Copyright
©The Author(s) 2022.
World J Clin Oncol. May 24, 2022; 13(5): 323-338
Published online May 24, 2022. doi: 10.5306/wjco.v13.i5.323
Published online May 24, 2022. doi: 10.5306/wjco.v13.i5.323
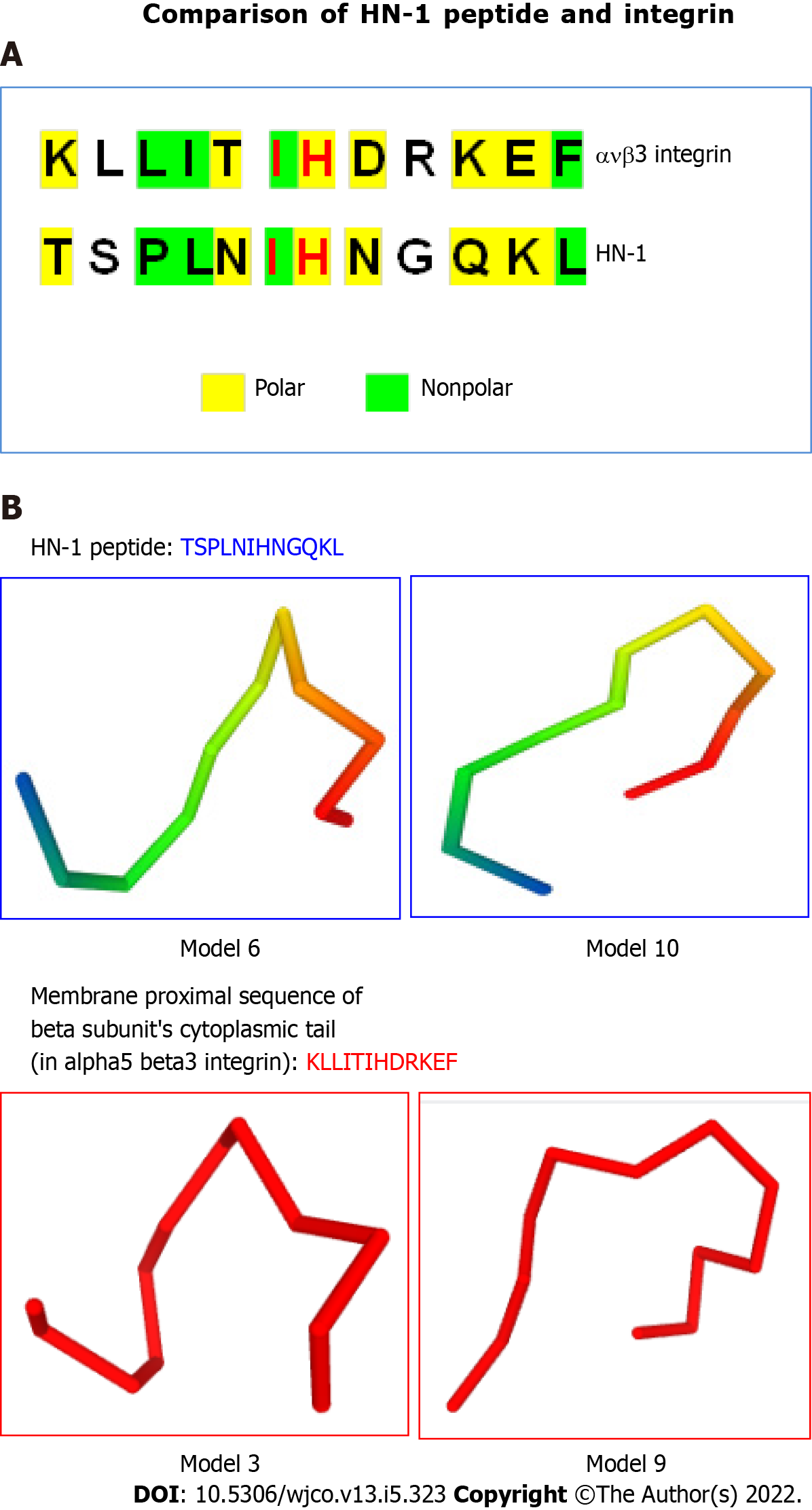
Figure 3 HN-1 peptide exhibits similarity to integrin peptide.
A: The similarities between the HN-1 sequence (TSPLNIHNGQKL) and a stretch of amino acids in alpha5 beta3 integrin (KLLITIHDRKEF) are highlighted. As HN-1 peptide lacks the recognition motif (RGD) of integrin, HN-1 may interact with an "integrin-like" molecule. HN-1 is internalized by attached cells but not by suspended cells. As tissue culture plates are typically coated with collagen, collagen-binding DDR1 receptor (expressed by adherent but not suspended cells) may represent the receptor for HN-1 consistent with that DDR1 is overexpressed in human head and neck cancer (also breast cancer) targeted by HN-1. avb3: alpha5 beta3; B: Comparison of 3D models generated by PEP-FOLD (INSERM, France) in RPBS bioinformatics web portal: https://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD/ TSPLNIHNGQKL: HN-1 peptide (top panels); KLLITIHDRKEF, membrane proximal sequence of beta subunit’s cytoplasmic tail in alpha5 beta3 integrin (bottom panels); TSPLNIHNGQKL: Thr-Ser-Pro-Leu-Asn-Ile-His-Asn-Gly-Gln-Lys-Leu; KLLITIHDRKEF: Lys-Leu-Leu-Ile-Thr-Ile-His-Asp-Arg-Lys-Glu-Phe.
- Citation: Hong FU, Castro M, Linse K. Tumor specifically internalizing peptide ‘HN-1’: Targeting the putative receptor retinoblastoma-regulated discoidin domain receptor 1 involved in metastasis. World J Clin Oncol 2022; 13(5): 323-338
- URL: https://www.wjgnet.com/2218-4333/full/v13/i5/323.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i5.323