Published online Apr 24, 2024. doi: 10.5306/wjco.v15.i4.566
Peer-review started: November 16, 2023
First decision: December 31, 2023
Revised: January 20, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: April 24, 2024
Low-grade myofibroblastic sarcoma (LGMS) is an extremely rare tumor characterized by the malignant proliferation of myofibroblasts. LGMS most commonly develops in adults, predominantly in males, in the head and neck region, oral cavity, especially on the tongue, mandible, and larynx. This article presents 2 cases of LGMS localized to the maxillary sinus and provides an overview of the available literature.
Two patients with LGMS located in the maxillary sinus underwent surgery at the Department of Head and Neck Surgery. Case 1: A 46-year-old patient was admitted to the clinic with suspected LGMS recurrence in the right maxillary sinus (rT4aN0M0), with symptoms of pain in the suborbital area, watering of the right eye, thick discharge from the right nostril, and augmented facial asymmetry. After open biopsy-confirmed LGMS, the patient underwent expanded maxillectomy of the right side with immediate palate reconstruction using a microvascular skin flap harvested surgically from the middle arm. The patient qualified for adjuvant radiotherapy for the postoperative bed, with an additional margin. Currently, the patient is under 1.5 years of observation with no evidence of disease. Case 2: A 45-year-old man was admitted to our clinic with facial asymmetry, strabismus, exophthalmos, and visual impairment in the right eye. Six months earlier, the patient had undergone partial jaw resection at another hospital for fibromatosis. A contrast-enhanced computed tomography scan revealed a tumor mass in the postoperative log after an earlier procedure. An open biopsy confirmed low-grade fibrosarcoma (rT4aN0M0). The patient qualified for an extended total right maxillectomy with orbital excision and right hemimandibulectomy with immediate microvascular reconstruction using an anterolateral thigh flap. The patient subsequently underwent adjuvant radiotherapy to the postoperative area. After 9 months, recurrence occurred in the right mandibular arch below the irradiated area. The lesion infiltrated the base of the skull, which warranted the withdrawal of radiotherapy and salvage surgery. The patient qualified for palliative chemotherapy with a regimen of doxorubicin + dacarbazine + cyclophosphamide and palliative radiotherapy for bone metastases. The patient died 26 months after surgical treatment. The cases have been assessed and compared with cases in the literature.
No specific diagnostic criteria or treatment strategies have been developed for LGMS. The treatment used for LGMS is the same as that used for sinonasal cancer radical tumor excision; adjuvant radiotherapy or chemoradiotherapy should also be considered. They have low malignant potential but are highly invasive, tend to recur, and metastasize to distant sites. Patients should undergo regular follow-up examinations to detect recurrence or metastasis at an early stage. Patients should be treated and observed at the highest referral centers.
Core Tip: Low-grade myofibroblastic sarcomas are tumors of low malignant potential; however, they are highly invasive and a high tendency to recur and metastasize to distant sites. Since only 55 cases of low-grade myofibroblastic sarcoma have been described, it is impossible to establish guidelines. As there are no specific diagnostic criteria, it is necessary to consider the occurrence of myofibroblastic sarcoma more often than reported in the literature.
- Citation: Mydlak A, Ścibik Ł, Durzynska M, Zwoliński J, Buchajska K, Lenartowicz O, Kucharz J. Low-grade myofibrosarcoma of the maxillary sinus: Two case reports. World J Clin Oncol 2024; 15(4): 566-575
- URL: https://www.wjgnet.com/2218-4333/full/v15/i4/566.htm
- DOI: https://dx.doi.org/10.5306/wjco.v15.i4.566
Low-grade myofibroblastic sarcoma (LGMS) is characterized by malignant proliferation of myofibroblasts. LGMS is extremely rare and most commonly presents on the tongue in the head and neck region. According to the literature, LGMS may also be present in the limbs, abdominal cavity, pelvis, and long bones and pelvis. Sarcomas are histologically atypical with infiltrating myoepithelial cells and morphological, immunochemical, and ultrastructural features of myofi
Myofibroblasts, also called modified fibroblasts, are myoepithelial cells or stellate cells of mesenchymal origin, disco
Myofibroblasts have an irregular, hyperchromatic, enlarged nucleus with moderate atypia in amphophilic cytoplasm[4]. They are characterized by the expression of α-Smooth muscle actin (SMA), vimentin and extra domain A of the fibr
LGMS most frequently occurs in men and is extremely rare in children. It is highly malignant and characterized by metastasis to distant sites. To the best of our knowledge only 5 cases of maxillary sinus LGMS are available[2,7,8]. Patients rarely report symptoms, and the primary complaint is painless edema. Radiologically, LGMS can present a destructive growth pattern.
Case 1: A 46-year-old male previously treated at another hospital was admitted to the outpatient clinic of the Maria Sklodowska-Curie National Research Institute of the Oncology Department of Head and Neck Oncology. The patient presented with right-sided pain in the suborbital area, watering of the right eye, and thick discharge from the right nostril with augmented facial asymmetry.
Case 2: A 45-year-old male was admitted to Maria Sklodowska-Curie National Research Institute of Oncology presenting with strabismus, exophthalmos, and visual impairment.
Case 1: The patient had previously undergone surgery at another hospital for LGMS. The patient underwent resection of the maxilla using a lateral rhinotomy. A second operation was performed because of the positive surgical margins. Histopathological examination confirmed radical resection and the patient qualified for observation. Thirty months after the surgery, clinical examination confirmed an advanced tumor infiltrating the right nasal cavity, hard palate, and soft palate.
Case 2: Six months earlier, the patient underwent partial resection of the maxilla because of fibromatosis.
Case 1: Generally healthy, did not report chronic diseases, allergies, or medications taken regularly. At the age of 4 years, there was an electric burn on the index finger of the left hand and subsequent amputation.
Case 2: Overall healthy. He does not take medications regularly. Allergy to penicillin.
