoperative adjuvant chemotherapy, an anthracycline plus cyclophosphamide and taxane-based regimens are standard treatments in Japan and Western countries. In 2009, however, the results of two large clinical studies designed to determine whether intravenous or oral treatment was superior for postoperative adjuvant chemotherapy were reported in Japan. Both studies showed that relapse-free survival and overall survival (OS) at 5 years after surgery were similar for a combination of cyclophosphamide, methotrexate, and 5-fluorouracil and for tegafur/uracil. Many chemotherapeutic agents that are used to treat recurrent or metastatic breast cancer have not yet been approved in Japan. As for molecular targeted therapy, some agents that target the human epidermal growth factor receptor family have been approved in Japan, whereas angiogenesis inhibitors have not. The results of many clinical trials have been incorporated into clinical practice in Japan, therefore, the outcomes of breast cancer therapy have surpassed those in other countries. Many pivotal clinical trials have been conducted outside Japan. Treatment regimens that have been developed on the basis of these studies might be suitable for the management of breast cancer in Western women, but not for Japanese women because of differences in genetic factors, physique, body mass index, pharmacokinetics, and drug metabolism. Such regimens should be modified on the basis of the characteristics of breast cancer in Japan to develop treatment that is optimally suited for Japanese women. In particular, local studies of pharmacokinetics, pharmacodynamics, and optimal dose levels and treatment intervals should be carefully performed. The establishment of treatment regimens optimally suited for Japanese patients with breast cancer could put the brakes on the trend towards increasing mortality from breast cancer in Japan.
- Citation: Park Y, Kitahara T, Takagi R, Kato R. Current status of therapy for breast cancer worldwide and in Japan. World J Clin Oncol 2011; 2(2): 125-134
- URL: https://www.wjgnet.com/2218-4333/full/v2/i2/125.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i2.125
Breast-cancer-related morbidity and mortality are lower in Japan than in Western countries. The 10-year survival rate after breast-conservation therapy is 85%-95% in Japan, as compared with only 60%-80% in Western countries. Since 1990, however, mortality from breast cancer has shown a decreasing tendency in the United States, but has continued to increase in Japan. The results of clinical trials conducted in Europe and North America have been incorporated into treatment strategies for breast cancer in Japan. Despite the use of similar treatment regimens, why has mortality from breast cancer been increasing in Japan?
In this review, we focus on differences in the current status of treatment for breast cancer between Japan and the rest of the world, particularly Europe and North America, and take into account recent trends in mortality and outcomes.
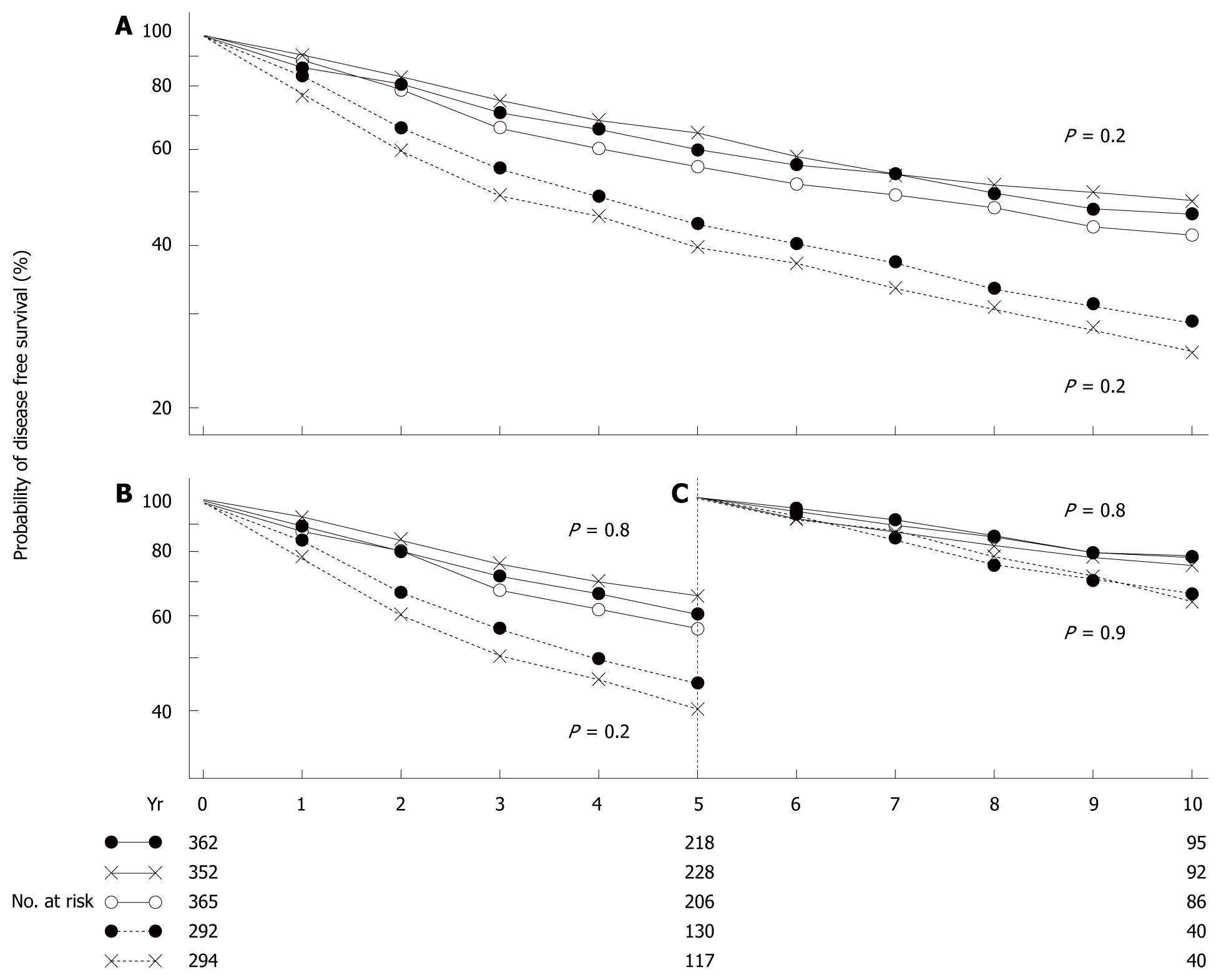
As for the evolution of surgical procedures for breast cancer in Western countries, since the beginning of the 20th century, radical mastectomy with removal of the pectoral muscles (Halstead mastectomy) has been performed as a standard procedure for about 70 years. Subsequently, the results of large clinical trials conducted during the latter half of the 1970s, such as the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-06 study (Figure 1)[1], have led to the widespread use of breast-conserving surgery. In 1989, the National Cancer Institute (NCI) in the United States positioned breast-conserving surgery as the standard procedure for early breast cancer.
