Published online Nov 26, 2014. doi: 10.4331/wjbc.v5.i4.457
Revised: August 1, 2014
Accepted: September 6, 2014
Published online: November 26, 2014
Processing time: 157 Days and 17.5 Hours
AIM: To study the response to silver nanoparticles (Ag NP) of human microvascular endothelial cells, protagonists of angiogenesis.
METHODS: We cultured human microvascular endothelial cells and endothelial colony-forming cells in their corresponding growth medium. Stock solutions of Ag NP were prepared in culture medium and sonicated before use. They were added at different concentrations and for different times to culture media. The toxicity of Ag NP was investigated by measuring the reduction of yellow tetrazolium salt to dark purple formazan (MTT assay) at 575 nm. After staining with trypan blue, cell proliferation was assessed by counting viable cells. The lactate dehydrogenase leakage assay was performed on culture media by following the oxidation of NADH to NAD+ and monitoring the reaction kinetically at 340 nm. Reactive oxygen species production was quantified using 2’-7’-dichlorofluorescein diacetate. The alkaline comet assay was performed after mixing the cells with low melting-point agarose. Electrophoresis was then conducted and the samples were stained with ethidium bromide and analyzed with a fluorescence microscope.
RESULTS: Ag NP are cytotoxic in a dose and time dependent fashion for HMEC. At high concentrations, Ag NP determine loss of membrane integrity as demonstrated by the increased activity of lactate dehydrogenase in the culture medium. Ag NP rapidly stimulate the formation of free radicals. However, pre-incubation with Trolox, apocynin, or N-acetyl-L-cysteine, antioxidants which have different structure and act through different mechanisms, is not sufficient to prevent cytotoxicity. Ag NP also induce DNA damage dose-dependently, as shown by comet assay. When exposed to sublethal concentrations of Ag NP for long times, the cells remain viable but are growth retarded. Interestingly, removal of Ag NP partially rescues cell growth. Also genotoxicity is reversible upon removal of Ag NP from culture medium, suggesting that no permanent modifications occur. It is noteworthy that Ag NP are cytotoxic and genotoxic also for endothelial progenitors, in particular for endothelial colony-forming cells, which participate to angiogenesis.
CONCLUSION: Silver nanoparticles are cytotoxic and genotoxic for human microvascular endothelial cells and might become a useful tool to control excessive angiogenesis.
Core tip: We studied the sensitivity to silver nanoparticles of microvascular endothelial cells, which are responsible for tissue homeostasis and fundamental in angiogenesis. Silver nanoparticles are cytotoxic and lead to membrane leakage. Cytotoxicity is not prevented by the antioxidants Trolox, N-acetyl-L-cysteine or apocynin. Silver nanoparticles also induce DNA damage as demonstrated by comet assay. When exposed to sublethal concentrations of silver nanoparticles for long times, the cells remain viable but are growth retarded. Interestingly, removal of silver nanoparticle rescue cell growth, suggesting that no permanent modifications occur. Silver nanoparticles are cytotoxic and genotoxic also for endothelial progenitors, which contribute to angiogenesis.
- Citation: Castiglioni S, Caspani C, Cazzaniga A, Maier JA. Short- and long-term effects of silver nanoparticles on human microvascular endothelial cells. World J Biol Chem 2014; 5(4): 457-464
- URL: https://www.wjgnet.com/1949-8454/full/v5/i4/457.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i4.457
Angiogenesis is a complex multistep process resulting in the formation of new capillaries from pre-existing vessels[1]. Protagonists in angiogenesis are the microvascular endothelial cells which are stimulated by angiogenic factors to proliferate, invade the surrounding tissues and undergo differentiation to form new vessels[1]. Therefore, impairing endothelial cell survival and growth can effectively block angiogenesis when excessive, as described in tumors, chronic inflammatory diseases, retinopathies, psoriasis among others[1]. Indeed, anti-angiogenic drugs are currently used in therapy to control angiogenesis in different pathological conditions[2].