Case 1: Professional driver by profession. No family history of malignancy.
Case 2: No family history of malignancy.
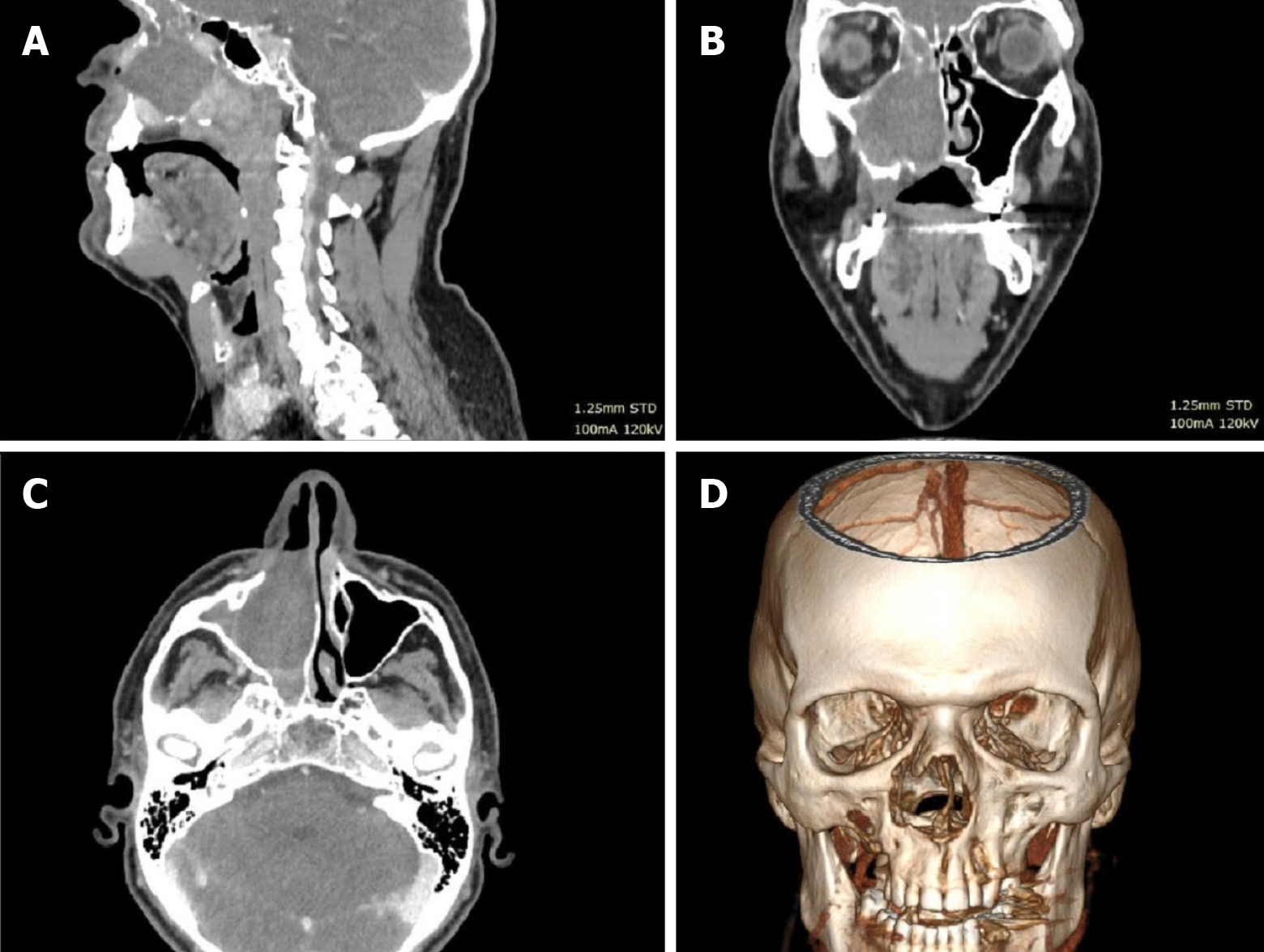
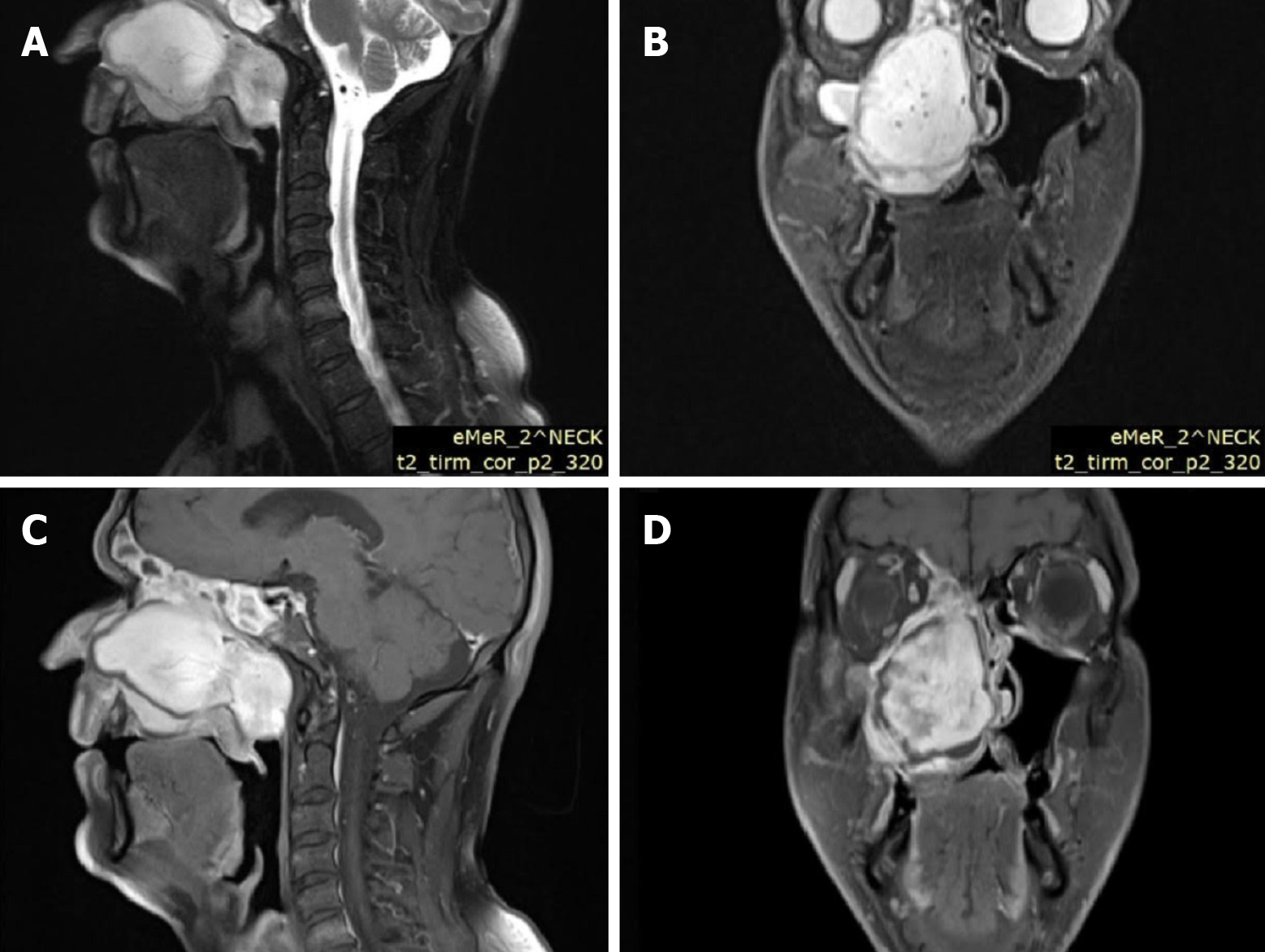
Case 1: Computed tomography (CT) (Figure 1) and magnetic resonance imaging (MRI) (Figure 2) of the head and neck region revealed extensive soft tissue masses in the right maxillary sinus, nasal