Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. Aug 26, 2014; 5(3): 321-333
Published online Aug 26, 2014. doi: 10.4331/wjbc.v5.i3.321
Published online Aug 26, 2014. doi: 10.4331/wjbc.v5.i3.321
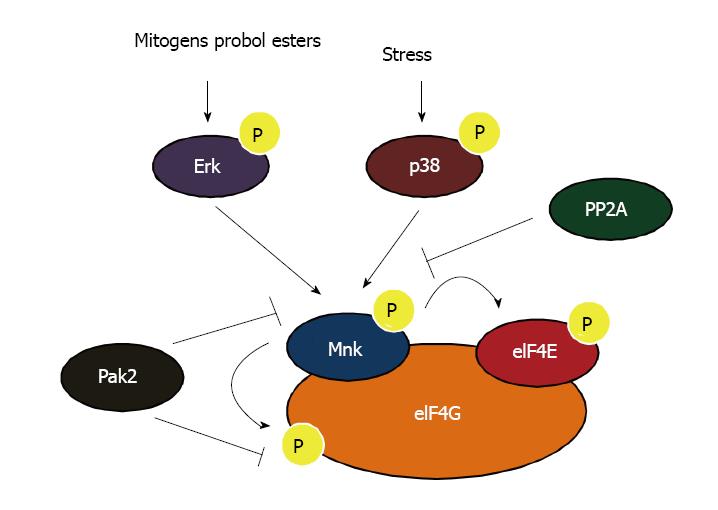
Figure 1 Regulation of Mnk kinases.
The Mnk kinases are phosphorylated on Thr 197/202 by the p38 and Erk1/2 mitogen-activated protein kinases (MAPKs). They can associate with eIF4G and this interaction is essential for the efficient phosphorylation of their target eIF4E. The Mnk kinases are also known to phosphorylate eukaryotic initiation factor 4G (eIF4G) but its functional consequences remain to be determined. Pak2 can phosphorylate Mnk1 on Thr22/Ser27 resulting in decreased affinity for eIF4G and potentially interferes with Mnk1 mediated phosphorylation of eIF4E. Additionally Pak2 also phosphorylates eIF4G inhibiting its interaction with eIF4E. Protein phosphatase 2A (PP2A) is a phosphatase for Mnk1 and thereby negatively regulates Mnk kinase activity.
- Citation: Joshi S, Platanias LC. Mnk kinase pathway: Cellular functions and biological outcomes. World J Biol Chem 2014; 5(3): 321-333
- URL: https://www.wjgnet.com/1949-8454/full/v5/i3/321.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i3.321