Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Apr 26, 2011; 2(4): 67-72
Published online Apr 26, 2011. doi: 10.4331/wjbc.v2.i4.67
Published online Apr 26, 2011. doi: 10.4331/wjbc.v2.i4.67
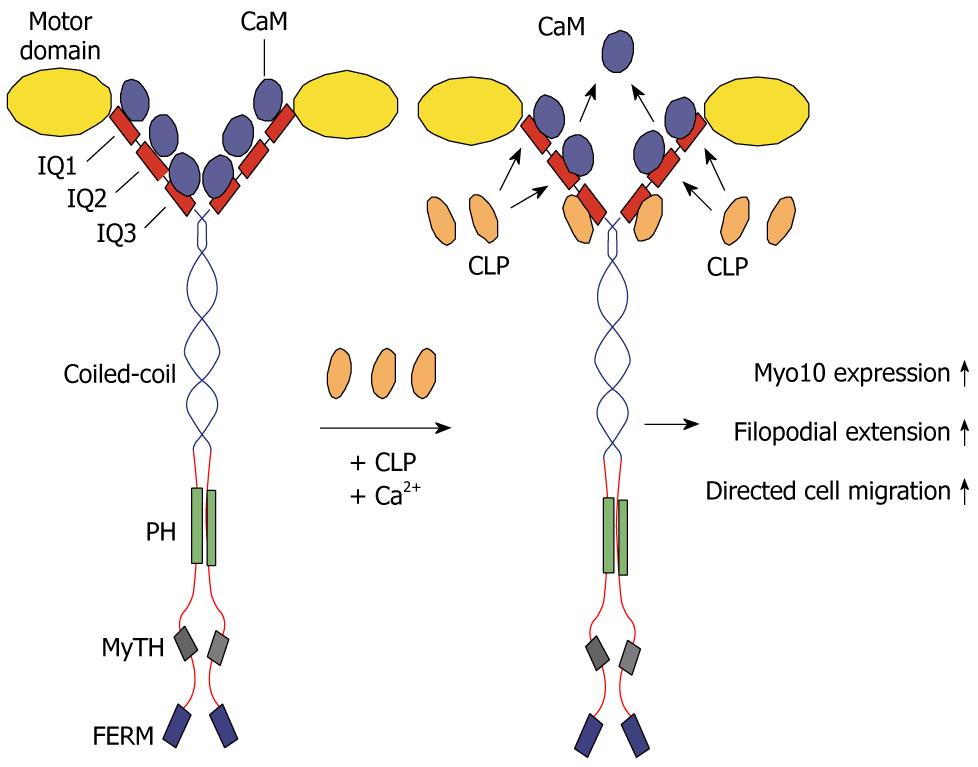
Figure 3 Calmodulin-like protein serves as light chain for Myo10 and increases its expression and function.
Myo10 consists of two heavy chains with an N-terminal motor domain, a “neck” region of three IQ domains involved in light chain binding, a putative coiled-coil region, and a tail region that contains pleckstrin homology (PH) domains, a myosin tail homology (MyTH) domain, and a C-terminal FERM (4.1, ezrin, radixin, moesin) domain. The IQ motifs bind calmodulin (CaM) or calmodulin-like light chains, which regulate Myo10 activity and Ca2+ sensitivity. Upon expression of calmodulin-like protein (CLP) (in the presence of Ca2+), it competes successfully with calmodulin and binds tightly to IQ3 and possibly IQ1 and IQ2 of Myo10. CLP binding results in increased Myo10 expression and function, as demonstrated by increased filopodial extension and enhanced directional cell migration.
- Citation: Strehler EE. Emanuel Strehler’s work on calcium pumps and calcium signaling. World J Biol Chem 2011; 2(4): 67-72
- URL: https://www.wjgnet.com/1949-8454/full/v2/i4/67.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i4.67