cavity, nasopharynx, ethmoid cells, and frontal sinus. Infiltration and partial osteolysis were observed in the bone structures on the right side, including the sinus walls, hard palate, medial and suborbital bones, and pterygoid plates.
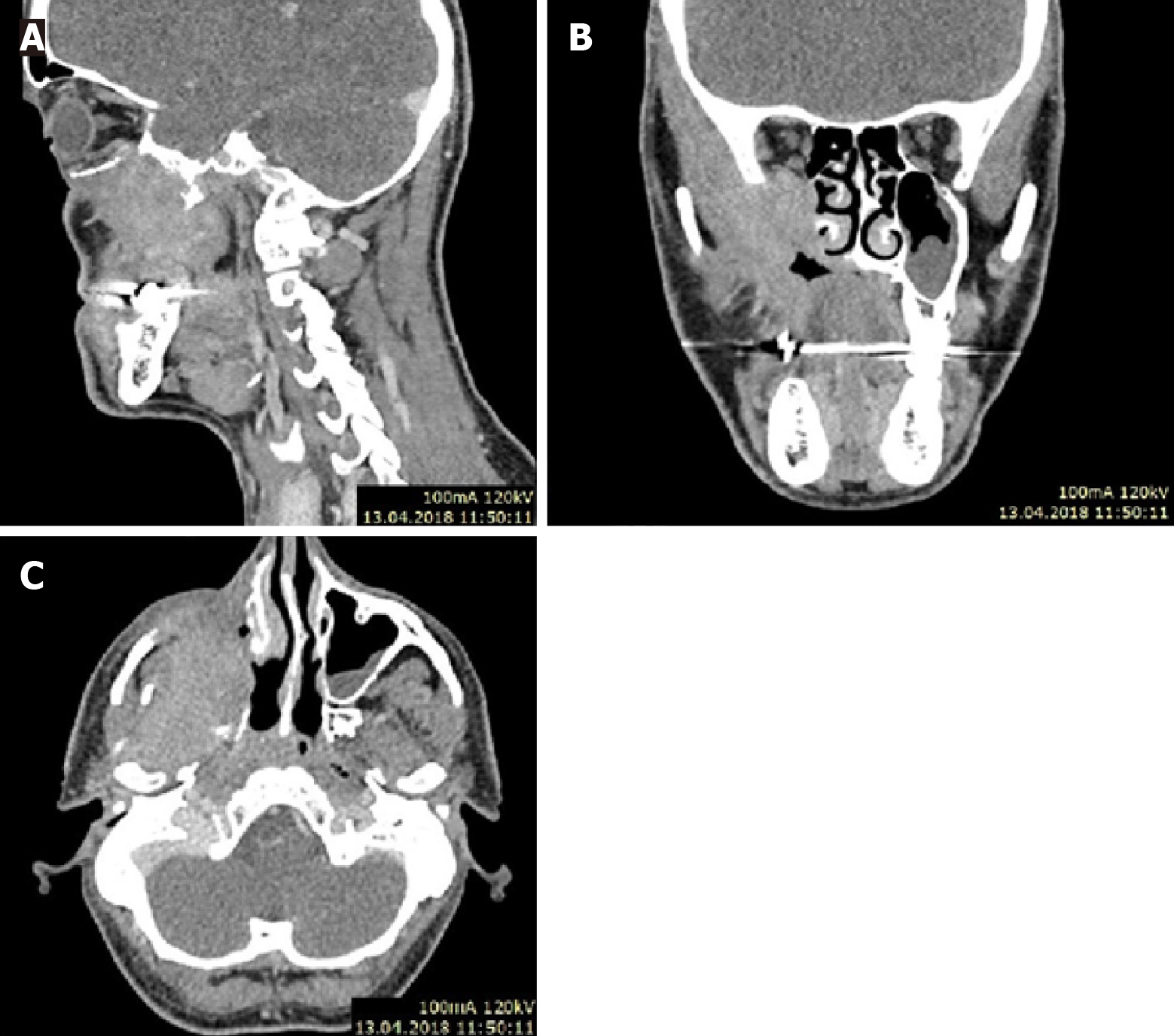
Case 2: CT, with contrast scan (Figure 3), revealed a tumor mass in the postoperative lobe after the first surgery.