Silver nanoparticles (Ag NP) are the most widely commercialized among all nanomaterials, because they show antiseptic properties. Consequently, they are used in the production of catheters or implantable devices as well as in cosmetics and household products[3]. Many studies have reported the cytotoxicity of Ag NP in several systems in vitro and in vivo[4]. Few studies focused on endothelial cells. Ag NP induce cell injury through the activation of the NFkB pathway in human endothelial cells from the umbilical vein (HUVEC)[5]. Exposure of rat brain endothelial cells to Ag NP leads to a marked cytotoxicity which is independent from the Ag ion release[6] and partially related to the induction of an inflammatory response[7]. Ag NP inhibit Vascular Endothelial Growth Factor (VEGF)-induced cell proliferation and migration in bovine retinal endothelial cells and promote apoptosis[8,9]. They also reduce VEGF and interleukin 1β-induced permeability in porcine retinal endothelial cells[10]. In contrast with these studies, Kang et al[11] showed that Ag NP stimulated the murine endothelial cell line SVEC4-10 to produce angiogenic factors and nitric oxide and to activate VEGF-receptor pathways. This study also reports that Ag NP increase angiogenesis in Matrigel plug assay.
To our knowledge, no data are available about the effects of Ag NP on human dermal microvascular endothelial cells (HMEC). It is noteworthy that endothelial cells show a high degree of phenotypic heterogeneity[12] and it is therefore relevant to analyze cells from different districts. Indeed, because of the diversity of hemodynamics and of signals from different tissue microenvironments, microvascular endothelial cells diverge from endothelial cells of the large vessels in gene expression profile[13].
We here show the short- and long-term effects of Ag NP on HMEC viability and growth, crucial events in angiogenesis. Interestingly, also endothelial colony-forming cells (ECFC), rare circulating endothelial cells which participate to angiogenesis[14], are sensitive to Ag NP.
Ag NP were purchased from NanoAmor (Houston, United States). According to the manufacturer, Ag NP chemical specifications are the following: purity, 99.5%; average particle size, 35 nm; specific surface area, 30-50 m2/g; particle morphology, spherical; crystallographic structure, cubic. Stock solutions of these nanoparticles were prepared in MCDB131 and EBM-2 media without fetal bovine serum (FBS) at concentration of 1080 μg/mL and kept at 4 °C for 1 mo. Stock solutions were sonicated several times just before preparing appropriate dilutions in culture medium. Unless otherwise specified, all the reagents were from Sigma (Oakville, Canada).
HMEC were obtained from Centers for Disease Control and Prevention (Atlanta, United States). They exhibit typical cobblestone morphology, express cell-surface molecules, including CD31 and CD36, secrete von Willebrand’s Factor, and take up acteylated low-density lipoprotein[15]. HMEC were grown in MCDB131 (Invitrogen, Milan, Italy) containing Epidermal Growth Factor (EGF) (10 ng/mL), hydrocortisone (1 μg/mL) and 10% FBS on 2% gelatin-coated dishes. ECFC were from Lonza (Milano, Italy) and grown in EBM-2 medium supplemented with 10% FBS and hydrocortisone, human EGF, VEGF, human basic Fibroblast Growth Factor, ascorbic acid, heparin and gentamicin/amphotericin B, according to the manufacturer’s instruction[16].
For MTT assay the cells were seeded in 96 well/plates. MTT is a sensitive quantitative colorimetric assay measuring the reduction of yellow tetrazolium salt MTT to dark purple formazan by succinate dehydrogenase, mainly in mitochondria, and it is now widely accepted as a reliable way to examine cytotoxicity[17]. Briefly, the medium was replaced with medium containing 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT, 0.5 mg/mL). At the end of the incubation, media were removed and formazan crystals generated by the cellular reduction activity were dissolved in dimethyl sulfoxide (DMSO). Absorbance was measured at 575 nm. In long-term experiments, cells were trypsinized, stained with trypan blue (0.4%) and viable cells were counted[16].
To evaluate cell membrane integrity, the lactate dehydrogenase (LDH) leakage assay was performed. Culture medium was collected and centrifuged at 4000 rpm for 10 min. 50 μL of medium was resuspended in a solution containing phosphate buffer 0.1 mol/L pH 7.0, 25 μL sodium pyruvate 23 mmol/L, 12.5 μL NADH 14 mmol/L. LDH catalyzes the reduction of pyruvate to lactate in the presence of reduced nicotinamide adenine dinucleotide (NADH) at pH 7.0. The reaction was monitored kinetically at 340 nm by the rate of decrease in absorbance resulting from the oxidation of NADH to NAD+ proportional to the activity of LDH in the sample. Data are expressed as enzyme unit/ml culture medium.
