SPHINGOLIPID METABOLISM
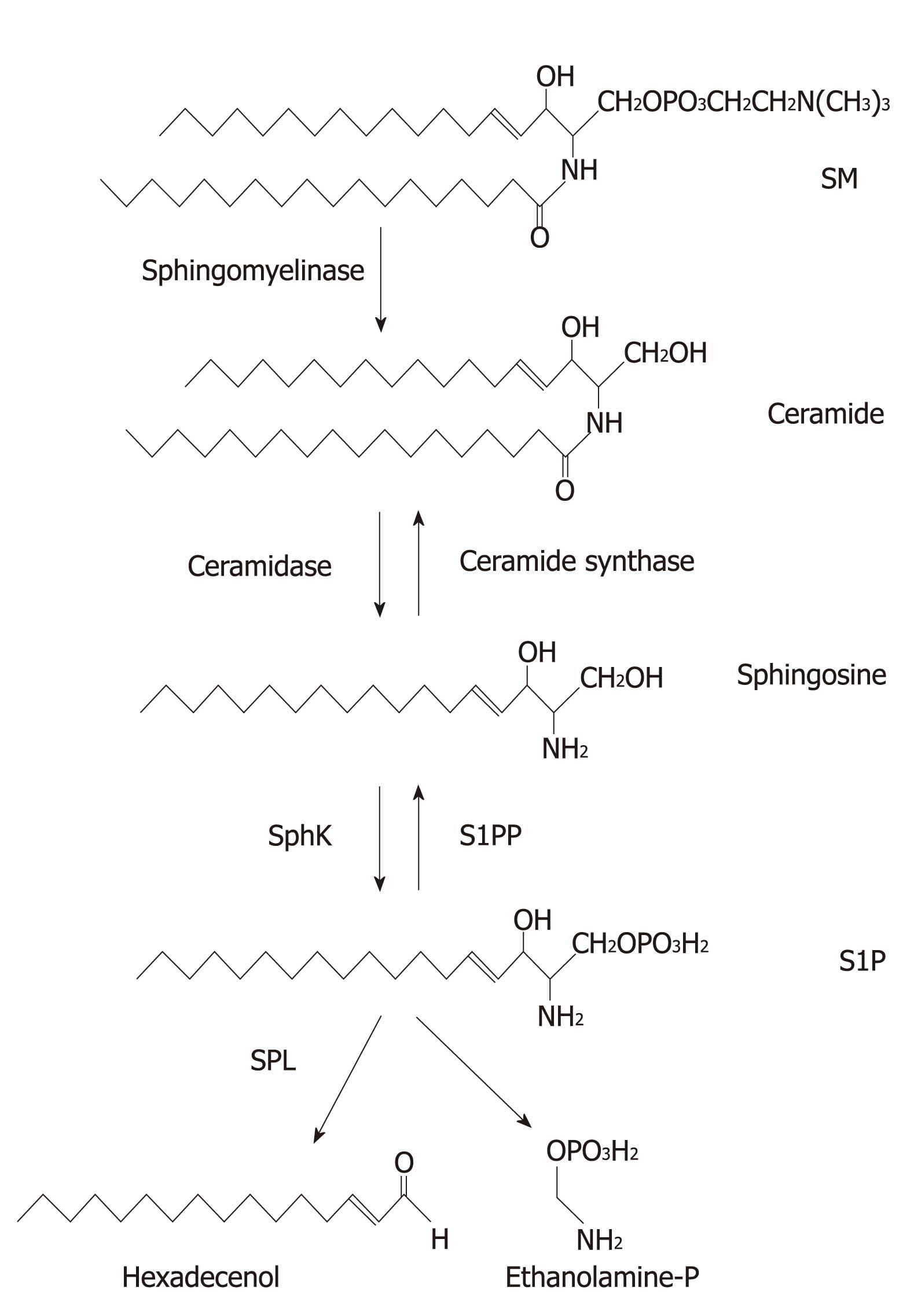
Sphingolipids are present mainly in the cell membranes of most eukaryotic species[1]. All sphingolipids are characterized by the presence of a long chain base that can be acrylated at the free amino group to form a ceramide, which is the simplest structural form of sphingolipid (Figure 1). Diverse sphingolipids result from different hydrophobic sphingolipid bases combined with fatty acids, and are further modified by adding phosphate, phosphorylcholine and sugar groups (Figure 1). Besides providing structural integrity in cell membranes, sphingolipids activate multiple signal transduction pathways that are associated with diseases including cancers[2-4]. The key sphingolipid metabolites are ceramide, sphingosine, and sphingosine 1-phosphate (S1P). Ceramide, a pro-apoptotic lipid that accumulates in cells in response to various stresses, can be further modified by adding phosphorylcholine to form sphingomyelin (SM), a structural component of membranes, or by linking sugar residues that results in formation of glycosphingolipids. Ceramide can either be generated de novo or by breakdown of SM through the activities of sphingomyelinase (Figure 1). By the activity of ceramidase, ceramide can be deacrylated to generate sphingosine. Subsequently, sphingosine can either be acrylated back to ceramide by ceramide synthase or further phosphorylated by sphingosine kinases (Sphks) to form S1P. S1P can be dephosphorylated back to sphingosine by S1P phosphatases (S1PPs) or type 2 phosphatidate phosphohydrolases[5]. Alternatively, S1P is irreversibly degraded in the