Tumor infiltration was observed in the pterygopalatine and right temporal fossa. Infiltration also involved the lateral pterygoid and masseter muscle, the lateral wall of the nasal cavity and the oral cavity.
Soft tissue mass protruding from the tumor into the posterior orbit through the superior orbital fossa.
Tumor progression and rapid recurrence after primary surgery. The histopathological examination results were verified at the Maria Sklodowska-Curie National Research Institute of Oncology. After additional examinations and multispecialty consultation, the primary diagnosis was changed from fibromatosis to inflammatory myofibroblastic tumor.
Case 1: Laboratory tests without deviations.
Case 2: Laboratory tests without any significant deviations.
Case 1: Facial asymmetry, highlighting of the right cheek. Eyeball movement was preserved, and the patient denied diplopia or any other deviation from the norm. On intraoral examination, an exophytic tumor of the hard palate reached the midline. Lymphadenopathy was not present during the physical examination.
Case 2: Facial asymmetry, swelling of the right cheek. Scars on the right cheek from previous surgery. Strabismus and exophthalmos of the right eye, significant visual impairment, preserved response to light.
During intraoral examination, a palpable tumor on the palate on the right side was observed. Palpable cervical bulb on the right in group 2.
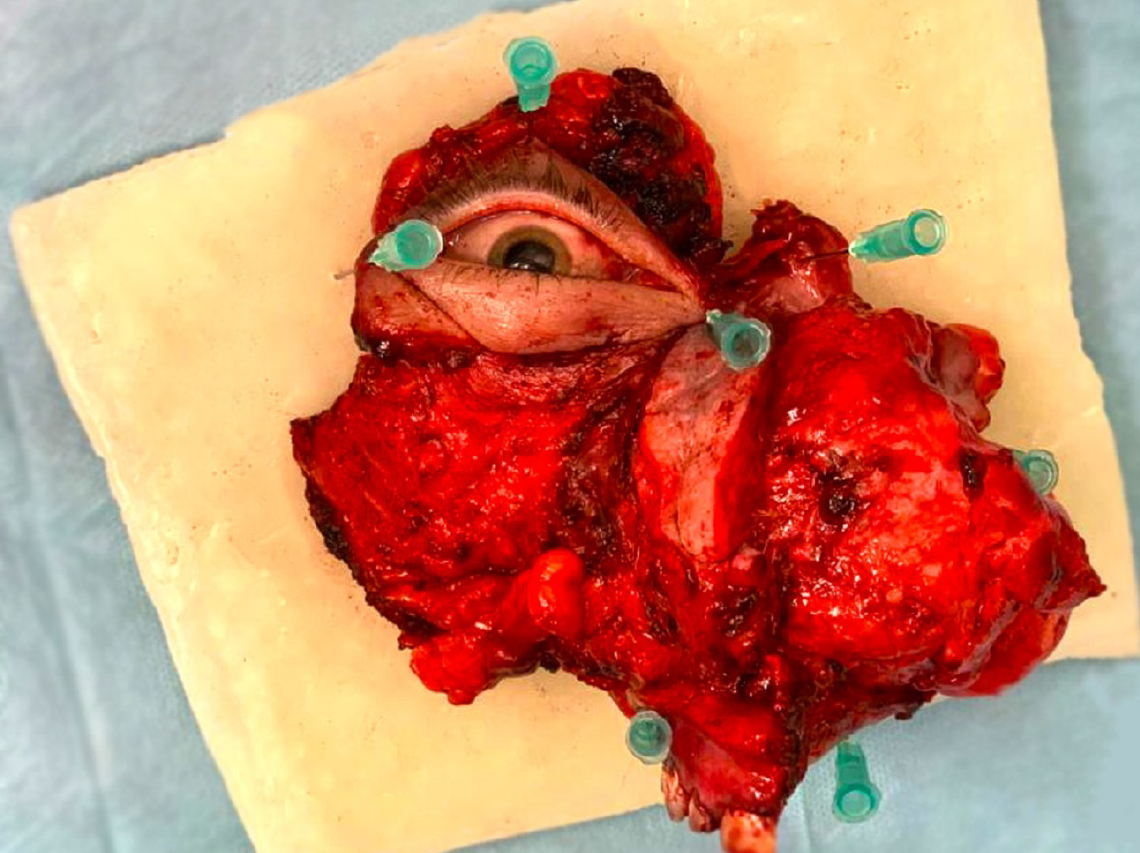
Histological examination confirmed the recurrence of LGMS (rpT4aN0M0) (8th Edition, American Joint Committee on Cancer) of the right maxilla, 8 cm in size. Neoplasms with spindle-cell proliferation and moderate cellular atypia.
Mitotic activity was low [four mitoses per 10 high power field (HPF)], without atypical mitosis. The collagenous stroma was partially myxoid and contained an increased number of thick-walled capillaries; no necrosis was observed. Bone destruction was also observed.