Intracellular oxidative stress was quantified using 2’-7’-dichlorofluorescein diacetate (DCFH). Cells were seeded into black bottomed 96 plates (Greiner bio-one, Frickenhausen, Germany) and 24 h later exposed for 30 min to different concentrations of NP in a 20 μmol/L DCFH solution. Intracellular oxidative stress was measured by monitoring the emission at 529 nm of the DCFH dye using Promega Glomax Multi Detection System. H2O2 was used as a positive control. The results are the mean of three independent experiments performed in quadruplicate. Data are shown as the fold increase in reactive oxygen species (ROS) levels of Ag NP treated cells compared to control ± SD.
The alkaline comet assay was performed on HMEC and ECFC cells. Cells were seeded at 10000/cm2 in 24 well-plates 24 h prior to treatment. After treatment, cells were trypsinized, mixed with low melting-point agarose and spread on pretreated slides which were allowed to dry. The slides were then immersed in ice cold lysis solution (Tris-HCl 0.01 mol/L pH 10, NaCl 2.5 mol/L, EDTA 0.1 mol/L, NaOH 0.3 mol/L, Triton 1%, DMSO 10%) and incubated at 4 °C for 60 min. Electrophoresis was conducted in ice cold running buffer (NaOH 0.3 mol/L, EDTA 0.001 mol/L) for 30 min at 300 mA. The slides were then rinsed, fixed in ice-cold methanol for 3 min and dried at room temperature. Cells were stained with ethidium bromide and analyzed with a fluorescence microscope.
Statistical significance was determined using the Student’s t test and set at P values less than 0.05. In the figures aP < 0.05; bP < 0.01; dP < 0.001.
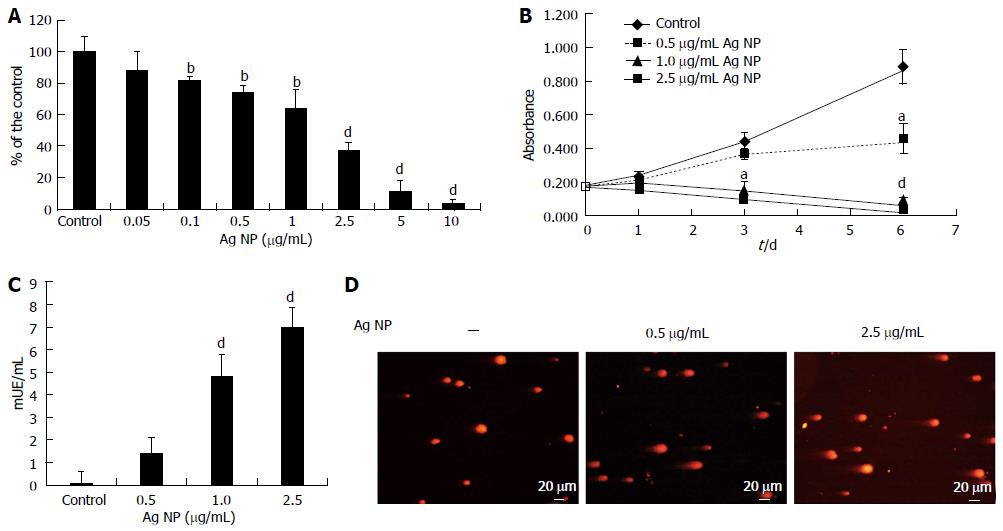
HMEC cells were exposed to various concentrations of Ag NP for 72 h. By MTT assay we found that Ag NP reduced the viability of HMEC cells dose dependently (Figure 1A). Cytotoxicity was statistically significant at a concentration 0.1 μg/mL (P < 0.01) with an Inhibitory Concentration 50 (IC50) 1.86 ± 0.13 μg/mL. The kinetics of NP-induced cytotoxicity were then examined using 0.5, 1.0 and 2.5 μg/mL Ag NP (Figure 1B). 1.0 and 2.5 μg/mL Ag NP had dramatic effects on cell viability, while HMEC survived with 0.5 μg/mL Ag NP. This experiment was also performed by counting the cells. We found no viable cells with the high concentrations of Ag NP while we observed an increase of cell number in HMEC treated with 0.5 μg/mL Ag NP (data not shown). These results demonstrate that Ag NP are cytotoxic in a dose and time dependent fashion. Interestingly, HMEC displayed the same sensitivity to Ag NP coated with PolyVinylPyrrolidone (data not shown), thus indicating that the toxic effect is not due to the release of Ag ions from their surface upon the contact with aqueous solutions.
