Copyright
©2011 Baishideng Publishing Group Co.
World J Biol Chem. Mar 26, 2011; 2(3): 48-58
Published online Mar 26, 2011. doi: 10.4331/wjbc.v2.i3.48
Published online Mar 26, 2011. doi: 10.4331/wjbc.v2.i3.48
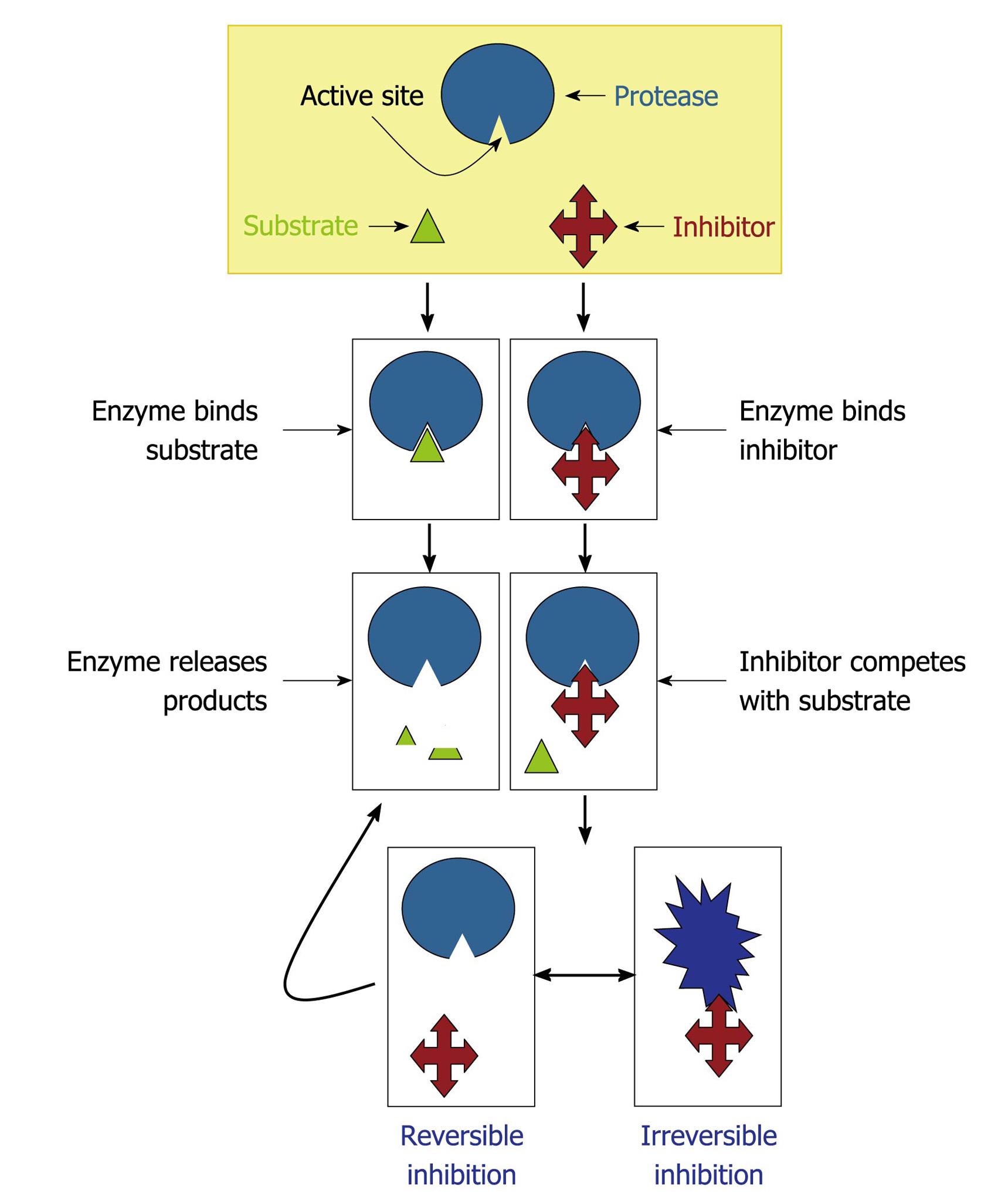
Figure 6 Mechanisms of protease inhibition.
The protease inhibitor competes with the substrate to bind to the active site of a protease and two distinct possibilities arise: (1) substrate binds to the catalytic site and then is cleaved by the protease, which releases the products or (2) inhibitor binds to the active site and by steric hindrance blocks the substrate attachment. In this last case, the inhibitor can promote an irreversible (the conformational structure of the protease is completely lost) or reversible inhibition (when the inhibitor disconnects from the enzyme, the substrate can bind to it).
- Citation: Santos ALSD. Protease expression by microorganisms and its relevance to crucial physiological/pathological events. World J Biol Chem 2011; 2(3): 48-58
- URL: https://www.wjgnet.com/1949-8454/full/v2/i3/48.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v2.i3.48