endoplasmic reticulum (ER) by S1P lyase (SPL) to hexadecenol (Hex) and ethanolamine phosphate (EP) (Figure 1). Ceramide and sphingosine are associated with the induction of apoptosis, whereas S1P promotes cell growth, proliferation, migration, and survival in various cell types[6-10]. These sphingolipid metabolites with opposite functions are inter-convertible within cells. Therefore, the balance between ceramide/sphingosine and S1P forms a sphingolipid rheostat model[11] (Figure 2), which suggests that it leads to cell death when this balance moves towards ceramide or sphingosine, but to cell survival or proliferation when S1P levels are increased[12,13]. Indeed, several reports have suggested that reduction of S1P level that leads this rheostat towards ceramide/sphingosine might provide a potential target for cancer therapies[14-18]. This review is focused on discussing the roles of S1P in proliferation, migration, angiogenesis, and autophagy, which are all closely associated with tumorigenesis.
Figure 1 Sphingolipid structure and metabolism.
All sphingolipids contain a long chain base. Sphingomyelin (SM) is metabolized to ceramide by sphingomyelinase. Ceramide is further metabolized to sphingosine and sphingosine 1-phosphate (S1P) by ceramidase and sphingosine kinases (SphKs), respectively. Subsequently, S1P is irreversibly degraded to hexadecenal and ethanolamine phosphate by S1P ligase (SPL). S1P can be recycled to sphingosine and then ceramide by S1P phosphatase (S1PP) and ceramide synthase, respectively.
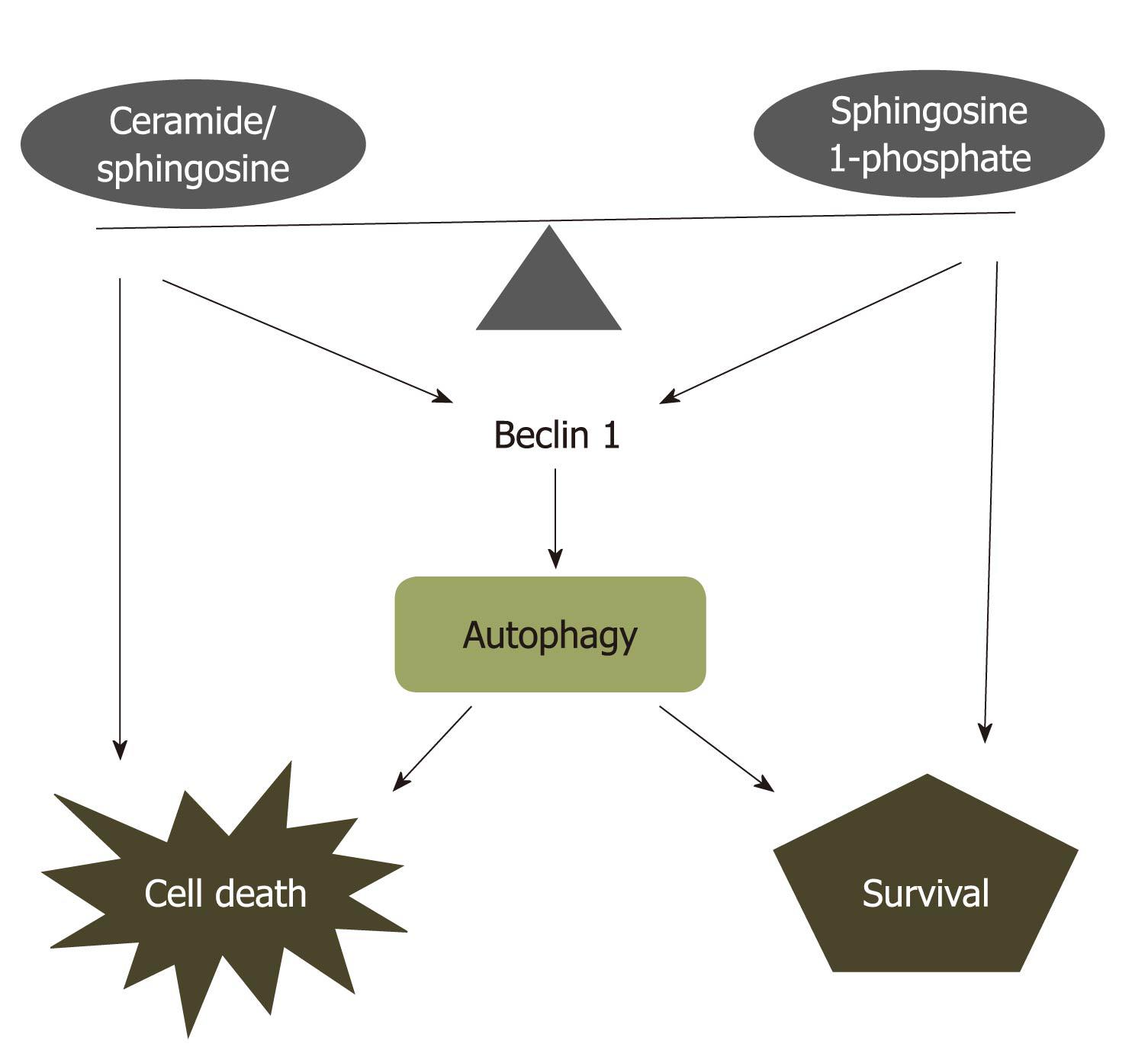
Figure 2 Ceramide-sphingosine-sphingosine 1-phosphate rheostat model.