LDH is a soluble cytosolic enzyme that is released into the culture medium following loss of membrane integrity. We measured LDH activity after treating HMEC for 24 h with Ag NP and observed a marked dose dependent increase of LDH activity in the medium after exposure to 1.0 and 2.5 μg/mL Ag NP (Figure 1C), while the leakage was very low in HMEC treated with 0.5 μg/mL of nanoparticles. These results indicate that high concentrations of Ag NP induce damage to the cellular membranes.
We then evaluated whether exposure to Ag NP for 24 h induced DNA strand breaks in HMEC cells by comet assay. As shown in Figure 1D, Ag NP induced a dose dependent level of genotoxic stress as demonstrated by the formation of comets.
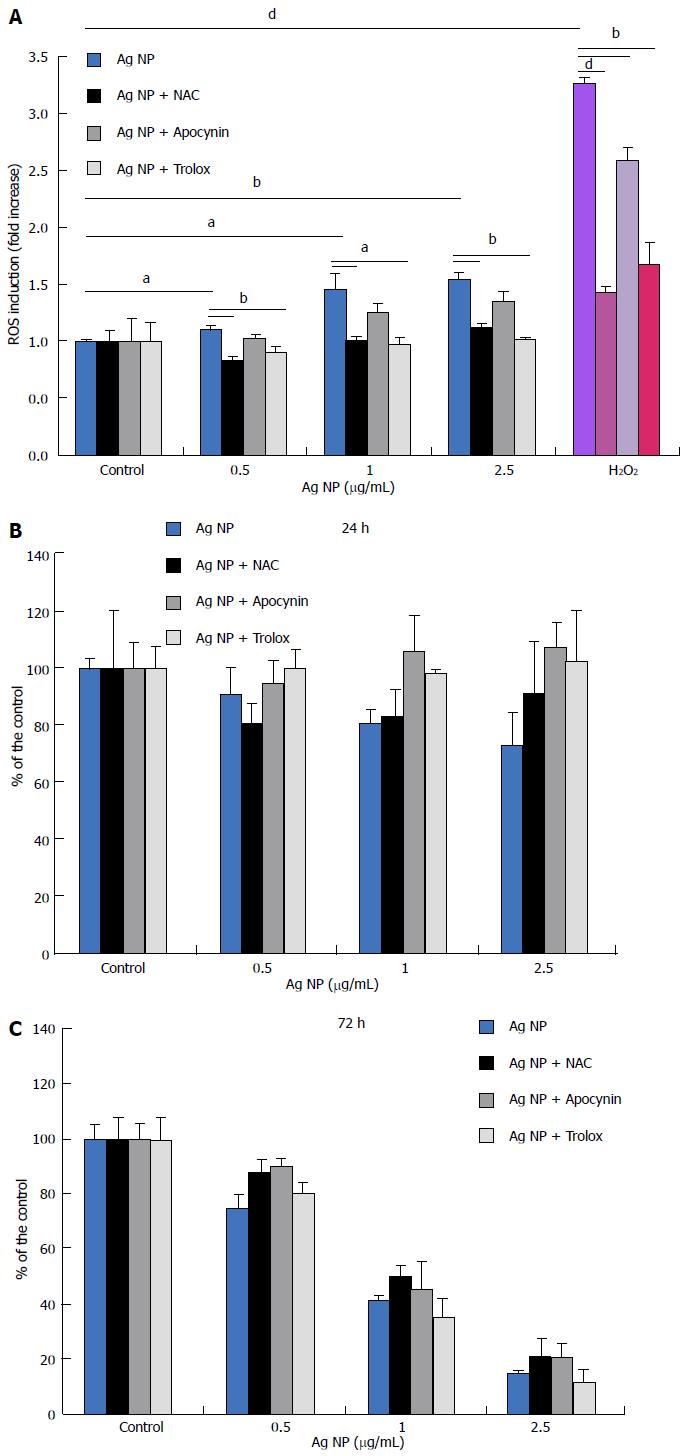
Initially, we measured the generation of ROS in response to Ag NP for 30 min. In HMEC, we found a significant dose-dependent increase of ROS (Figure 2A). We also evaluated the capability of three antioxidants with different mechanisms of action to inhibit ROS formation in response to Ag NP in HMEC. To this purpose, we used Trolox, a synthetic cell-permeable analogue of α-tocopherol which scavenges peroxyl and alkoxyl radicals, and N-acetyl-L-cysteine (NAC), a thiol compound that is a precursor of reduced glutathione and increases the activity of superoxide dismutase[18]. We also utilized apocynin, an inhibitor of NADPH oxidase[19]. We pre-incubated the cells with Trolox (40 μmol/L), apocynin (10 μmol/L), or NAC (5 mmol/L) for 2 h before adding different concentrations of Ag NP or H2O2 as a positive control. Figure 2A shows that the three antioxidants reduced the formation of ROS induced by Ag NP and by H2O2. To evaluate the role of oxidative stress in Ag NP-mediated toxicity, we treated the cell with the three antioxidants before adding Ag NP for 24 and 72 h. As shown in Figure 2B, antioxidants did not exert any protective effect against Ag NP in HMEC. Accordingly, we detected DNA damage by comet assay also when HMEC were pre-incubated with antioxidants prior to the addition of Ag NP (data not shown).