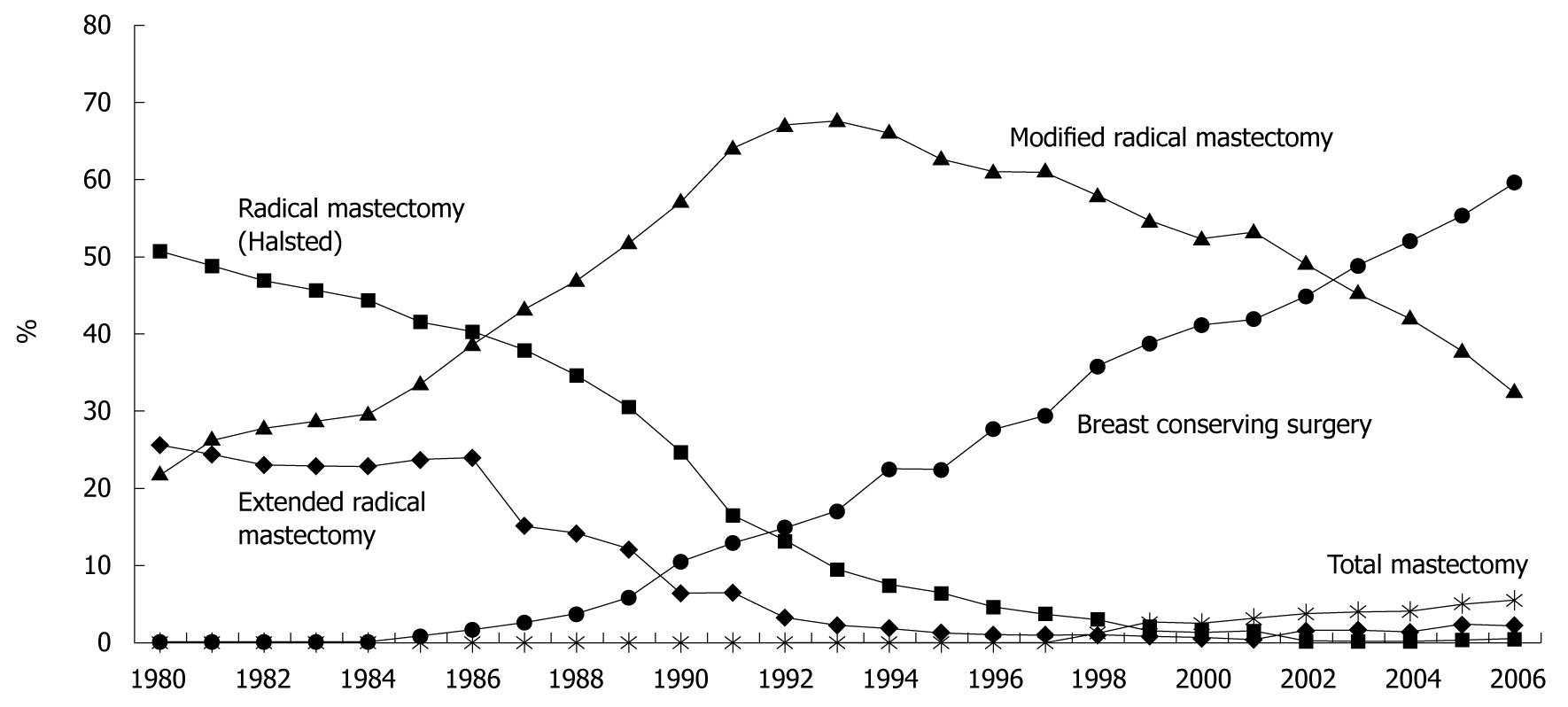
As for changes in surgical procedures for breast cancer in Japan[2], the standard procedure was radical mastectomy (Halsted mastectomy) in the 1980s and modified radical mastectomy (pectoral muscle-preserving mastectomy) from the latter half of the 1980s. Subsequently, breast-conserving surgery was introduced around 1986 and has been the most widely used procedure since 2003 (Figure 2).
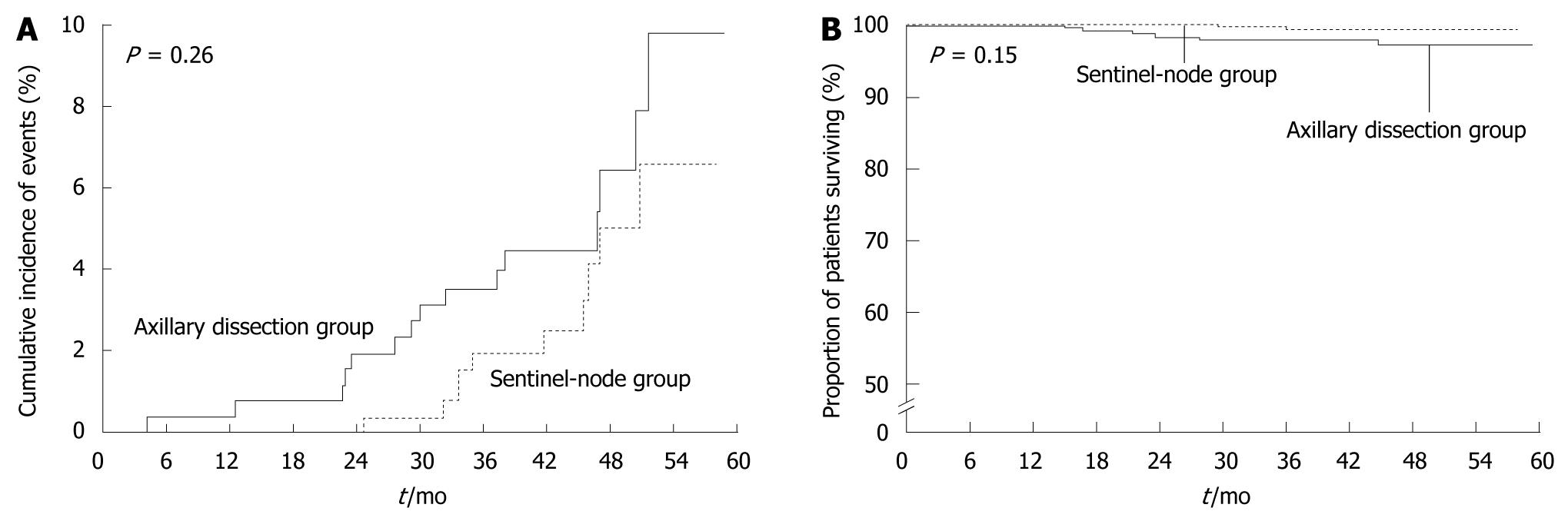
With the advent of sentinel node biopsy, the need for axillary node dissection has been questioned. A randomized controlled study that has compared sentinel node biopsy with routine axillary dissection in breast cancer[3] has shown no significant difference in the rates of axillary recurrence or survival between the biopsy and axillary dissection groups; at least in the short term. Sentinel node biopsy was approved and has been used as a standard examination in Europe and North America since about 2005 (Figure 3). In Japan, clinical trials of sentinel node biopsy have been delayed, and the procedure was not approved until April 2010.
At present, there appear to be no major differences in surgical therapy or sentinel node biopsy between Western countries and Japan.
Breast-conservation therapy includes breast-conserving surgery and postoperative radiotherapy. Radiotherapy after breast-conserving surgery has an important role in the radical treatment of very small, residual breast cancer nests in the remaining breast tissue.
Owing to the relatively few radiologists and radiotherapy hospitals in Japan, some patients could not receive breast irradiation previously. At present, however, breast irradiation is recommended after breast-conserving surgery. In 2006, 82% of patients received radiotherapy after breast-conserving surgery in Japan; similar to the proportion in Western countries.
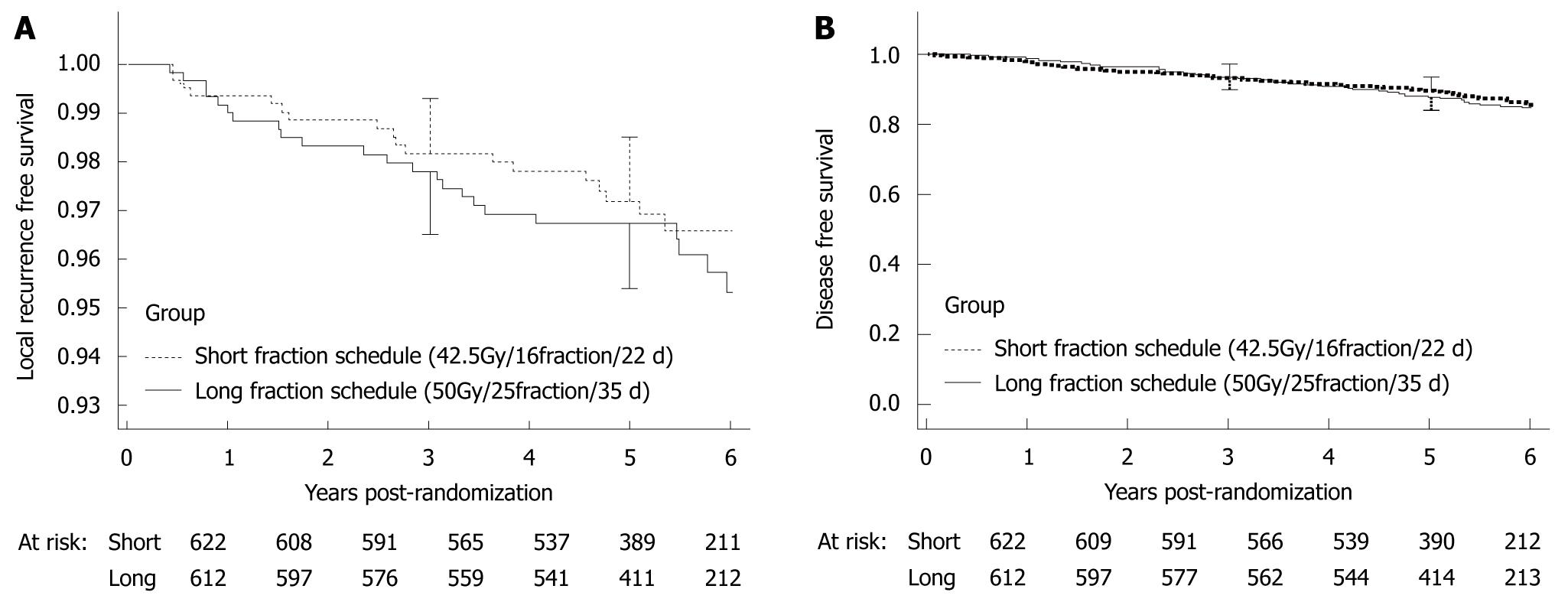
In Canada and the United Kingdom, treatment regimens that require fewer sessions of radiotherapy have been examined to enhance patients’ convenience. In a randomized controlled study that has compared long-term irradiation (42.5 Gy, 16 fractions, 22 d) with short-term irradiation (50 Gy, 25 fractions, 35 d) in Canada[4], the 5-year rates of local recurrence and disease free survival (DFS) did not differ significantly between the groups. Therefore, hypo-fractionated radiotherapy that uses a lower total dose of radiation given in larger fractions is now used (Figure 4).