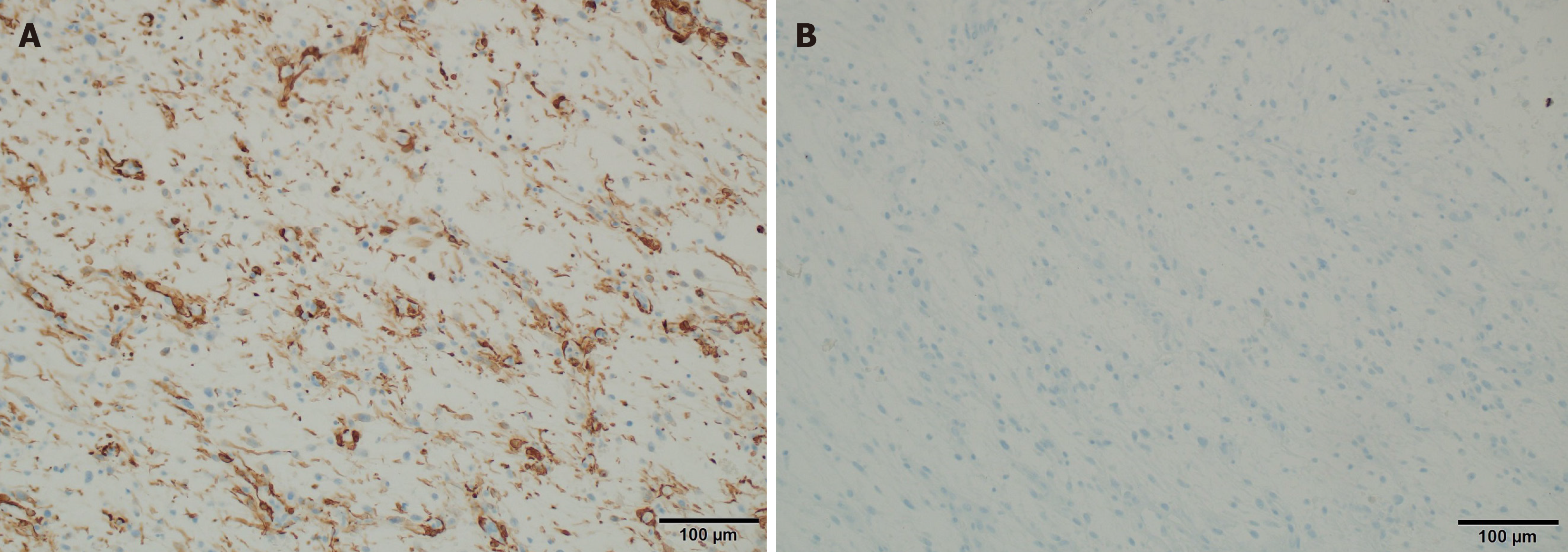
Immunohistochemistry staining performed: SMA (+, in parts of cell population), reaction type "tram truck", cytokeratin AE1 and AE3 (CKAE1/3) (-/+, insufficient focal reaction), Mucin 4 (MUC4) (-), CD34 (-), Desmin (-), SOX10 (-), S100 protein (-), Ki-67 protein (5%), hHf35 (-), Epithelial membrane antigen (EMA) (-) (-/+), Caldesmon (-/+, trace), H3K27me3 (+, expression prohibited), ALK (-), ROS1 (-), HMB45 (-), Melan-A (-), Myogenin (-), MyoD1 (-).
Histopathological examination confirmed LGMS. The tumor was poorly demarcated, cream-gray in color, macroscopically without necrosis, and 8 cm in diameter with endophytic growth (rpT4aN0M0) (8th Edition, American Joint Committee on Cancer).
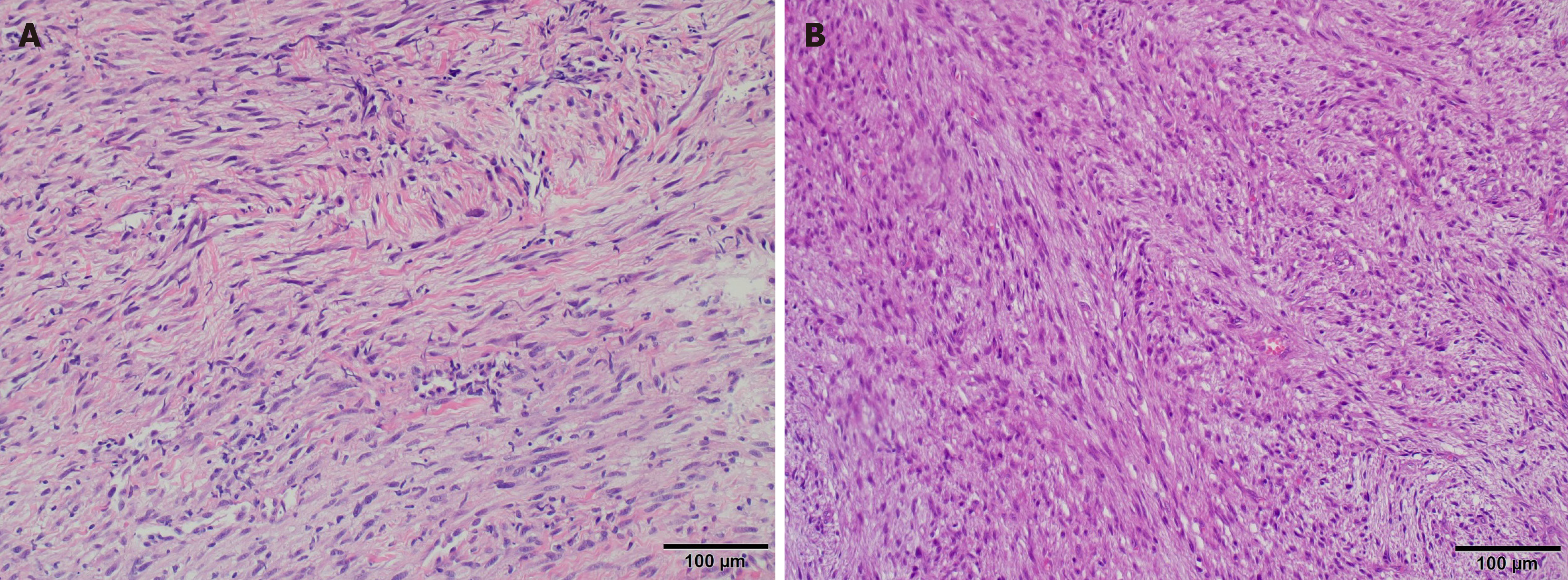
Microscopic examination revealed proliferation of spindle cells with moderate cellularity and focal moderate cellular atypia; mitotic activity was low (four mitoses per 10-HPF) without atypical mitosis. The collagen stroma was partially edematous without necrosis. Natural invasion was also observed.