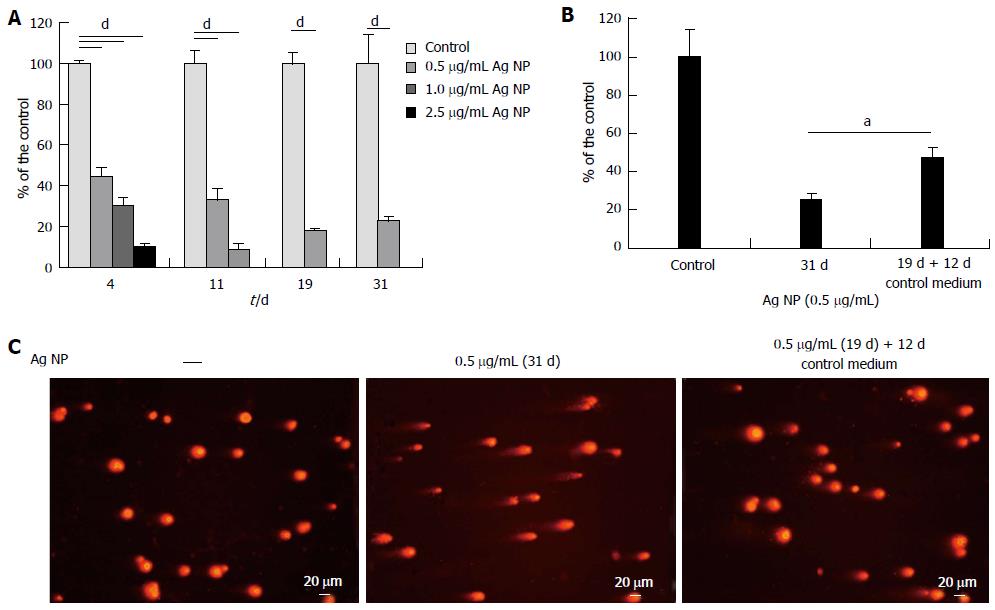
We then evaluated the effects of long term exposure to Ag NP. HMEC were treated with different concentrations of Ag NP and counted on the day in which the control untreated cells reached confluence. After counting, Ag NP-treated or untreated cells were re-seeded at the same density.
As shown in Figure 3A, 2.5 μg/mL Ag NP are highly cytotoxic and almost no viable cells are observed after 4 d treatment, while 11 d were necessary to have no viable cells when 1.0 μg/mL Ag NP was used. Only the 0.5 μg/mL Ag NP treated cells could be cultured for 31 d. At day 19, the cells were counted, re-seeded and propagated with or without 0.5 μg/mL Ag NP for additional 12 d. We show that HMEC partially recovered their proliferative potential after removing Ag NP from the medium (Figure 3B). In parallel, we performed comet assay and show that also genotoxicity was reversible upon removal of Ag NP from culture medium (Figure 3C).
