Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 85-92
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.85
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.85
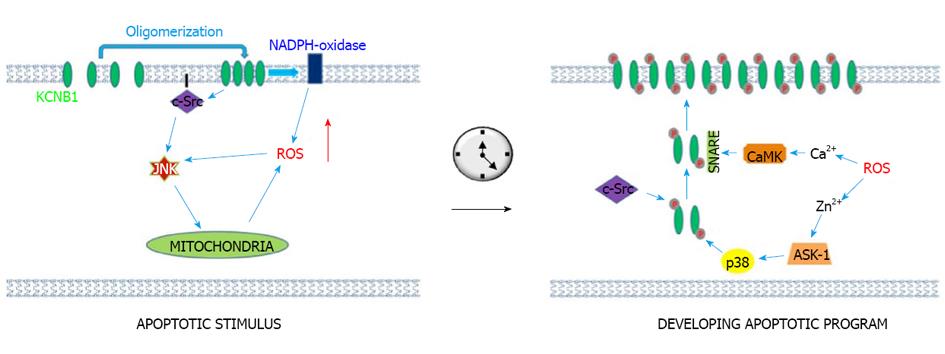
Figure 1 A two-step model for the pro-apoptotic actions of KCNB1.
Upon exposure to oxidants, KCNB1 oligomers are formed. They accumulate in the plasma membrane thereby perturbing the organization of lipid rafts. This results in activation of an apoptotic stimulus mediated by c-Src and downstream, JNK kinases. As result of activation of c-Src and JNK kinases and in part of NADPH-oxidase (Xilong Wu, private communication) which is localized in the plasma membrane, ROS levels increase in the cell. ROS induce a raise in cytosolic Ca2+ and Zn2+ that initiate a phosphorylation-mediated surge of KCNB1 channels that further drives apoptosis. The signaling pathway activated by Zn2+ proceeds through activation of p38 by ASK-1 and independently, of c-Src tyrosine kinases (Zn2+ inhibits the activity of the tyrosine phosphatase PTP epsilon) which phosphorylate KCNB1 at S800 and Y124 thereby allowing interaction with SNARE family protein syntaxin. The Ca2+ signaling pathway results in activation of CaMKII kinase which in turn acts to modulate the interaction of KCNB1 with syntaxin. It is not known whether Src and p38 phosphorylation directly act to increase KCNB1 current. ROS: Reactive oxygen species.
- Citation: Sesti F, Wu X, Liu S. Oxidation of KCNB1 K+ channels in central nervous system and beyond. World J Biol Chem 2014; 5(2): 85-92
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/85.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.85