Ceramide, sphingosine, and sphingosine 1-phosphate (S1P) with opposing functions are interconvertible within cells. The balance between ceramide/sphingosine and S1P forms a sphingolipid rheostat model, that is primed for cell death when the balance shifts towards ceramide or sphingosine, or to cell survival or proliferation when S1P levels are increased. This rheostat model also regulates autophagic cell death or survival through targeting to Beclin 1 which interacts with Bcl-2 protein.
DUAL MESSENGER SIGNALINGS OF S1P
Extracellular action mode of S1P
Like other sphingolipids, S1P is thought to be mainly a degradative metabolite of sphingolipids formed during turnover of eukaryotic cell membranes. S1P plays important roles in diverse physiological and pathological processes in cancer cells. It regulates cell growth, proliferation, differentiation, cell survival, migration, and angiogenesis[8,10,19-23]. S1P exerts most of its function as a specific ligand for a family of G-protein-coupled receptors (GPCRs), termed S1P1-S1P5[24-27]. In addition to binding to its cell surface receptors, S1P also mediates several biological functions, including calcium homeostasis, cell growth, and protection of apoptosis through a receptor-independent intracellular mechanism[28]. Thus, S1P can function both as an extracellular first messenger and also as an intracellular second messenger. The dual functional mode, as well as five specific receptors could explain the diverse biological functions regulated by S1P.
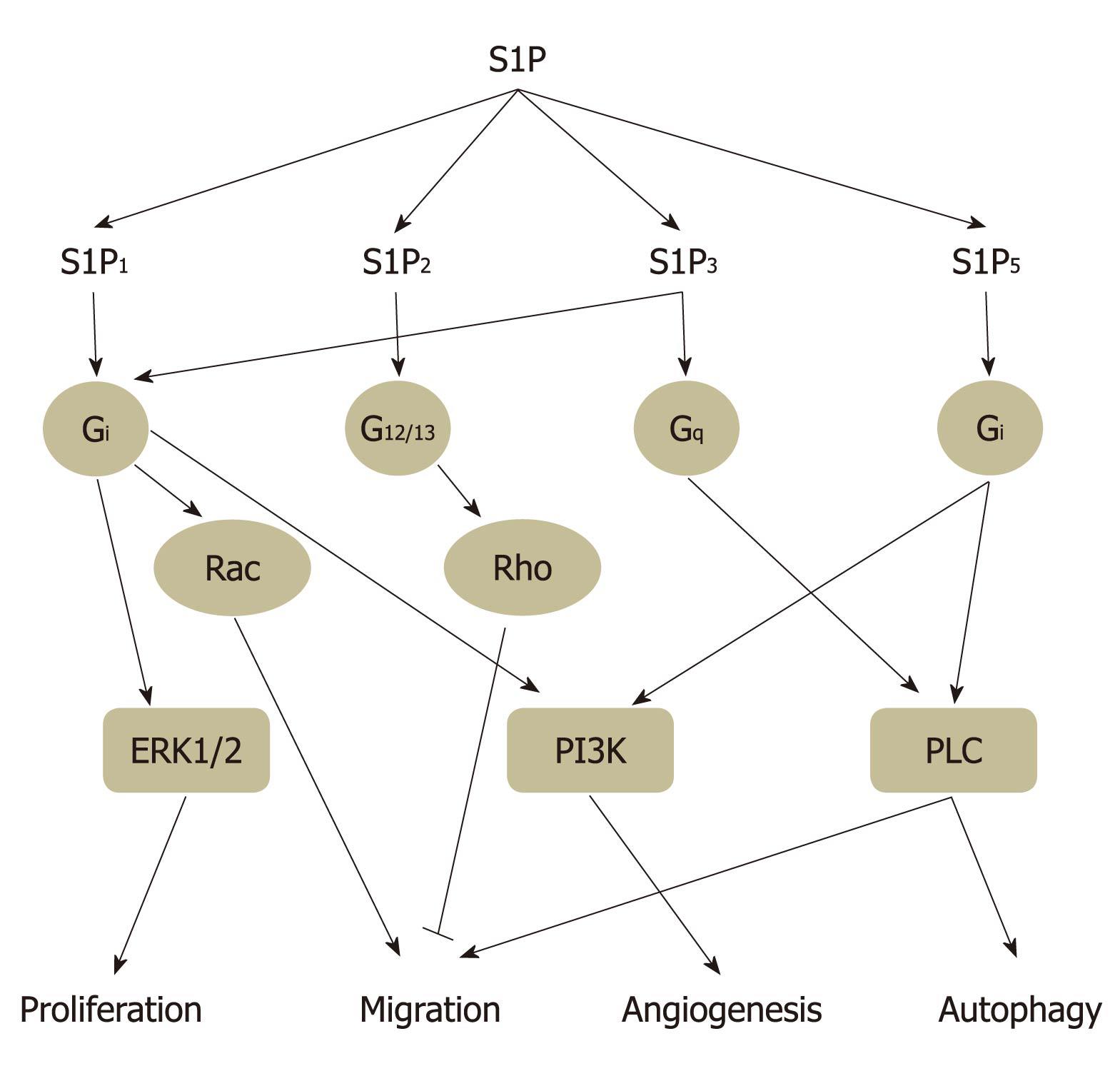
S1P can potentially stimulate diverse signal transduction pathways in different cell types, as well as within the same cell, depending on the patterns of S1P receptor expressed. S1P1 is coupled exclusively via Gi protein to activate Ras, mitogen activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), Akt, and phospholipase C (PLC) pathways. Both S1P2 and S1P3 coupled to Gi, Gq and G12/13 and activating Ras, MAPK, PI3K, Akt, PLC, and Rho-dependent pathways (Figure 3). G protein coupling properties of S1P4 and S1P5 remain largely unclear at present. Through coupling with Gi and G12/13, but not Gq, S1P4 mediates cell shape change and motility via a Rho-dependent pathway in CHO-K1 and Jurkat T cells[29]. S1P5 appears to activate G12/13 protein and subsequent Rho/ROCK signaling pathway to attenuate cell migration in oligodendrocyte precursor cells[30]. These studies strongly suggested that bioactive lipid S1P mediates diverse cytoprotective functions through activation of its extracellular receptors.