As an alternative to whole breast irradiation, accelerated partial breast irradiation, in which short-term intensive irradiation is delivered only to the tumor bed intraoperatively, or by small radiation source therapy, has been introduced into clinical practice in Europe and North America, but not in Japan.
At present, procedures for radiotherapy differ slightly between Western countries and Japan.
Several differences exist between women with breast cancer in Japan and Western countries. In Japan, premenopausal breast cancer is more common, whereas in Western countries postmenopausal breast cancer is predominant. Japanese women with breast cancer have a better prognosis than Western women with breast cancer. Differences between Japanese and Western women for various other factors, such as postmenopausal blood estrogen concentrations and body mass index, have also been reported. The optimal dose of hormonal therapy might thus be influenced by racial and other genetic factors.
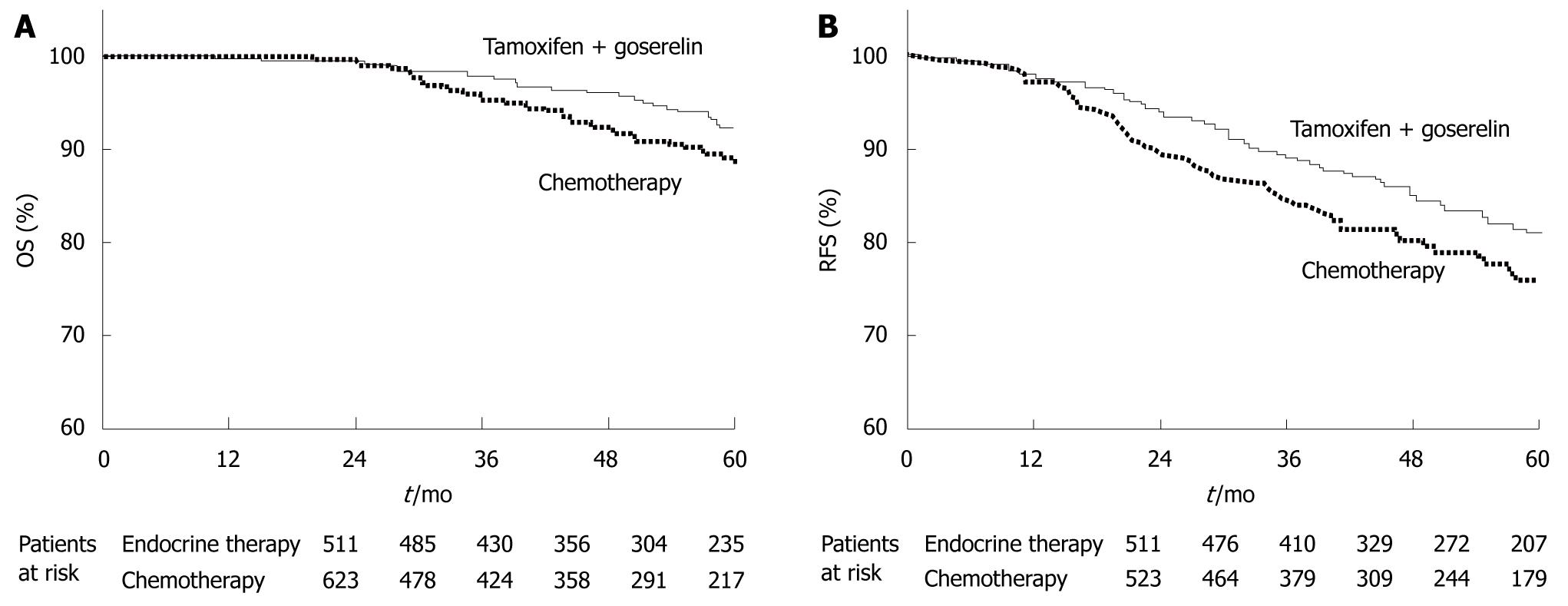
As for postoperative adjuvant hormonal therapy for premenopausal beast cancer, the Austrian Breast and Colorectal Cancer Study Group Trial 5 (ABCSG-05)[5] has reported that tamoxifen plus a luteinizing hormone-releasing hormone agonist is at least equivalent to cyclophosphamide, methotrexate, and 5-fluorouracil (CMF). This regimen for hormonal therapy has thus become a standard therapy in Japan and Western countries (Figure 5).
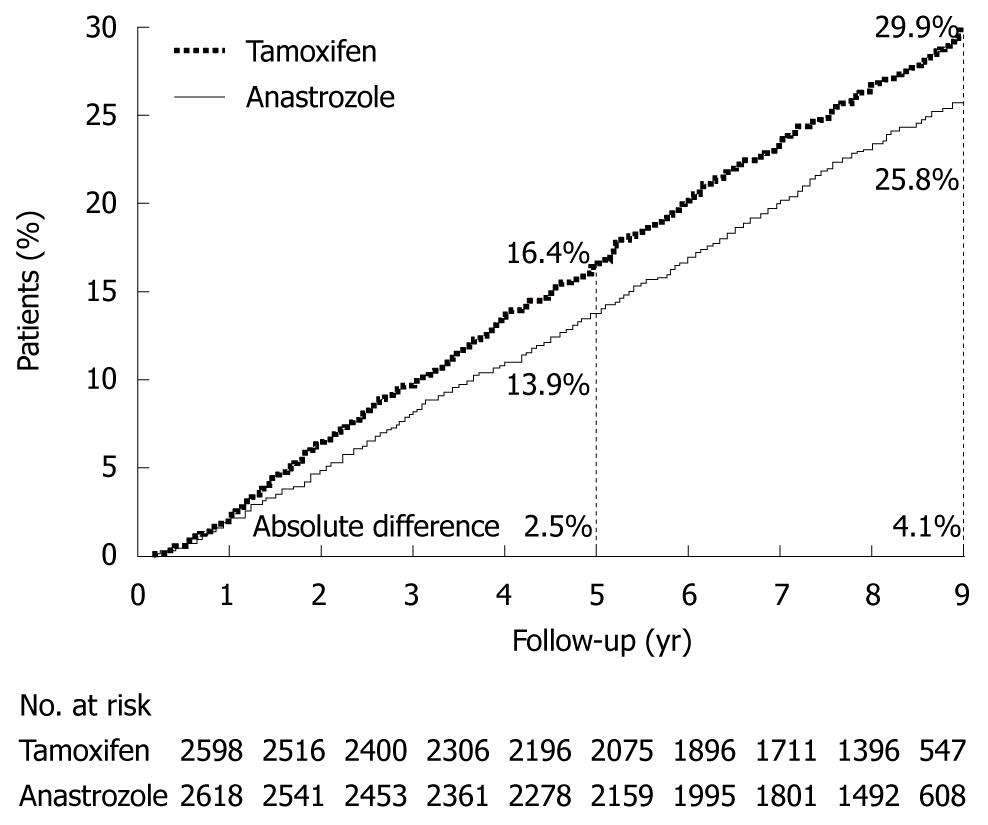
The effect of postoperative adjuvant hormonal therapy has been evaluated in postmenopausal breast cancer in the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial[6]. An aromatase inhibitor has been reported to improve significantly DFS and other variables as compared with tamoxifen, which was previously considered to be the gold standard for hormonal therapy. Consequently, aromatase inhibitors have become standard treatment in both Japan and Western countries (Figure 6).