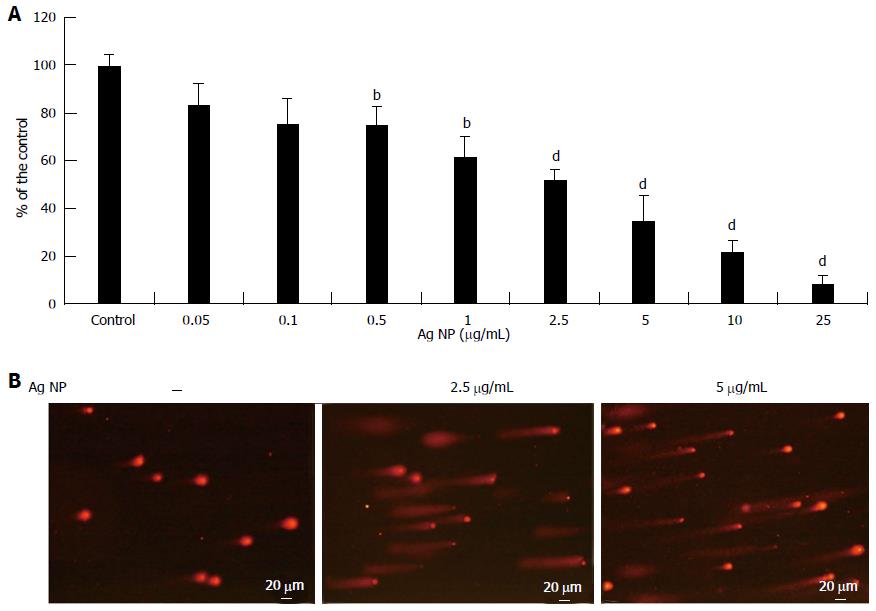
We extended part of our studies to ECFC, which are known to contribute to new vessel formation[14]. We evaluated the effects of different concentrations of Ag NP and found that, similarly to HMEC, Ag NP were cytotoxic in a dose dependent fashion (Figure 4A). It is noteworthy that ECFC are less sensitive to Ag NP than HMEC, since the IC50 we observed was 2.40 ± 0.24 μg/mL. We also found that Ag NP induced a marked DNA damage as detected by comet assay (Figure 4B).
The possibility of exploiting nanoparticles as alternative candidates to treat different diseases is under intensive investigation and represents a major challenge for modern medicine. Indeed, nanoparticles could serve as active components or could be useful to deliver functional moieties into their targets.
In particular, Ag NP are cytotoxic and genotoxic in various cell types including cancer cell lines[9,20], and induce DNA damage in human peripheral blood cells[21]. Consequently, the possibility to exploit their cytotoxic activity to treat proliferative diseases is of interest. Little is known about the effects of Ag NP on endothelial cells[5-7] and, to our knowledge, no data are available on human microvascular endothelial cells, pivotal regulators of tissue homeostasis and fundamental players in angiogenesis.
We here show that Ag NP are cytotoxic and genotoxic for HMEC. These results suggest that Ag NP might represent a challenging tool to treat all those diseases characterized by exaggerated angiogenesis[1]. Considering that: (1) angiogenesis is one of the hallmark of cancer[22]; and (2) human cancer cells are susceptible to the cytotoxic effects of Ag NP, Ag NP might also be promising to control proliferative diseases since they could target both the vasculature and the neoplastic cells. To this purpose, it is worth underscoring that also endothelial progenitors, specifically ECFC, circulating endothelial cells with intrinsic in vivo vessel forming ability[14], are sensitive to Ag NP. Interestingly, ECFC are emerging as important players in angiogenesis and also as useful tools in regenerative medicine[14].
Several mechanisms have been proposed to explain how Ag NP exert their activity. Ag NP are cytotoxic through inducing oxidative stress[23], impairing mitochondrial function[24], damaging DNA[25] and promoting inflammation[5]. In HMEC we show that Ag NP damage DNA, and this might account for the decreased viability of Ag NP-treated cells.
The contribution of free radicals to Ag NP toxicity in HMEC is puzzling. Indeed, while the production of ROS rapidly increases upon exposure to Ag NP, the three antioxidants used, which have different structure and act through different mechanisms[18,19], do not prevent Ag NP cytotoxicity. On the contrary, the antioxidant N-acetyl cysteine inhibited Ag NP cytotoxicity in HUVEC[5]. This discrepancy might be due to the well known differences occurring between micro- and macro-vascular endothelial cells and to different culture conditions used[12].
