Copyright
©The Author(s) 2017.
World J Biol Chem. Aug 26, 2017; 8(3): 163-174
Published online Aug 26, 2017. doi: 10.4331/wjbc.v8.i3.163
Published online Aug 26, 2017. doi: 10.4331/wjbc.v8.i3.163
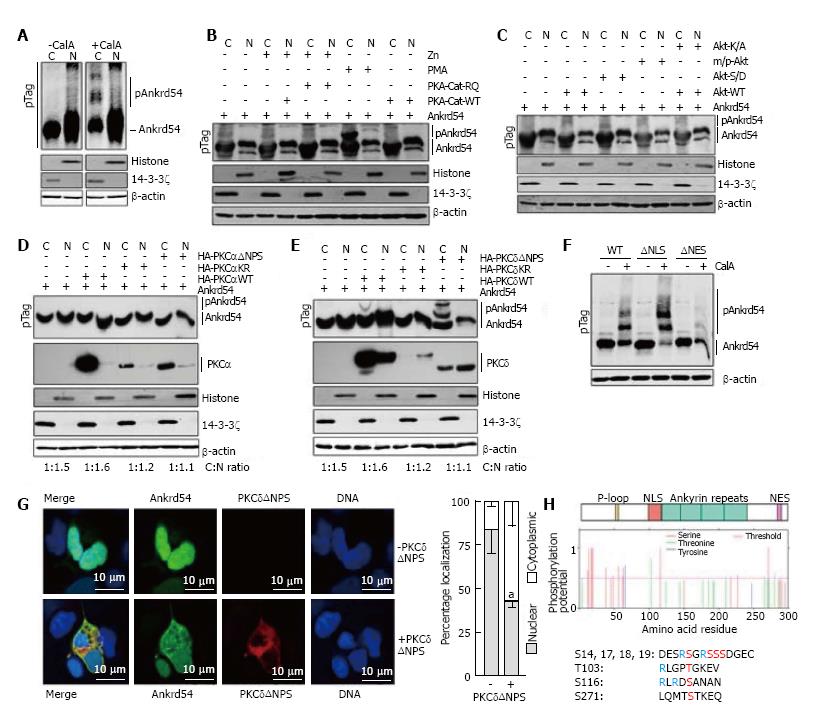
Figure 2 Phosphorylation by PKCδ regulates subcellular compartmentalization of Ankrd54.
A: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), with (+CalA) and without (-CalA) CalyculinA treatment (50 nmol/L, 60 min). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); B: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with PKA expression plasmids, or with addition of PMA (200 nmol/L, 60 min). The wild-type (PKA-Cat-WT) catalytic subunit of PKA, or a dominant active mutant (PKA-Cat-RQ) were induced to express by the addition of Zn. Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); C: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with Akt expression plasmids. Plasmids expressed either wild-type Akt (Akt-WT), a kinase inactive mutant (Akt-K/A), an oncogenic myristoylated/palmitylated Akt (m/p-Akt), or an activation phospho-mimic (Akt-S/D). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); D: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with HA-tagged PKCα expression plasmids. Plasmids expressed either wild-type PKCα (HA-PKAαWT), a kinase inactive mutant (HA-PKCαKR), or an activation mutant (HA-PKCαΔNPS). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-HA (PKCα), anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54). Quantitation of cytoplasmic:nuclear ratios are displayed; E: Immunoblot analysis of nuclear (N) and cytoplasmic (C) fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), co-transfected with HA-tagged PKCδ expression plasmids. Plasmids expressed either wild-type PKCδ (HA-PKAδWT), a kinase inactive mutant (HA-PKCδKR), or an activation mutant (HA-PKCδ∆NPS). Transfected HEK293 cells were immunoblotted using anti-Ankrd54, anti-HA (PKCδ), anti-Histone (H3), anti-14-3-3ζ, and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54). Quantitation of cytoplasmic:nuclear ratios are displayed; F: Immunoblot analysis of cytoplasmic fractionation of eGFP-Ankrd54 using Phos-Tag™ SDS-PAGE (pTag), with and without the addition of CalyculinA (50 nmol/L, 60 min). Transfected HEK293 cells were immunoblotted using anti-Ankrd54 and anti-β-actin antibodies. Mobility shifted Ankrd54, due to phosphorylation, is indicated (pAnkrd54); G: Localization analysis of eGFP-Ankrd54 with and without co-expression of dominant active PKCδ (PKCδΔNPS) in HEK293 cells. Delineation of the nucleus by Hoechst staining of the DNA is on the right, eGFP fluorescence (green), PKCδ (HA-tag, red), with merged image (left). Nuclear and cytoplasmic localization of eGFP-Ankrd54 was enumerated (graph at right), aP < 0.05; H: Prediction of phosphorylated residues in Ankrd54. Top, schematic of Ankrd54 domain structure showing location of P-loop (yellow), NLS (red), ankyrin repeats (green) and NES (purple). Middle, graph of potential phosphorylated residues, predicted by NetPhos3.1. Below, amino acid sequences surrounding top predicted motifs as indicated.
- Citation: Samuels AL, Louw A, Zareie R, Ingley E. Control of nuclear-cytoplasmic shuttling of Ankrd54 by PKCδ. World J Biol Chem 2017; 8(3): 163-174
- URL: https://www.wjgnet.com/1949-8454/full/v8/i3/163.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v8.i3.163