Figure 3 Sphingosine 1-phosphate receptor subtype-specific signaling.
Sphingosine 1-phosphate (S1P)1, S1P2, S1P3, and S1P5 activate partially overlapping downstream signaling via coupling different G proteins and then regulate several biological functions, such as cell proliferation, migration, angiogenesis, and autophagy. Through coupling to Gi protein, S1P1 activates extracellular signal-regulated kinase (ERK)1/2, Rac, and phosphoinositide 3-kinase (PI3K) signaling to regulate cell proliferation, migration, and angiogenesis, respectively. S1P2 mediates inhibition of cell migration via G12/13-Rho signaling, whereas S1P3 enhances cell migration and angiogenesis through Gi-Rac and Gq-phospholipase C (PLC) signaling, respectively. Our unpublished results have revealed that Gi coupled-S1P5 induces autophagy which is mediated through PI3K and PLC signaling.
S1P acts as a second messenger
Besides binding to its cell surface receptors, S1P also acts as a second messenger to mediate several cellular functions, such as proliferation, cell survival, autophagy, and suppression of apoptosis[31-33]. As mentioned above, S1P is generated by the phosphorylation of sphingosine via the activity of SphKs. Several growth factors and cytokines, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), tumor necrosis factor-α (TNF-α), and nerve growth factor (NGF), which are well known inducers of cell survival and proliferation, also activate SphKs and thereby increase intracellular S1P levels[34,35]. The levels of S1P in cells are tightly regulated by the balance between SphK-dependent synthesis and S1PP- or SPL-dependent degradation. Early studies have proposed that S1P may directly activate intracellular calcium channels via an inositol triphosphate-independent action[36], which suggests that S1P acts as a second messenger. However, this event has not been molecularly characterized. Several studies have supported this view by demonstrating that increasing intracellular S1P levels also upregulate intracellular calcium concentrations[37]. In addition, N, N-dimethy sphingosine (DMS), an inhibitor of SphK, inhibits calcium release elicited by activating m2 and m3 muscarinic acetylcholine receptors (mAChRs) that are expressed in human embryonic kidney (HEK)-293 cells[38]. Furthermore, elevation of intracellular S1P either by depleting S1PP or overexpressing SphK1 causes autophagy in human breast cancer MCF-7 cells[31,32]. These results support the intracellular second messenger role of S1P. However, the intracellular targets for S1P remain largely elusive.
Inside-out signaling by S1P
As mentioned above, S1P is mainly generated within cells and it functions as an extracellular mediator. The polar nature of the head group on S1P, which contains both phosphate and ammonium ion pairs, as well as a hydroxyl group, suggests that S1P cannot cross the plasma membrane easily. Thus, an export or secretion system for S1P may be necessary. Indeed, it has been suggested that intracellular S1P, generated by SphKs, is released through a family member of ABC transporter proteins, which are originally defined as multidrug transporter proteins, into the extracellular milieu close to S1P receptors[39-42]. PDGF-directed cell motility requires cross-talk to S1P1via activation of SphKs and formation of S1P[43]. Thus, intracellular S1P can either be secreted or diffuse across the plasma membrane through transporter proteins and then activate S1P receptors in an autocrine or paracrine manner. In addition, SphKs translocate to the leading edge of migrating cells following PDGF stimulation, which results in the formation of an extracellular S1P gradient at the leading edge, thereby directing cell movement[44]. Furthermore, phorbol ester induces the translocation of SphK1 to the plasma membrane, which is accompanied by the extracellular appearance of S1P in HEK 293 cells[45]. Ancellin et al[46] have proposed a novel mechanism of S1P generation in the extracellular milieu by export of SphK1. They have revealed that depletion of SphK1 by siRNA significantly prevents SphK1 export in human umbilical vein endothelial cells (HUVECs), whereas SphK1 is released into the extracellular milieu by transfection with SphK1 in HEK293 cells. In addition, overexpression of SphK1 results in the release of SphK, which may induce angiogenesis and vascular maturation in AE1-2a cells. These observations suggest that S1P is generated in the extracellular milieu, and that extracellular export of SphK may be responsible for the action of S1P in the vascular system. These studies strongly suggest that S1P possess two action modes, including both inside-out and outside-in signaling.
The sphingolipid rheostat model
It has been suggested that S1P regulates apoptosis through activating extracellular signal-regulated kinase (ERK) and inhibiting c-Jun N-terminal kinase (JNK)[11,47,48]. Moreover, ceramide induces apoptosis by these pathways[11]. Due to the opposing effects of S1P and ceramide, which can be inter-convertible within cells, on the induction of apoptosis, the dynamic balance between S1P and ceramide/sphingosine may therefore determine the cell fate[11] (Figure 2). Thus, agents that regulate the inter-conversion of ceramide-sphingosine-S1P may direct the cell towards either an apoptotic or a survival program depending on the levels of ceramide/sphingosine and S1P, respectively (Figure 2). Despite the fact that thespecific intracellular targets of S1P remain elusive, several intracellular targets of ceramide have been identified that mediate apoptotic action of ceramide, including protein phosphatases PP1 and PP2A[49], protein kinase C[50], and cathepsin D[51]. Moreover, S1P may protect cells from apoptosis through inside-out signaling to activate its cell surface receptors. Indeed, exogenous S1P has been shown to upregulate anti-apoptotic Bcl-2 and Mcl-1 proteins, as well as downregulate pro-apoptotic Bad and Bax[52-54]. Furthermore, overexpression of SphK1, which upregulates S1P level, also enhances the expression of Bcl-2 and suppresses the expression of Bim in endothelial cells[55]. In addition, overexpression of Bcl-2 also upregulates, and knockdown of Bcl-2 downregulates the expression of SphK[56], which strongly suggests that S1P is an important regulator for apoptosis.
Several in vivo and in vitro studies have supported the sphingolipid rheostat model in cancer, especially studies of SphK-1. Overexpression of SphK1 induces the transformation of NIH3T3 cells, based on the observation of colony formation on soft agar and the ability to form tumors in nude mice[57]. Furthermore, overexpression of SphK1 inhibits apoptosis and induces chemoresistance[12,58]. By comparing two different human prostate cancer cell lines, PC-3 and LNCaP, which are camptothecin (CPT)-resistance and -sensitive, respectively, Akao et al[59] have shown that PC-3 cells exhibit higher expression levels of SphK1, and also elevate S1P receptor expression, as compared with those in LNCaP cells. Knockdown of SphK1 significantly inhibits PC-3 cell growth, which suggests that SphK1 signaling is involved in the proliferation of PC3 cells[59]. Indeed, orthotopic implantation of SphK1-overexpressing PC-3 cells into nude mice develops remarkably larger tumors that are resistant to docetaxel treatment[60]. Similar results have also been observed in other cancer cells, such as pancreatic cancers and chronic myeloid leukemia cells are resistant to gemcitabine[61] and imatinib[12], respectively, following the overexpression of SphK1. Alternatively, administration of a specific monoclonal antibody against S1P reduces tumor progression and eliminates measurable tumors in murine xenograft and allograft models[14]. These results have suggested that SphK1 acts as a sensor of chemotherapy, thereby inhibition of SphK1 could be a target for chemotherapy-induced apoptosis in cancer. In addition, S1P signaling may play important roles in regulating tumorigenesis and tumor progression.
S1P AND TUMORIGENESIS
S1P as a regulator for cell proliferation
As mentioned above, S1P acts as a ligand for a family of GPCRs to activate various signaling cascades, that are implicated in promoting cell growth, survival, migration, and invasion, as well as inhibiting apoptosis[34,62-65]. Several reports have indicated that S1P serves as a mitogen to increase DNA synthesis and cell division in diverse quiescent cell types, including Swiss 3T3 cells, rat-1 fibroblasts, vascular smooth muscle cells, and endothelial cells[66-68]. This S1P-induced cell proliferation is suppressed by inhibitors of different G proteins, which indicates that the effects of S1P on proliferation induction are through extracellular receptors[69]. In addition to extracellular action mode, intracellular S1P signaling also plays important roles in facilitating cell proliferation. Two distinct SphK isoforms have been identified, SphK1 and SphK2, both of which are responsible for conversion of sphingosine to S1P[70]. Recent, evidence has suggested that SphK1 possess many characteristics of oncogenes[12,55,57,58,71]. Furthermore, elevated expression of SphK1 is observed in multiple types of cancer, such as gastric, lung, brain, colon, kidney, and breast cancers, and associated with tumor grading as well as reduced patient survival[72-80].
In contrast to SphK1 activity as a potential oncogene, the effect of SphK2 on cell proliferation remains controversial. Overexpression of SphK2 causes cell cycle arrest, caspase-3 activation, cytochrome c release, and inhibits cell growth; these effects require its membrane localization[81,82]. Moreover, SphK2 contains a nine-amino acid motif similar to that present in BH3-only protein, a pro-apoptotic Bcl-2 family member. Like other BH3-only Bcl-2 proteins, SphK2 binds and inhibits Bcl-xL and induces apoptosis[82]. We and others have revealed that S1P attenuates cell growth in human prostate cancer PC-3 cells[83,84] and human keratinocytes[85]. Further experiments are needed to determine whether this S1P-mediated anti-proliferative effect results from SphK2 activity. On the other hand, knockdown of SphK2 in some cancer cells unexpectedly attenuates cell growth. In breast cancer MCF-7 cells and colon cancer HCT116 cells, down-regulation of SphK2 with siRNA decreases G2/M phase arrest and markedly enhances apoptosis induced by doxorubicin[86]. Likewise, knockdown of SphK2 inhibits cell proliferation in U14242 and U87 MG glioblastoma cells[76]. Moreover, the growth of SphK2-deficient MCF-7 breast tumor xenografts is markedly delayed and displays a prominent anti-tumor phenotype, based on the observation of increasing expression of pro-inflammatory mediators such as NO, TNF-alpha, IL-12 and MHCII, and lower expression of anti-inflammatory IL-10 and CD206[87]. These observations suggest that either exogenous S1P mediated through its cell surface receptors or intracellular S1P produced by SphKs is important for cell proliferation.
As mentioned above, although both SphK1 and SphK2 increase S1P level, their biological effects are diverse in some cells. A possible explanation for the opposing effects of SphK1 and SphK2 is that the two proteins are located in, or translocated to, different compartments within cells. Indeed, SphK1 and SphK2 are predominantly localized in cytosol and nucleus, respectively, thereby, creating a potential for distinct functional pools of S1P[88]. It is critical for S1P to mediate diverse biological functions, not only by activation of SphKs, but also by their subcellular localization[89]. Additionally, SphK1 activation involves its translocation to the plasma membrane, where it, subsequently activates cell surface S1P receptor[43]. Nevertheless, S1P generated by SphK2 does not transactivate S1P receptor[82]. This translocation of SphK1 is regulated by ERK1/2-mediated phosphorylation of SphK1 on Ser225, which is also required for SphK1 catalytic activity[89]. Despite exhibiting SphK1 activity that is required for transformation of NIH3T3 fibroblasts[57], S225A SphK1 mutant prevents Ras-dependent transformation. Targeting of either wild-type or S225A SphK1 to the plasma membrane, which is necessary for S1P generation, promotes transformation of NIH3T3 fibroblasts and increases S1P levels[90]. Furthermore, artificial targeting of SphK1 to ER converts the normally anti-apoptotic kinase into one with a pro-apoptotic function[91].
S1P as a regulator for cell motility
Pharmacological inhibitors, such as DMS and D, L-threo-dihydrosphingosine (DHS), that inhibit both SphK1 and SphK2, suppress chemotaxis following growth factor stimulation in diverse cell types[28]. Likewise, knockdown of SphK1, but not SphK2, with specific siRNA prevents EGF-, prolactin-, and estrogen-induced migration in MCF-7 cells[92,93]. Furthermore, down-regulation of both SphK1 and SphK2 suppresses EGF-induced migration, while overexpression of either SphK1 or SphK2 enhances migration towards EGF in MDA-MB-453 breast cancer cells[94]. These results suggest that SphK2 has a distinct or an overlapping function on migration regulation from that of SphK1.
Matrix metalloproteinases (MMPs) are zinc-dependent proteolytic enzymes, which are involved in degradation of the extracellular matrix and play critical roles in cell migration. Through activation of S1P1, S1P upregulates the expression of urokinase plasminogen activator (uPA), a potent stimulator involved in cancer cells invasion, in human glioblastoma multiforme U118 cells[95]. Our previous studies have revealed that S1P enhances MMP-2 expression via the MAPK kinase/ERK-, nuclear factor-κB-, and calcium influx-dependent pathways in HUVECs and EAby925 endothelial cells[96]. Furthermore, we also have shown that endothelial cell invasion is enhanced by S1P stimulation, and the induction can be prevented by an MMP inhibitor, GM6001, which suggests that S1P plays important roles in endothelial cell invasion through regulating the expression of MMP-2[96]. Recently, we have shown that overexpression of mutant (F19, 22F) S1P1, which lacks tyrosine sulfation sites, suppresses native S1P1 effects on migration, actin rearrangement and lamellipodia formation, which indicates that tyrosine sulfation of S1P1 is necessary for S1P-induced Src phosphorylation and migration in HUVECs[10]. In contrast, S1P also prevents migration in other cell types. This unexpected anti-migration effect results from binding to S1P2 and involves the inhibition of Rac, as well as activation of Rho, and the subsequent phosphorylation of focal adhesion kinases, and paxillin in melanoma cells[97,98].
S1P as a regulator for angiogenesis
S1P stimulates cell proliferation and migration, both of which are required for inducing angiogenesis. Angiogenesis is a critical component for growth and metastasis of tumors. Accumulating evidence has suggested that S1P is a potent pro-angiogenic factor. S1P enhances endothelial cell migration as effectively as vascular endothelial growth factor (VEGF), thereby promoting blood vessel formation[99]. Moreover, administration of a monoclonal antibody against S1P suppresses human xenograft tumor growth, endothelial migration, and capillary formation, similar to treatment with anti-VEGF antibody[14]. Recent studies have demonstrated that S1P induces VEGF[21,100] and MMP-2 production via ZNF580[21]; a transcriptional factor implicating in angiogenesis, in endothelial cells. We also have demonstrated that S1P induces VEGF-C expression in human prostate cancer PC-3 cells (unpublished results). Furthermore, the monoclonal antibody against S1P prevents the release of pro-angiogenic cytokines, such as VEGF, IL-6, and IL-8, from tumor cells[14]. Monoclonal antibody against S1P also suppresses VEGF- and FGF-induced angiogenesis in Matrigel plugs in mice[18], which suggests that endogenous S1P plays important roles in angiogenesis and may acted as a down-stream regulator of VEGF and FGF.
S1P1 is required for stabilization of nascent blood vessels during embryonic development[62]. Knockdown of S1P1 in endothelial cells markedly attenuates angiogenic response and abolishes the expressions of platelet-endothelial cell adhesion molecule-1 and VE-cadherin[101]. In addition, VEGF treatment of vascular endothelial cells markedly upregulates S1P1 expression and enhances S1P-mediated signaling pathways leading to endothelial nitric oxide synthase activation[102]. Furthermore, expression of S1P1 is enhanced in tumor vasculature during angiogenesis, and knockdown of S1P1 inhibits the growth of neovessels into subcutaneous implants of Matrigel in vivo[103]. These studies strongly suggest that S1P1 is a crucial receptor in mediating angiogenesis and metastasis in tumors.
S1P as a regulator for cell autophagy
Autophagy plays a vital housekeeping role for maintaining cellular homeostasis, including the turnover of damaged organelles and mis-folded proteins[104]. Increased autophagy is often observed in response to diverse stresses during the progression of cancer formation, such as nutrient starvation, unfolded protein response, hypoxia, and cytotoxic chemotherapeutic agent treatments[105]. Therefore, autophagy has been proposed to promote tumorigenesis. However, genetic evidence suggests otherwise. For example, the expression of Beclin 1, a tumor suppressor protein implicating in the formation of autophagosome, is decreased in human breast carcinomas compared to normal breast tissue. Additionally, ectopic Beclin 1 expression suppresses human breast cancer MCF-7 cell proliferation in vitro and attenuates tumorigenic potential in vivo[106]. Hence, the role of autophagy during tumorigenesis remains controversial, since autophagic vacuoles are observed in several types of tumor. Further investigations are required to determine whether these autophagic vacuoles are associated with cell survival or cell death. However, targeting autophagy has been served as an adjuvant therapy for several cancers.
It has been demonstrated that tamoxifen increases intracellular levels of ceramide, which is responsible for enhancing Beclin1 expression and inhibiting Akt/PKB phosphorylation, thereby inducing autophagic cell death in human breast cancer MCF-7 cells[107]. In addition, inhibition of ceramide synthesis by its pharmacological inhibitor fumonisin B1 markedly prevents tamoxifen-induced autophagy. Furthermore, ceramide-induced autophagic cell death is mediated through decreasing mitochondrial membrane potential and activating the expression of BNIP3, a death-inducing mitochondrial protein in malignant glioma cells[108]. Overexpression of SphK1[31] or knockdown of S1PP[32], both of which increase intracellular S1P levels, induces autophagy in human breast cancer MCF-7 cells. We have also revealed that exogenous S1P induces autophagy through S1P5 activation and mTOR inhibition in human prostate cancer PC-3 cells[83]. This S1P-induced autophagy is responsible for cell survival. Our current study further demonstrated that the cytoprotective effect of S1P-induced autophagy resulted from triggering ER stress in PC-3 cells (unpublished results). Thus, S1P may mediate the nutrient deprivation-induced autophagic cell survival during the early phase of cancer development and tumorigenesis.
Both ceramide and S1P induce autophagy. However, their effect on cell fate regulation is very different. The rheostat system between ceramide and S1P in controlling cell fate may be partially mediated through the mechanism of autophagy induction. Ceramide, but not S1P, inhibits Akt phosphorylation, a pro-survival pathway that is also associated with autophagy inhibition[109]. In addition, elevated intracellular S1P-induced autophagy is associated with moderately increasing Beclin 1 expression during nutrient deprivation[31]. In contrast, ceramide significantly upregulates Beclin 1 expression during autophagy induction[107]. Furthermore, the interaction between the anti-apoptotic protein, Bcl-2, and Beclin 1, represents a potentially important point of convergence of the apoptotic and autophagic machinery[110] (Figure 2). Increased SphK1 expression and activity are required for Bcl-2-inhibited apoptosis triggered by ceramide[56]. Therefore, the rheostat system between ceramide and S1P in controlling cell fate may be resulted from regulating the ratio of Bcl-2/Beclin 1[111].
S1P SIGNALING: A CANCER CHEMOTHERAPEUTIC TARGET?
Based on the above observations, S1P signaling is implicated in cell proliferation, migration, angiogenesis, and autophagy; all processes that facilitate cancer progression. Therefore, blocking of S1P signaling may be a potential target for cancer therapy. Indeed, LT1009, a S1P specific monoclonal antibody, has been formulated for phase I clinical trials in cancer as ASONEP (LPath, Inc.). Besides, L-threo-dihydrosphingosine (Safingol), a potent PKC inhibitor with SphK-inhibiting activities, has also been assessed in Phase I trials. Moreover, FTY720, an analog of sphingosine that can be phosphorylated by SphK2 in vivo, is currently being assessed as an immunomodulator in phase III trials for patients with multiple sclerosis.
The sphingosine analog DMS and sphinganine (D, L-threo-dimethylsphingosine), which are widely used as a pharmacological inhibitors for SphK, inhibit gastric and lung tumor growth and reduce metastasis in vivo[112]. However, these compounds, without SphK isoform specificity, also inhibit PKC and ceramide kinase, as well cause hemolysis and hepatoxicity[113-115]. Since SphK1 and SphK2 may have different functions in cancer progression, the specific targets for these isoforms are needed. SphK1-I (BML-258), a specific SphK1 inhibitor, prevents tumor growth and vascularization, and induces apoptosis in glioblastoma xenografts. In addition, SphK1-I enhances survival in orthotopic glioblastoma[116]. Recently, a SphK2-selective inhibitor, (3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridine-4-yl-methy)amide (ABC294640) has been identified. It inhibits tumor growth, induces apoptosis and autophagic cell death in kidney tumor xenografts[117]. As stated above, S1P regulates several biological functions that are implicated in tumorigenesis, through its cell surface receptors. Thus, targeting of the receptors of S1P may also be a therapeutic strategy. FTY720, a potent immunosuppressive drug that induces lymphopenia, is phosphorylated by SphK2 and then binds to four out of five S1P receptor subtypes, except S1P2. This compound leads to an ubiquitin-dependent degradation of S1P1 in T lymphocytes, thereby preventing these cells leaving lymphoid organs[118]. FTY720 has become a good target for cancers in different ways, including suppression of tumor growth, tumor vascularization, angiogenesis, and metastasis, as well as induction of apoptosis[119-122].