The reduced viability observed in HMEC exposed to Ag NP correlates with increased leakage of LDH, a cytosolic enzyme detected out of the cells when membrane integrity is lost. Recently, based on the evidence that discrepancies exist between the results of LDH release assay and MTT assay, the interference of Ag NP with LDH assay has been investigated[26]. While it is possible that the presence of Ag NP underestimates LDH leakage, in our system the results from LDH assay match the data from MTT assay.
When treated with sub-lethal, low concentrations of Ag NP, HMEC can be propagated for a month and are growth retarded for the duration of the experiment. This growth inhibition is partially reversible. Indeed, after 19 d, part of the cells were not exposed to Ag NP anymore and began to grow at a faster rate than HMEC maintained in the presence of Ag NP, suggesting that no permanent modifications occur as a consequence of a long-term exposure to Ag NP.
Most reports indicate that Ag NP promote an anti-angiogenic phenotype in endothelial cells of various species and derived from different districts by impairing their viability and inhibiting their growth, migration and differentiation in response to angiogenic factors[5-10]. Moreover, Ag NP were anti-angiogenic when evaluated in chick chorioallantoic membranes and in matrigel plugs in mice[27]. At the moment, we are aware of a single report demonstrating that Ag NP induce angiogenesis[11]. These discordant findings might result from the use of a transformed endothelial cell line[11]. While cell lines are useful for some experiments, they appear to be less suited for studies focused on the regulation of cell survival, proliferation and apoptosis[28].
In conclusion, our results demonstrate that Ag NP are toxic for microvascular endothelial cells and for endothelial precursors, thus indicating that they could be exploited to contrast angiogenesis. Moreover, it should be recalled that any substance that enters the blood stream will be in direct contact with the endothelium. Consequently, in case Ag NP are used to deliver drugs, the possibility of their cytotoxic effect on the endothelium lining the microvasculature should be taken into account.
More studies are necessary to delineate the mechanisms involved in Ag NP toxicity on HMEC and to translate our findings in in vivo models.
Because of their strategic location at the interface between blood and vessels, endothelial cells are readily exposed to various molecules, some of which may promote maladaptive functional changes or direct injury. Recently, the therapeutic potential of nanoparticles as active component or as vehicle to deliver drugs has been investigated. The study of the effects of nanoparticles on endothelial cells is therefore of paramount interest. Little is known about the sensitivity to silver nanoparticles of microvascular endothelial cells, which are highly-specialized regulators of tissue homeostasis and are fundamental in angiogenesis, i.e., the formation of new capillaries from pre-existing vessels.
The possibility of exploiting nanoparticles as alternative candidates to treat different diseases is under intensive investigation and represents a major challenge for modern medicine. This work shows that Ag NP are cytotoxic and genotoxic for capillary endothelial cells. Therefore, these results suggest that Ag NP might represent a challenging tool to treat all those diseases characterized by exaggerated angiogenesis, i.e., the formation of new capillaries from pre-existing vessels. Indeed, angiogenesis is a crucial pathogenic event in malignant tumors, chronic inflammatory diseases, retinopathies, and psoriasis.
This is the first study to report the short and long term effects of Ag NP on capillary endothelial cells.
By demonstrating the cytotoxic effect of Ag NP on capillary endothelial cells, this study may represent a future strategy for therapeutic intervention in all those diseases characterized by pathologic angiogenesis.
Angiogenesis is a complex multistep process resulting in the formation of new capillaries from pre-existing vessels. Protagonists in angiogenesis are the capillary endothelial cells which are stimulated by angiogenic factors to proliferate, invade the surrounding tissues and undergo differentiation to form new vessels. Nanoparticles are microscopic particles with dimension less than 100 nm. In particular, silver nanoparticles (Ag NP) are the most widely commercialized among all nanomaterials, because of their antiseptic properties.
This is a very well written and designed manuscript. The purpose of the study is clear. Method, results and discussion are well documented.