Immunohistochemical staining performed: SMA (+), Desmin (-), CD34) (-), EMA (-), CKAE1/3 (-), Caldesmon (-), MUC1 (-), S100 protein (-), ALK1 (-), Signal transducer and activator of transcription 6 (+/-), B-creatinin (-).
Based on the physical, histopathological, and radiological examinations, the patient qualified for an expanded maxill
The patient qualified for adjuvant radiotherapy radiation therapy (IMRT) and cone beam CT for postoperative treatment, with additional margins. The patient received a fractional dose of 200 centiGray (cGy) for a total dose of 6600 cGy.
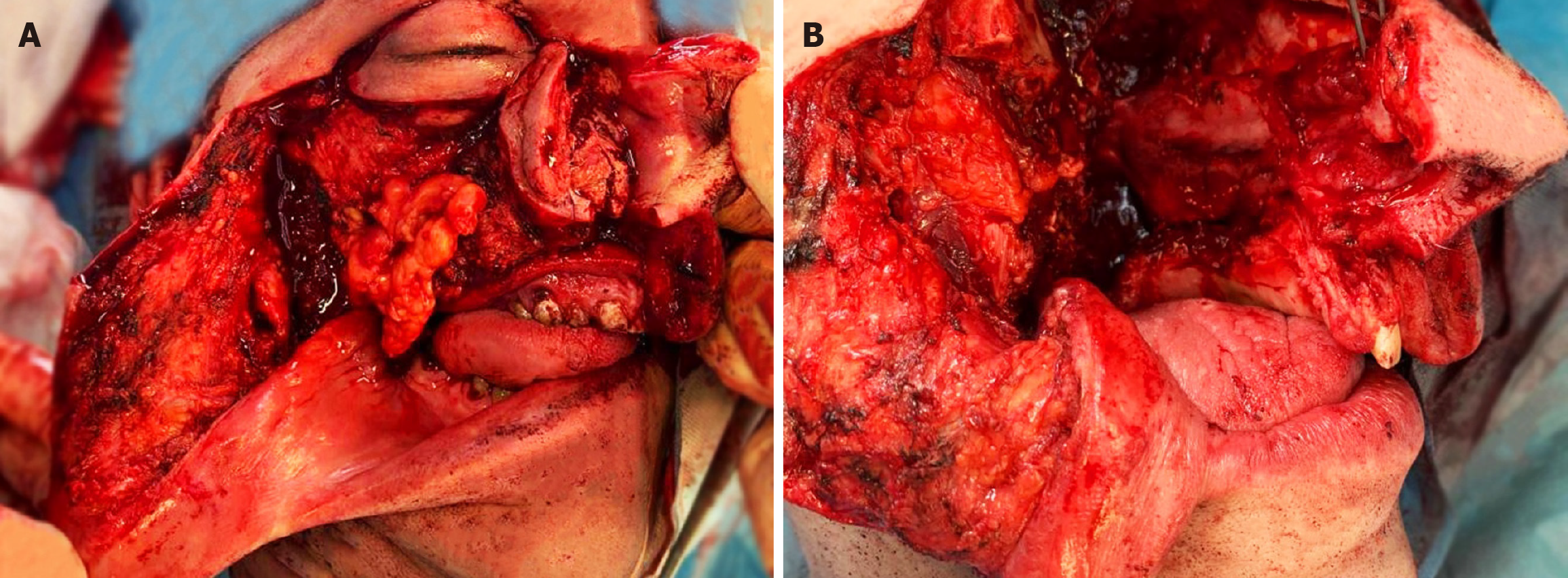
Open biopsy confirmed recurrence of low-grade myofibrosarcoma. Based on clinical, histopathological, and radiological results, the patient qualified for expanded complete right-sided maxillectomy and right-sided hemimandibulectomy with immediate microvessel reconstruction using an anterolateral thigh flap and selective resection of the lymph nodes on the right side. Because of infiltration of the orbital tissues, right-sided exenteration was performed.
The patient qualified for adjuvant radiation therapy (IMRT) of the postsurgical bed with a fraction dosage of 200 cGy to a total dose of 6600 cGy.
Currently, the patient is under observation with no evidence of disease.
After 9 months of observation, recurrence appeared in the right mandibular arch below the irradiated area. CT confirmed the progression in both the irradiated and the previously irradiated areas. The lesion is located at the base of the skull.
There were increasing postoperative risks, which justified refrainment from radiotherapy and salvage surgery.
The patient qualified for palliative chemotherapy with doxorubicin + dacarbazine + cyclophosphamide regimen. Due to pathological L1 and L5 fractures, metastases to L1 and L2, and metastasis to the right hip bone, the patient was eligible for Radiation Therapy [fractions of 3 Gray (Gy) to a total dose of 36 Gy] and second-line chemotherapy (gemcitabine + docetaxel). The patient died 26 months after surgical treatment.
A comparison of the immunohistochemical studies of Case 1 and Case 2 is shown in Table 1.
| Case | Vimentin | SMA | CK AE 1/3 | Desmin | h-Caldesmon | CD 34 | S100 protein | EMA | ALK 1 |
| 1 | + | + | -/+ | - | -/+ trace | - | - | -/+ | - |
| 2 | + | + | - | - | - | - | - | - | - |
LGMS is a recently discovered and extremely rare malignant tumor. The first such case was diagnosed in the 1998s. In 2002, the World Health Organization made the LGMS a separate unit in the pathology and genetics of soft tissue and bone tumors[9]. Clinically, it manifests as a slow-growing and infiltrating tumor. LGMS is a low-grade malignant tumor with a high tendency for recurrence and distant metastases, even after several years[10,11].