Currently used hormonal therapy is thus based on scientific evidence, and similar regimens are used in Japan and Western countries. However, many clinical trials of hormonal therapy are ongoing in Western countries. The results of these trials could lead to revisions of current regimens for standard treatment, mainly in Western countries, in the future.
Intravenous regimens such as CMF were initially used for postoperative adjuvant chemotherapy. At present, a combination of an anthracycline and cyclophosphamide and taxane-based regimens are regarded as standard treatment in Japan and Western countries. In Japan, highly toxic intravenous chemotherapy has not become popular. Instead, oral chemotherapy with drugs such as tegafur/uracil (UFT) has been widely used because of its ease of administration.
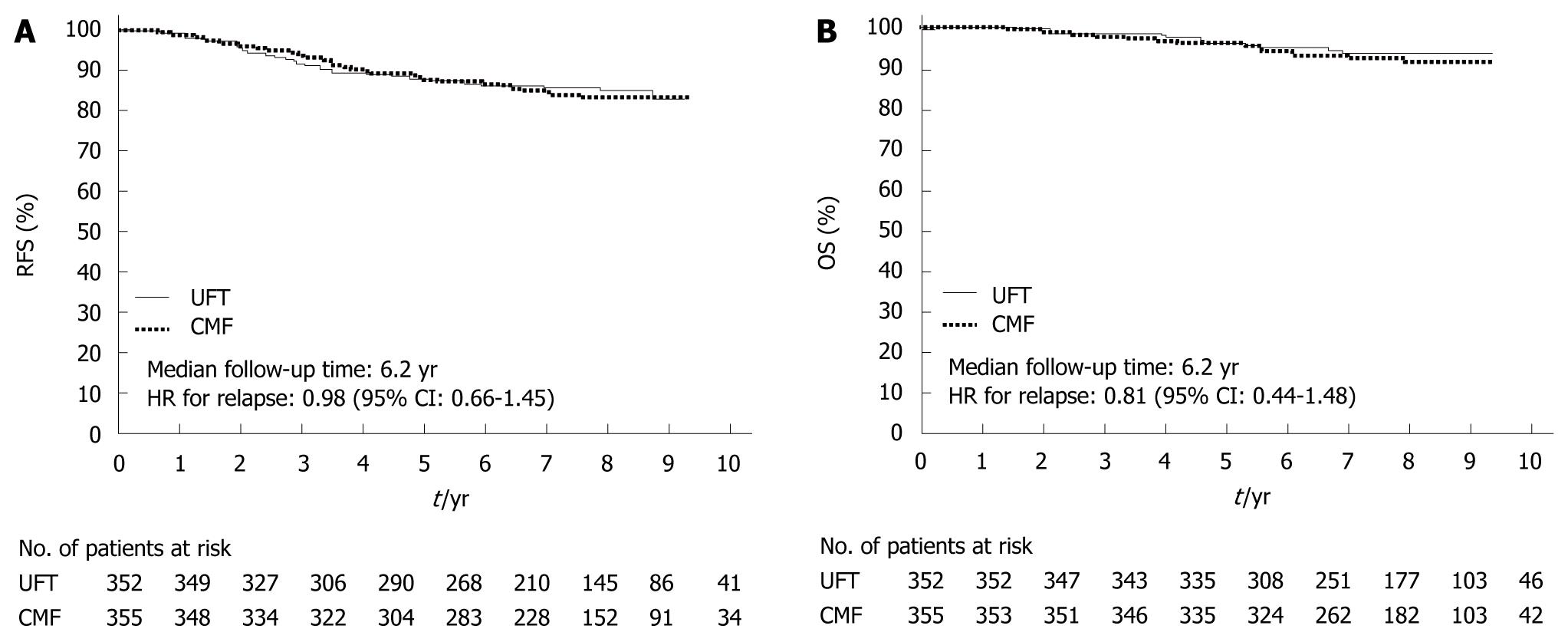
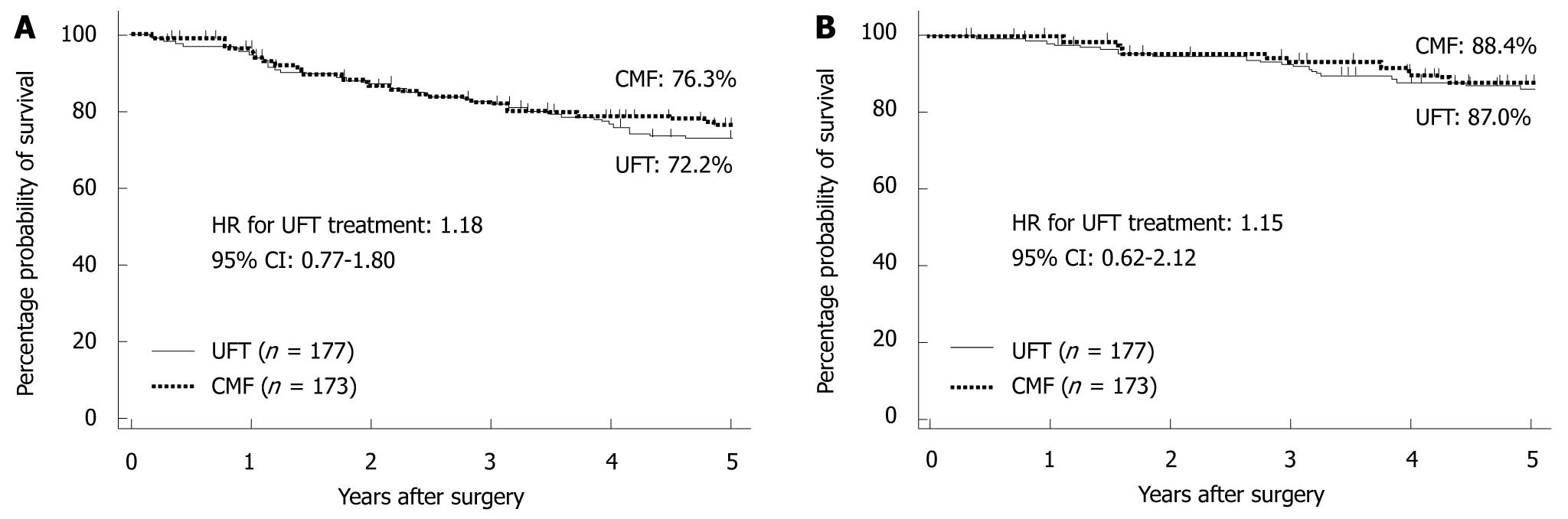
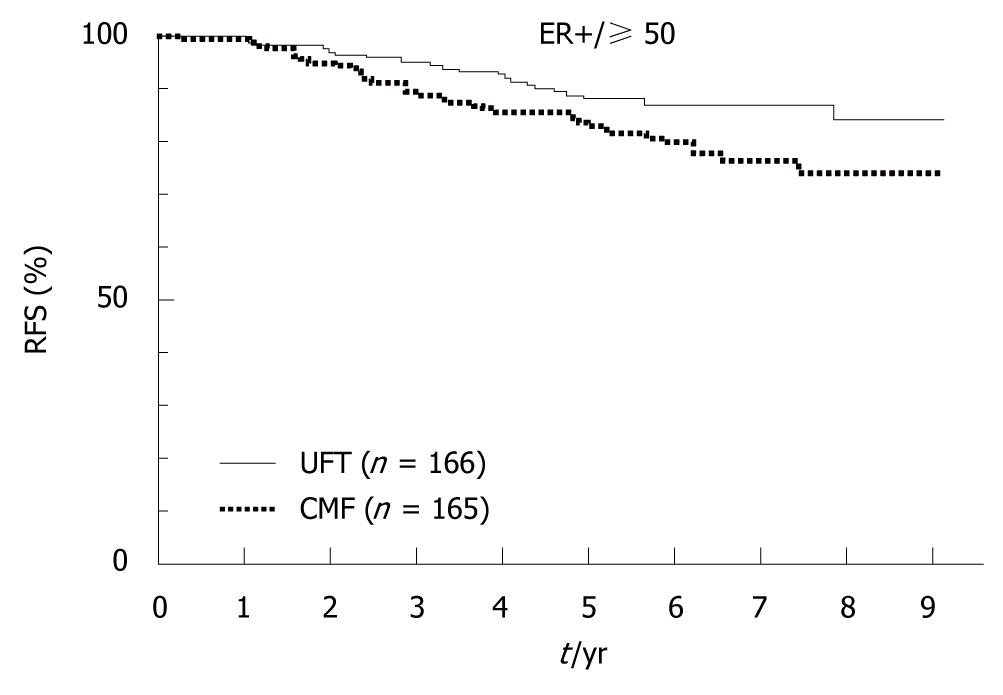
In 2009, the results of the National Surgical Adjuvant Study for Breast Cancer 01 Trial (NSAS-BC01)[7] and the Comparative Trial of UFT + Tamoxifen with CMF + Tamoxifen in Adjuvant Therapy for Breast Cancer (CUBC)[8] - two recent Japanese clinical trials designed to establish whether intravenous or oral treatment was superior for postoperative adjuvant chemotherapy - were reported. The NSAS-BC01 trial compared CMF with UFT in high-risk patients with axillary node negative breast cancer. The CUBC trial compared CMF plus tamoxifen with UFT plus tamoxifen in patients with axillary lymph node positive breast cancer. In both studies, relapse-free survival (RFS) and OS at 5 years after surgery were similar in the CMF and UFT groups (Figures 7 and 8). The CUBC trial showed a trend towards better RFS in the UFT group than in the CMF group among women whose tumors were estrogen receptor (ER) and progesterone receptor positive[8]. A