P- Reviewer: Cheng TH, Schattner M, Wang J S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 2. | Gerger A, LaBonte M, Lenz HJ. Molecular predictors of response to antiangiogenesis therapies. Cancer J. 2011;17:134-141. [PubMed] |
| 3. | Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 798] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 4. | de Lima R, Seabra AB, Durán N. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 2012;32:867-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 318] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Shi J, Sun X, Lin Y, Zou X, Li Z, Liao Y, Du M, Zhang H. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-κB pathways. Biomaterials. 2014;35:6657-6666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Grosse S, Evje L, Syversen T. Silver nanoparticle-induced cytotoxicity in rat brain endothelial cell culture. Toxicol In Vitro. 2013;27:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Trickler WJ, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Oldenburg SJ, Paule MG, Slikker W. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci. 2010;118:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Kalishwaralal K, Banumathi E, Ram Kumar Pandian S, Deepak V, Muniyandi J, Eom SH, Gurunathan S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf B Biointerfaces. 2009;73:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Gurunathan S, Lee KJ, Kalishwaralal K, Sheikpranbabu S, Vaidyanathan R, Eom SH. Antiangiogenic properties of silver nanoparticles. Biomaterials. 2009;30:6341-6350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 354] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Sheikpranbabu S, Kalishwaralal K, Venkataraman D, Eom SH, Park J, Gurunathan S. Silver nanoparticles inhibit VEGF-and IL-1beta-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J Nanobiotechnology. 2009;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Kang K, Lim DH, Choi IH, Kang T, Lee K, Moon EY, Yang Y, Lee MS, Lim JS. Vascular tube formation and angiogenesis induced by polyvinylpyrrolidone-coated silver nanoparticles. Toxicol Lett. 2011;205:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017-6022. [PubMed] |
| 13. | Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. 2003;100:10623-10628. [PubMed] |
| 14. | Reinisch A, Hofmann NA, Obenauf AC, Kashofer K, Rohde E, Schallmoser K, Flicker K, Lanzer G, Linkesch W, Speicher MR. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716-6725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683-690. [PubMed] |
| 16. | Baldoli E, Maier JA. Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis. 2012;15:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Castiglioni S, Casati S, Ottria R, Ciuffreda P, Maier JA. N6-isopentenyladenosine and its analogue N6-benzyladenosine induce cell cycle arrest and apoptosis in bladder carcinoma T24 cells. Anticancer Agents Med Chem. 2013;13:672-678. [PubMed] |
| 18. | Monticone M, Taherian R, Stigliani S, Carra E, Monteghirfo S, Longo L, Daga A, Dono M, Zupo S, Giaretti W. NAC, tiron and trolox impair survival of cell cultures containing glioblastoma tumorigenic initiating cells by inhibition of cell cycle progression. PLoS One. 2014;9:e90085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Petrônio MS, Zeraik ML, Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18:2821-2839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Satapathy SR, Mohapatra P, Preet R, Das D, Sarkar B, Choudhuri T, Wyatt MD, Kundu CN. Silver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53. Nanomedicine (Lond). 2013;8:1307-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Flower NA, Brabu B, Revathy M, Gopalakrishnan C, Raja SV, Murugan SS, Kumaravel TS. Characterization of synthesized silver nanoparticles and assessment of its genotoxicity potentials using the alkaline comet assay. Mutat Res. 2012;742:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 46661] [Article Influence: 3332.9] [Reference Citation Analysis (4)] |
| 23. | Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009;190:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 456] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 24. | Teodoro JS, Simões AM, Duarte FV, Rolo AP, Murdoch RC, Hussain SM, Palmeira CM. Assessment of the toxicity of silver nanoparticles in vitro: a mitochondrial perspective. Toxicol In Vitro. 2011;25:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, Ginzkey C, Koehler C, Hagen R, Kleinsasser N. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011;201:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 26. | Oh SJ, Kim H, Liu Y, Han HK, Kwon K, Chang KH, Park K, Kim Y, Shim K, An SS. Incompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generation. Toxicol Lett. 2014;225:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Kemp MM, Kumar A, Mousa S, Dyskin E, Yalcin M, Ajayan P, Linhardt RJ, Mousa SA. Gold and silver nanoparticles conjugated with heparin derivative possess anti-angiogenesis properties. Nanotechnology. 2009;20:455104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Boerma M, Burton GR, Wang J, Fink LM, McGehee RE, Hauer-Jensen M. Comparative expression profiling in primary and immortalized endothelial cells: changes in gene expression in response to hydroxy methylglutaryl-coenzyme A reductase inhibition. Blood Coagul Fibrinolysis. 2006;17:173-180. [PubMed] |