LGMS most commonly develops in adults, predominantly in males, and in the head and neck. The tumor most often appears in the oral cavity, especially in the tongue, mandible, and larynx[6]. Other localizations include the limbs, abdominal cavity, pelvis, and long bones[12].
LGMS of the maxillary sinus is extremely rare, and only five cases have been described so far. Here, we present two more cases (Table 2)[2,7,8,13]. In cases of soft tissue sarcomas of the head and neck, MRI with contrast and/or CT with contrast should be performed (NCCN Guidelines version 2. 2022)[14]. Radiologic imaging typically shows a well-limited tumor with visible margins of destructive growth[6,10].
| Ref. | Age | Sex | Size in cm | Side | Main symptoms | Necrosis | Mitotic rate, 10/10 HPF scale | Follow up in months | IHC | Results |
| Meng et al[7], 2007 | 33 | F | 6.5 | Left | Nasal obstruction and leakage | Yes | 10/10/HPF | 12 | Vimentin (+); SMA (+); Fibronectin (+); Calponin (+); Desmin (-); h-Caldesmon (-); Laminin (-); Type IV collagen (-); CD34 (-); CD68 (-); ALK1 (-) | Diagnostic testing of 1 yr |
| Meng et al[7], 2007 | 28 | F | 3 | Left | Nasal obstruction and leakage | Yes | 8/10 HPF | 21 | Vimentin (+); SMA (+); Fibronectin (+); Calponin (+); Desmin (-); h-Caldesmon (-); Laminin (-); Type IV collagen (-); CD34 (-); CD68 (-); ALK 1 (-) | Recurrent at 0.5 yr |
| Ghosh et al[2], 2019 | 35 | M | No data | Left | Exophthalmos | No data | < 2/10 HPF | 72 | α-SMA (+); MIC-2 (+); Desmin (-); CD 34 (-); S100 protein (-); Cytokeratin (-); EMA (-); Calponin (-); Bcl-2 (-) | Recurrent at 6 yr |
| Bisceglia et al[13], 2001 | 24 | M | 4 | Left | Pain, swelling of the midface | No data | 1/10HPF | 40 | Vimentin (+); α-SMA (+); MSA (+); CD34 (-); Desmin (-); S100 protein (-); Cytokeratin (-) | Observation 3 yr |
| Gómez-Oliveira et al[8], 2015 | 75 | F | No data | Left | Pain, swelling | Yes | No data | 12 | Vimentin (+); SMA (+); CD10 (+); Cytokeratin (+); h-Caldesmon (+); Desmin (-); CD34 (-); ALK (-); EMA (-); S100 protein (-) | After 1 yr metastasis-left femur |
| Current article | 46 | M | 8 | Right | Pain, swelling of the midface, nasal obstruction and leakage | No | 4/10HPF | 15 | SMA (+) in some cells; tram truck type reaction; CK AE1/3 (-/+) focal weak reaction; MUC4 (-); CD34 (-); Desmin (-); SOX10 (-); S100 (-); Ki-67 5%; hHf35 (-); EMA (-/+) trace; Caldesmon (-/+) trace; H3K27me3 (+) preserved expression; ALK (-); ROS1 (-); HMB45 (-) Melan-A (-); Myogenin (-); MyoD1 (-) | Observation 1.5 yr |
| Current article | 45 | M | 8 | Right | Exophthalmos, strabismus, visual impairment | No | 4/10HPF | 26 | SMA (+); Desmin (-); CD34 (-); EMA (-); CKAE1/3 -; Caldesmon (-); MUC1 (-); S100 (-); ALK1 (-); STAT6 (+/-); B-catenin (-) | Recurrent |
Histologically, the tumor is composed of spindle and stellate cells collected in clusters of different lengths, with a focal herringbone, spiral, or no pattern[13]. Cancerous cells are composed of a mild to moderate amount of pale eosinophilic cytoplasm and a spindle nucleus, which can be spiral or circular, and vesicles with cavities.
In most cases, focal atypia of the nucleus is observed; however, this is usually benign with enlarged hyperchromatic nuclei. Additionally, larger atypical cells can sometimes be observed[11].
Microscopic Figure 6A shows spindle cell infiltration, hypocellularity with mild atypia, and stromal collagen. Hyper
Neoplastic cells in LGMS have a variable immunophenotype: Actin positive (+)/Desmin negative (-), Actin negative (-)/Desmin positive (+), and Actin positive (+)/Desmin (+) positive. In addition, tumor cells may stain positively for fib
The differential diagnosis of LGMS includes both malignant and benign tumors such as nodular fasciitis, myofibroblastic tumors, fibromatosis, myofibroma, myopericytoma, monophasic synovial sarcoma, malignant peripheral nerve sheath tumors, spindle cell rhabdomyosarcoma, fibrosarcoma, leiomyosarcoma, and melanoma[5,6,16].
The gold standard procedure in cases of sarcoma infiltrating bones is radical excision of the tumor[12,17]. In cases of positive margins, the radicalization procedure should be primarily considered. When radicalization is impossible, soft tissue margins are narrow and large. If tumors infiltrate the blood vessels or nerves, radiotherapy or chemoradiotherapy should be considered[12,18,19]. The LGMS head and neck recurrence rate is 25%-40% and is the highest when the tumor is in the nasal cavity or paranasal sinuses. A higher frequency of recurrence was observed in patients who underwent adjuvant radiation therapy. This is probably a result of the qualification of patients with unfavorable prognostic factors. The most important prognostic factor was the resection state. Positive margins, regional lymph node involvement, and age > 60 years[12].