pooled analysis of the CUBC trial and NSAS-BC 01 trial[9] has shown that UFT was associated with better RFS in patients with ER-positive tumors who were aged ≥ 50 years (Figure 9). Previous studies have suggested that chemotherapy is less effective for patients with ER-positive breast cancer. It is particularly interesting that UFT is clinically effective in patients who are elderly or have ER-positive breast cancer, because these subgroups have been suggested to have a poor response to chemotherapy on the basis of previous clinical trials.
In Japan, many chemotherapeutic agents for recurrent or metastatic breast cancer have yet to be approved. In 2010, the National Comprehensive Cancer Network guidelines, version 2[10] recommended “optimal chemotherapeutic regimens for recurrent or metastatic breast cancer”. Unapproved drugs in Japan include pegylated liposomal doxorubicin, albumin-bound paclitaxel, ixabepilone, and carboplatin.
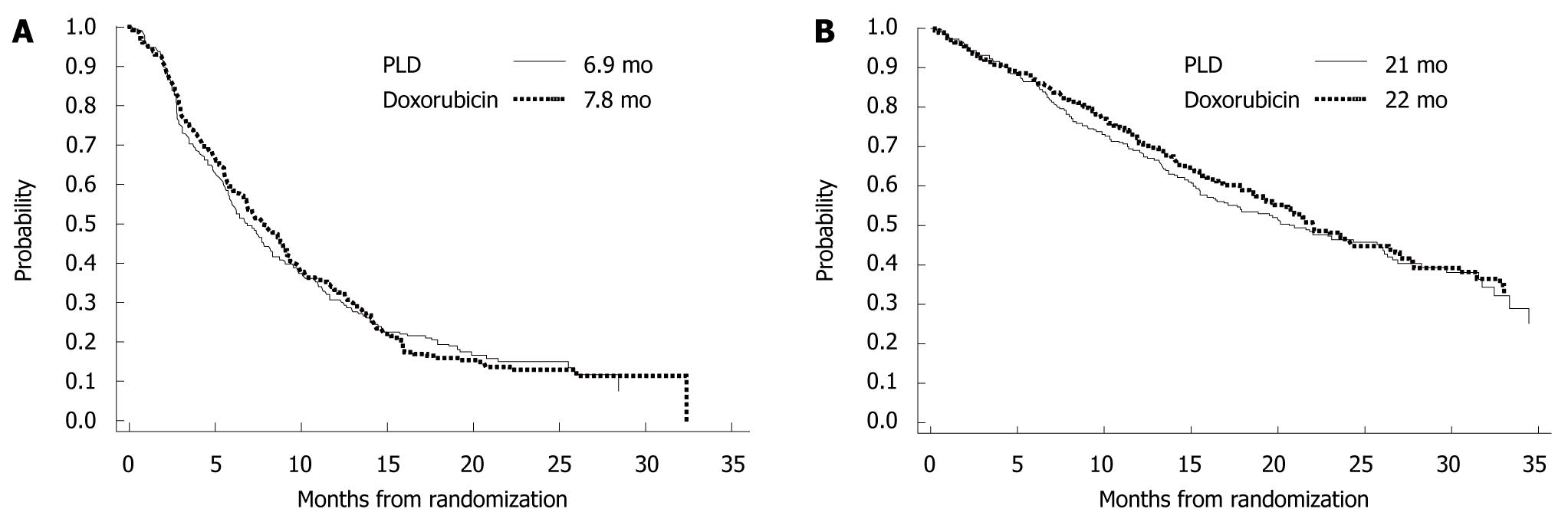
Pegylated-liposomal doxorubicin takes advantage of a unique drug delivery system in which doxorubicin is enclosed in liposomes coated with polyethylene glycol. With this preparation, high concentrations of liposomes are transferred to tumor tissue and gradually release active doxorubicin. Consequently, high concentrations of doxorubicin are retained in tumor tissue for a prolonged period, while free doxorubicin concentrations in plasma are decreased, which potentially reduces adverse events. In a phase III trial that has compared this preparation with conventional doxorubicin for first-line treatment of metastatic breast cancer[11], progression-free survival (PFS) and OS were similar in both treatment groups, whereas pegylated-liposomal doxorubicin was associated with reduced adverse events (e.g. cardiotoxicity, hair loss, nausea and vomiting, and neutropenia) (Figure 10).
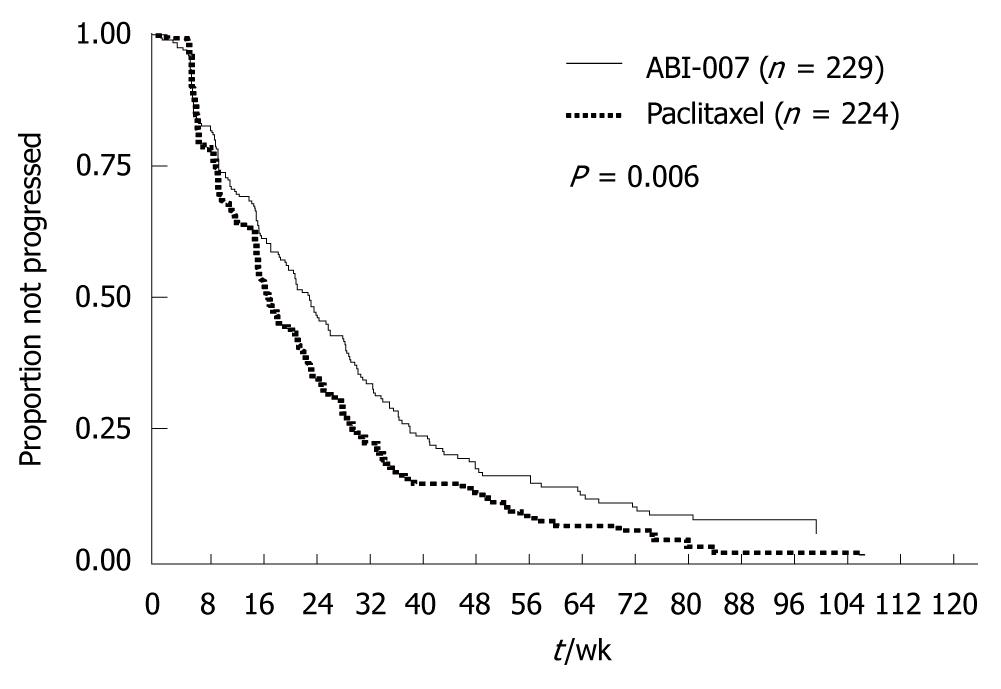
Albumin-bound paclitaxel is a water-soluble formulation of paclitaxel that is prepared by binding the drug to albumin. The need for using solvents such as Cremophor® EL and ethanol has thus been eliminated. A phase III trial has compared this new preparation with conventional paclitaxel in women with metastatic breast cancer[12], and has shown that albumin-bound paclitaxel provided a significantly higher response rate (RR) (33% vs 19%, P < 0.001) and a significantly longer time to progression (TTP) (23.0 wk vs 16.9 wk, HR = 0.75, P < 0.006) (Table 1 and Figure 11). Albumin-bound paclitaxel was approved in Japan in July 2010, and is expected to improve outcomes in clinical practice substantially. However, it remains unclear when other new chemotherapeutic agents will be approved in Japan.
| Response | No. of patients/total No. ofpatients (%) | P | |
| ABI-007 (260 mg/m2) | Paclitaxel (175 mg/m2) | ||
| Complete and partial response | |||
| All patients | 76/229 (33) | 42/225 (19) | 0.001 |
| First-line therapy | 41/97 (42) | 24/89 (27) | 0.029 |
| Second-line or greater therapy | 35/132 (27) | 18/136 (13) | 0.006 |
| Prior anthracycline therapy | |||
| Adjuvant and/or metastatic | 60/176 (34) | 32/175 (18) | 0.002 |
| Metastatic only | 31/115 (27) | 18/130 (14) | 0.010 |
| Dominant metastatic organ site | |||
| Visceral | 59/176 (34) | 34/182 (19) | 0.002 |
| Non-visceral | 17/50 (34) | 8/43 (19) | NS |
| Age (yr) | |||
| < 65 | 68/199 (34) | 36/193 (19) | < 0.001 |
| ≥ 65 | 8/30 (27) | 6/32 (19) | NS |
| Target | Drug name |
| HER2 | Trastuzumab |
| HER1/2 | Lapatinib |
| HER2 (dimerization domain) (Pertuzumab inhibits the dimerization of the HER2 with other HER family receptors) | Pertuzumab |
| HER1/2/4 | Neratinib |
| VEGF | Bevacizumab |
| Multi target (VEGFR, PDGFR etc) | Axitinib, sunitinib, pazopanib |
Molecular targeted agents currently under development are summarized according to their target receptors in Table 2.
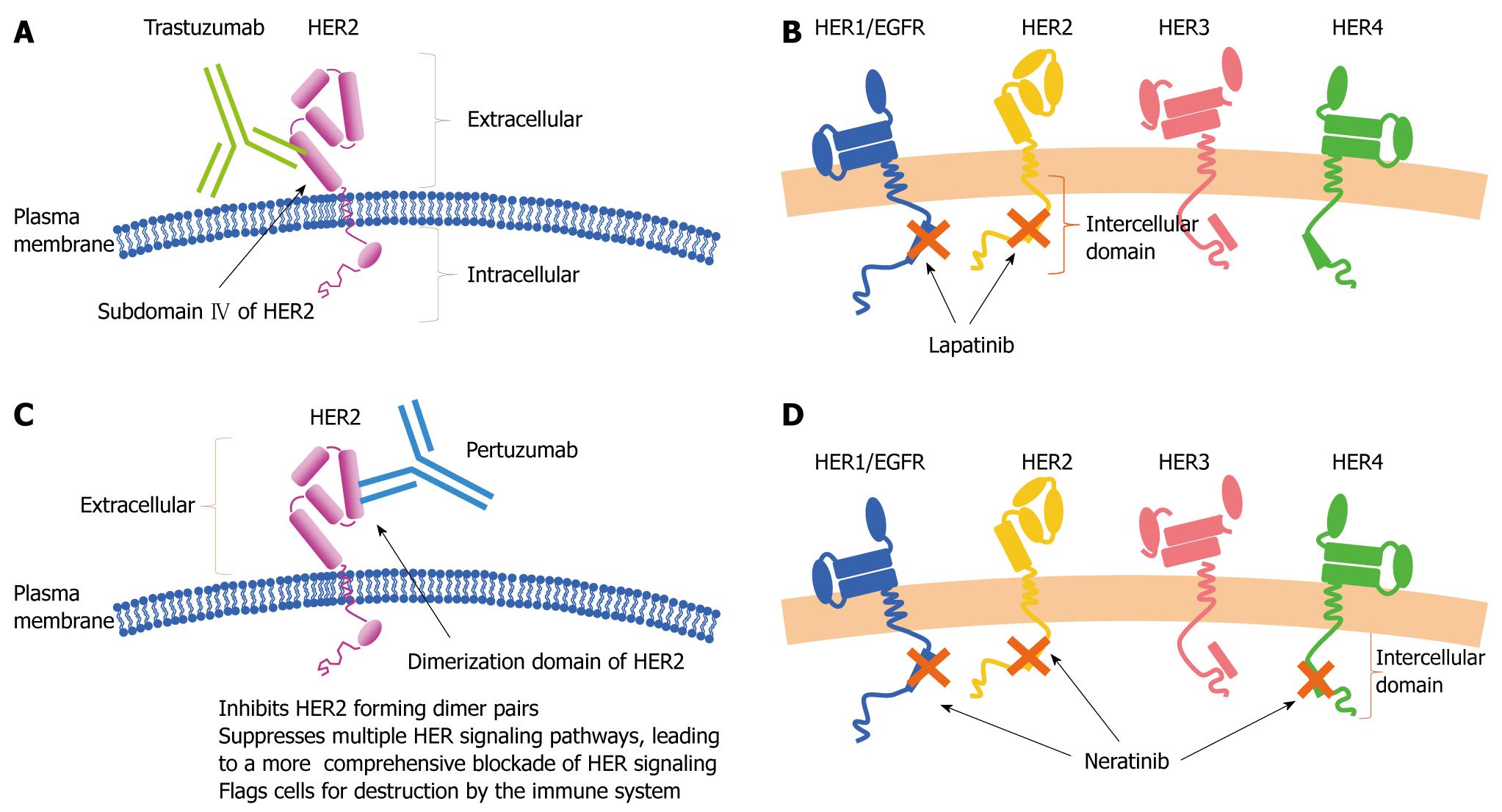
Drugs targeting the human epidermal growth factor receptor (HER) family: In molecular targeted therapy, the development of drugs that target the HER family (HER1/2/3/4) is most advanced. Agents that target the HER family can be broadly divided into two groups: antibodies that bind to extracellular receptors and low-molecular-weight compounds that bind to intracellular domains.
Trastuzumab is an antibody preparation that binds to extracellular domains of HER2 (Figure 12A). In 1998, trastuzumab was approved in the United States for the indication of metastatic breast cancer with HER2 overexpression. Subsequently, several large clinical trials of postoperative adjuvant therapy with trastuzumab plus chemotherapy[13-15] have been conducted (Table 3). These trials consistently have reported that postoperative adjuvant therapy with trastuzumab plus chemotherapy decreases the rates of recurrence and mortality. Therefore, trastuzumab combined with chemotherapy has become standard treatment for HER-2-positive breast cancer in Western countries. In Japan, trastuzumab was approved for the indication of metastatic breast cancer with HER-2 overexpression in 2001. In 2008, trastuzumab was approved as postoperative adjuvant chemotherapy for breast cancer with confirmed HER-2 overexpression.
| Trial | Regimen | DFS | OS |
| HERA[11] | CT alone | 36% events reduction, HR = 0.64, | 2HR = 0.66, P = 0.0115 at 2 yr |
| CT + H 1 yr | P < 0.0001 at 2 yr | ||
| NSABP B-31 and | AC × 4 → P × 4 | 52% events reduction, HR = 0.48, | 33% mortality reduction; |
| NCCTG N-9831[12] | AC × 4 → P × 4 + H1 | P < 0.0001 at 2 yr | HR = 0.66, P = 0.015 at 2 yr |
| BCIRG 006[13] | AC × 4 → T × 4 | HR = 0.61, P < 0.00013 | HR = 0.59, P < 0.0043 |
| AC × 4 → T × 4 + H2 | HR = 0.67, P = 0.00033 | HR = 0.66, P = 0.00173 | |
| T + Carbo × 6 + H2 | At 3 yr | At 3 yr |
Lapatinib is an oral low-molecular-weight compound that reversibly binds to ATP-binding sites of HER1 and HER2 tyrosine kinase in the cytoplasm (Figure 12B). In a phase II trial of lapatinib alone conducted in Japan[16], the RR was 24% in patients with breast cancer that was resistant to anthracycline, taxanes and trastuzumab. In a phase III study of lapatinib plus capecitabine versus capecitabine alone in patients with HER2-positive advanced or metastatic breast cancer[17], lapatinib improved the RR (22% vs 14%) and prolonged the TTP (8.4 mo vs 4.4 mo, P < 0.001). In contrast to the antibody trastuzumab, lapatinib can penetrate the blood-brain barrier because it is a low-molecular-weight compound[18]. Lapatinib is therefore expected to be effective in patients with brain metastases.
Pertuzumab and neratinib have not been approved in Japan. Pertuzumab, a monoclonal antibody, acts by binding to the dimerization domain of HER2 and inhibits the dimerization of HER2 with HER1/2/3/4 (Figure 12C). In a study of 33 patients with metastatic breast cancer who had progressive disease while receiving trastuzumab, pertuzumab plus trastuzumab had a complete response rate of 3% and a partial response rate of 18%[19].
Neratinib is an oral low-molecular-weight compound that irreversibly blocks intracellular ATP-binding sites of HER1/2/4, thereby suppressing signaling pathways (Figure 12D). In a phase II trial of neratinib monotherapy in patients with HER2-positive advanced or recurrent breast cancer[20], the PFS rate and RR after 16 wk of treatment were 60% and 26%, respectively, in patients who had previously received trastuzumab, and 77% and 51% in those who had not received trastuzumab. In Japan, a phase III trial of neratinib alone versus lapatinib plus capecitabine is ongoing in patients with HER2-positive locally advanced or metastatic breast cancer.
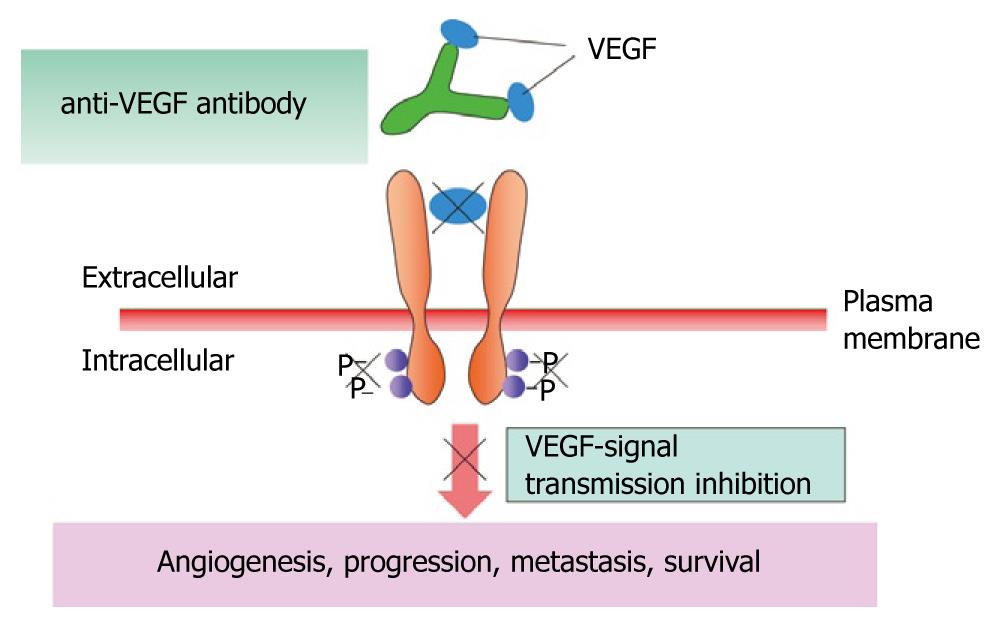
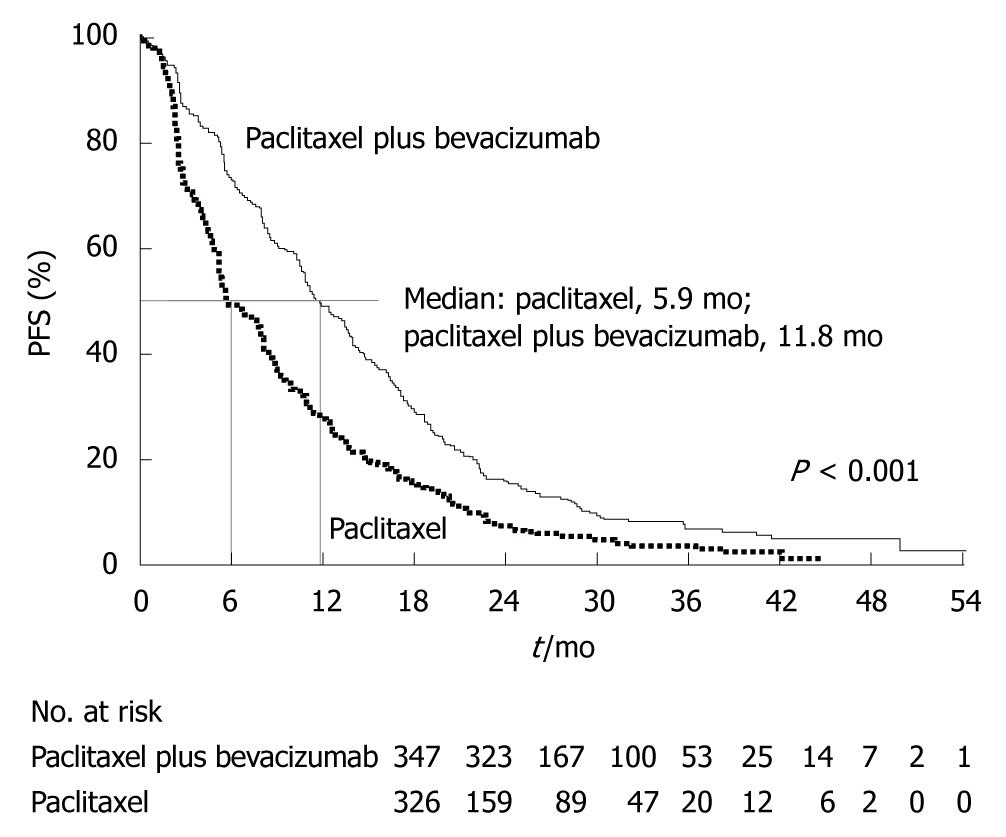
Angiogenesis inhibitors for breast cancer: A number of angiogenesis inhibitors have been developed for the treatment of breast cancer (bevacizumab, axitinib, sunitinib, and pazopanib), but none has been approved in Japan. Bevacizumab is a humanized monoclonal antibody that binds to vascular endothelial cell growth factor (VEGF) ligands (Figure 13). In the Eastern Cooperative Oncology Group (ECOG) 2100 phase III trial of paclitaxel alone versus paclitaxel plus bevacizumab in 722 patients with metastatic breast cancer[21], the primary endpoint of PFS was significantly better in the paclitaxel plus bevacizumab group (Figure 14). For the treatment of metastatic breast cancer, bevacizumab in combination with paclitaxel or docetaxel was approved by the European Commission in March 2007, and bevacizumab in combination with paclitaxel was approved by the FDA in February 2008.
Axitinib, sunitinib and pazopanib are oral low-molecular-weight tyrosine kinase inhibitors that target several molecules, such as VEGF receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR). A randomized controlled study has shown that docetaxel plus axitinib provides a significantly better RR and TTP than docetaxel plus placebo in patients with metastatic breast cancer[22]. A phase II study has reported that sunitinib monotherapy had an RR of 11% (n = 64) in patients with breast cancer that had been previously treated with an anthracycline and a taxane[23]. In a randomized controlled study that has compared lapatinib alone with lapatinib plus pazopanib in previously untreated patients with HER-positive advanced breast cancer[24], the TTP at 12 mo was slightly better in the combined treatment group. These drugs have not yet been approved even in the United States, but approval is expected to be granted as soon as good results are obtained.
Other molecular targeted drugs: Poly (ADP-ribose) polymerase-1 (PARP-1) plays an important role in the repair of DNA single-strand breaks caused by factors such as alkylation, X-ray irradiation, and active oxygen. Inhibition of PARP-1 blocks DNA repair, and induces genomic instability, cell cycle arrest, and cell death. PARP-1 inhibitors (BCI-201 and AZD 2281) are currently under development. In a randomized phase II clinical trial that has compared gemcitabine plus carboplatin (G/C) with BCI-201 plus G/C in patients with metastatic triple-negative breast cancer[25], RRs were 16% and 48% and clinical benefit rates were 21% and 62% in the G/C group and the BCI-201 + G/C group, respectively. These results show that BCI-201 substantially improves outcomes.
The results of many clinical trials have been incorporated into treatment strategies in Japan, therefore, the outcomes of breast cancer now surpass those of many other countries. In particular, remarkable progress has been made in the development of molecular targeted therapy. The future clinical introduction of currently unapproved molecular targeted agents is eagerly awaited in Japan. However, racial differences associated with genetic polymorphisms should be carefully evaluated before introducing these preparations to ensure the safety of Japanese patients.
Many pivotal clinical trials have been conducted outside Japan. Treatment regimens developed on the basis of these studies might be suitable for the management of breast cancer in Western women, but not for Japanese women because of differences in genetic factors, physique, body mass index, pharmacokinetics, and drug metabolism. Such regimens should be modified on the basis of the characteristics of breast cancer in Japan, to develop treatment that is optimally suited for Japanese women. In particular, local studies of pharmacokinetics, pharmacodynamics, and optimal dose levels and treatment intervals should be carefully performed. The establishment of treatment regimens that are optimally suited for Japanese patients with breast cancer could put the brakes on the trend towards increasing mortality from breast cancer in Japan.
Peer reviewers: Chao-Cheng Huang, MD, Associate Professor, Department of Pathology, Chang Gung Memorial Hospital-Kaphsiung Medical Center, 123 Ta-Pei Road, Niso-Sung Hsiang, Kaohsiung County 83301, Taiwan, China; Gary M Tse, MD, Department of Anatomical and Cellular Pathology, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, China
S- Editor Cheng JX L- Editor Kerr C E- Editor Ma WH
| 1. | Fisher B, Redmond C, Fisher ER, Bauer M, Wolmark N, Wickerham DL, Deutsch M, Montague E, Margolese R, Foster R. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674-681. [Cited in This Article: ] |
| 2. | Sonoo H, Noguchi S. Results of questionnaire survey on breast cancer surgery in Japan 2004-2006. Breast Cancer. 2008;15:3-4. [Cited in This Article: ] |
| 3. | Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546-553. [Cited in This Article: ] |
| 4. | Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, Lada B, Lukka H, Perera F, Fyles A. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143-1150. [Cited in This Article: ] |
| 5. | Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, Seifert M, Haider K, Mlineritsch B, Steindorfer P. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer--Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002;20:4621-4627. [Cited in This Article: ] |
| 6. | Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45-53. [Cited in This Article: ] |
| 7. | Watanabe T, Sano M, Takashima S, Kitaya T, Tokuda Y, Yoshimoto M, Kohno N, Nakagami K, Iwata H, Shimozuma K. Oral uracil and tegafur compared with classic cyclophosphamide, methotrexate, fluorouracil as postoperative chemotherapy in patients with node-negative, high-risk breast cancer: National Surgical Adjuvant Study for Breast Cancer 01 Trial. J Clin Oncol. 2009;27:1368-1374. [Cited in This Article: ] |
| 8. | Park Y, Okamura K, Mitsuyama S, Saito T, Koh J, Kyono S, Higaki K, Ogita M, Asaga T, Inaji H. Uracil-tegafur and tamoxifen vs cyclophosphamide, methotrexate, fluorouracil, and tamoxifen in post-operative adjuvant therapy for stage I, II, or IIIA lymph node-positive breast cancer: a comparative study. Br J Cancer. 2009;101:598-604. [Cited in This Article: ] |
| 9. | Ohashi Y, Watanabe T, Sano M, Koyama H, Inaji H, Suzuki T. Efficacy of oral tegafur-uracil (UFT) as adjuvant therapy as compared with classical cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) in early breast cancer: a pooled analysis of two randomized controlled trials (N.SAS-BC 01 trial and CUBC trial). Breast Cancer Res Treat. 2010;119:633-641. [Cited in This Article: ] |
| 10. | National Comprehensive Cancer Network. Practice Guidelines in Oncology. Available from: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. [Cited in This Article: ] |
| 11. | O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440-449. [Cited in This Article: ] |
| 12. | Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794-7803. [Cited in This Article: ] |
| 13. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [Cited in This Article: ] |
| 14. | Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684. [Cited in This Article: ] |
| 15. | Slamon D, Eiermann W, Robert N. BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ACT) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ACTH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients. San Antonio Breast Cancer Symposium, 2006. . [Cited in This Article: ] |
| 16. | Iwata H, Toi M, Fujiwara Y. Phase II clinical study of lapatinib (GW572016) in patients with advanced breast cancer. Breast Res Treat. 2006;100:A1091. [Cited in This Article: ] |
| 17. | Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733-2743. [Cited in This Article: ] |
| 18. | Lin NU, Carey LA, Liu MC. Phase II trial of lapatinib for brain metastases in patients with HER2 breast cancer. J Clin Oncol. 2006;24 suppl:A503. [Cited in This Article: ] |
| 19. | Baselga J, Imadalou K, Paton V. Efficacy, safety and tolerability of dual monoclonal antibody therapy with pertuzumab + trastuzumab in HER2+ metastatic breast cancer patients previously treated with trastuzumab. Cancer Res. 2009;69:A3138. [Cited in This Article: ] |
| 20. | Burstein HJ, Sun Y, Tan AR. Neratinib (HKI-272), an irreversible pan erbB2 receptor tyrosine kinase inhibitor: Phase II results in patients with advanced HER-2+ breast cancer. Cancer Res. 2009;69:A37. [Cited in This Article: ] |
| 21. | Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676. [Cited in This Article: ] |
| 22. | Rugo HS, Stopeck A, Joy AA, Chan S, Verma S, Lluch A, Liau KF, Kim S, Bycott P, Soulieres D. A randomized, double-blind phase II study of the oral tyrosine kinase inhibitor (TKI) axitinib (AG-013736) in combination with docetaxel (DOC) compared to DOC plus placebo (PL) in metastatic breast cancer (MBC). J Clin Oncol. 2007;25 suppl:A1003. [Cited in This Article: ] |
| 23. | Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810-1816. [Cited in This Article: ] |
| 24. | Slamon D, Gomez HL, Kabbinavar FF, Amit O, Richie M, Pandite L, Goodman V. Randomized study of pazopanib + lapatinib vs. lapatinib alone in patients with HER2- positive advanced or metastatic breast cancer. J Clin Oncol. 2008;26 suppl:A1016. [Cited in This Article: ] |
| 25. | O'Shaughnessy J, Osborne C, Pippen J, Yoffe M, Patt D, Monaghan G, Rocha C, Ossovskaya V, Sherman B, Bradley C. Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): Results of a randomized phase II trial. J Clin Oncol. 2009;27 suppl:A3. [Cited in This Article: ] |