The clinical cases presented above were characterized by characteristics specific to the described type of sarcoma, which enabled the identification of certain groups of tumors. As shown in Table 2, males are mostly affected (57%), which is also indicated in previous literature. The average age of the patients is 41 ± 17.2 years (females 45 ± 25.8 years, males 38 ± 10.6 years). The most common symptoms are nasal congestion, rhinorrhea, edema, and pain. Exophthalmos was present in two patients; however, visual impairment was present in one patient.
LGMSs are tumors of low malignancy; however, they are highly invasive, with a high tendency for recurrence and a high risk of distant metastases[6]. Several factors may contribute to this paradox of LGMS. Tumors with a low grade of malignancy may have a lower mitotic index, but this does not necessarily reflect their invasive potential or likelihood of metastasis. It is suspected that these tumors may show infiltrative growth patterns, making complete surgical removal difficult, and allowing residual microscopic disease left after surgery to cause recurrence.
Even within a specific histological subtype, tumors can be significantly heterogeneous in terms of biological behavior. Some cells may have more aggressive features. Tumor behavior is also influenced by genetic and molecular characteristics. Some low-grade tumors may contain genetic changes or mutations that contribute to their ability to recur or metastasize.
LGMSs are tumors of low malignant potential; however, they are highly invasive and have a high tendency to recur and metastasize to distant sites. A standard treatment strategy has not been developed yet for LGMS patients. Because of its low frequency of occurrence, it is impossible to establish guidelines. Therefore, the treatment used for LGMS is the same as that used for sino-nasal carcinoma.
It is important that LGMS patients be closely monitored by a multidisciplinary healthcare team to determine the most appropriate treatment plan and follow-up. Regular follow-up examinations are crucial to detect recurrence or metastasis at an early stage. Considering the lack of precise diagnostic criteria, LGMS occurs more often than the literature indicates and may include various clinicopathological forms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Yoshimoto S, Japan S-Editor: Luo ML L-Editor: Filipodia P-Editor: Zhao YQ
| 1. | Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1041] [Cited by in F6Publishing: 1001] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 2. | Ghosh A, Bandopadhyay A, Sarkar R. Low-grade myofibroblastic sarcoma of maxillary sinus and buccal mucosa: Two rare cases and review of the literature. Indian J Pathol Microbiol. 2019;62:119-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Eyden B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol. 2001;25:39-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Qiu JY, Liu P, Shi C, Han B. Low-grade myofibroblastic sarcomas of the maxilla. Oncol Lett. 2015;9:619-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Fisher C. Myofibrosarcoma. Virchows Arch. 2004;445:215-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Demarosi F, Bay A, Moneghini L, Carrassi A. Low-grade myofibroblastic sarcoma of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Meng GZ, Zhang HY, Bu H, Yang GH, Zhang XL, Yang G. Myofibroblastic sarcoma of the nasal cavity and paranasal sinus: a clinicopathologic study of 6 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:530-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Gómez-Oliveira G, Arribas-García I, Serrano Alvarez-Buylla A, Sánchez-Burgos R, Martínez-Pérez F, Alvarez-Flores M. Low-grade myofibroblastic sarcoma. Two rare tumours in two rare locations. Revista Española de Cirugía Oral y Maxilofacial. 2015;37:108-112. [DOI] [Cited in This Article: ] |
| 9. | Fletcher CDM, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. IARC Press; 2002. Accessed July 4, 2023. Available from: http://www.karger.com/Article/Pdf/78490. [Cited in This Article: ] |
| 10. | Mentzel T. Myofibroblastic sarcomas: a brief review of sarcomas showing a myofibroblastic line of differentiation and discussion of the differential diagnosis. Curr Diag Pathol. 2001;7:17-24. [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Yamada T, Yoshimura T, Kitamura N, Sasabe E, Ohno S, Yamamoto T. Low-grade myofibroblastic sarcoma of the palate. Int J Oral Sci. 2012;4:170-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Xu Y, Xu G, Wang X, Mao M, Wu H, Baklaushev VP, Chekhonin VP, Peltzer K, Wang G, Zhang C. Is there a role for chemotherapy and radiation in the treatment of patients with low-grade myofibroblastic sarcoma? Clin Transl Oncol. 2021;23:344-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Bisceglia M, Tricarico N, Minenna P, Magro G, Pasquinelli G. Myofibrosarcoma of the upper jawbones: a clinicopathologic and ultrastructural study of two cases. Ultrastruct Pathol. 2001;25:385-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Pollack SM, Poppe M, Riedel RF, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Hang LE, Sundar H, Bergman MA. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:815-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 70] [Reference Citation Analysis (0)] |
| 15. | Mikami Y, Fujii S, Kohashi KI, Yamada Y, Moriyama M, Kawano S, Nakamura S, Oda Y, Kiyoshima T. Low-grade myofibroblastic sarcoma arising in the tip of the tongue with intravascular invasion: A case report. Oncol Lett. 2018;16:3889-3894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 16. | Montgomery E, Goldblum JR, Fisher C. Myofibrosarcoma: a clinicopathologic study. Am J Surg Pathol. 2001;25:219-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Giraldo-Roldan D, Louredo BVR, Penafort PVM, Pontes HAR, Alves AP, Lima FCA, Fonseca TC, Abrahão AC, Romañach MJ, Fonseca FP, Delgado WA, Robinson L, Van Heerden WFP, de Almeida OP, Vargas PA. Low-Grade Myofibroblastic Sarcoma of the Oral and Maxillofacial Region: An International Clinicopathologic Study of 13 Cases and Literature Review. Head Neck Pathol. 2023;17:832-850. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 18. | Yu Y, Xiao J, Wang L, Yang G. Low-Grade Myofibroblastic Sarcoma in the Mandibular Canal: A Case Report. J Oral Maxillofac Surg. 2016;74:1505.e1-1505.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Mamikunian G, Ziegler A, Block A, Thorpe E. Risk Factors for Recurrence and the Role of Radiotherapy in Low-grade Myofibroblastic Sarcoma: A Systematic Review. Am J Clin Oncol. 2023;46:420-425. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |