INTRODUCTION
A principal function of eukaryotic cells is to mediate trafficking of proteins and lipids between organelles for maintaining homeostasis. Vesicles facilitate such transport between organelles. The different types of transport vesicles range from coat protein complex I (COPI) vesicles, COPII vesicles and clathrin-coated vesicles[1-3]. Of these, clathrin-coated vesicles mediate transport of proteins and lipids from the plasma membrane to early endosomes, and from the Golgi to endosomes[1]. COPI vesicles are involved in retrograde transport from the Golgi to the ER and in the intra-Golgi transport while COPII vesicles further the transport from the ER to the Golgi[2,3].
The level of proteins in a cell is dictated by protein synthesis and protein degradation. Some proteins are degraded in the proteasome while others are degraded at the site of the lysosome[4-7]. The proteasomal degradation pathway entails the use of E1, E2 and E3 enzymes for ubiquitinating target proteins[4,5]. This is followed by the subsequent targeting of these ubiquitinated proteins to the proteasome for degradation[4,5]. On the other hand, the lysosome degrades proteins and organelles using acid hydrolases[6,7]. The targeting of proteins for degradation at the lysosome occurs via numerous pathways[6,7]. Macroautophagy is one pathway that facilitates the degradation of such proteins. This non-selective catabolic process, which is regulated by ATG genes, enables cells to degrade their own components in the lysosome as a means to survive periods of nutrient deprivation and other stresses[8-11]. Besides the non-selective degradation of proteins, chaperone-mediated autophagy best exemplifies a selective autophagy pathway which utilizes luminal and cytosolic chaperones to target specific proteins (containing the KFERQ sequence) to the lysosome for degradation[12-15]. As such, autophagy is imperative for biological processes such as life span extension, cell development and growth[16,17]. Moreover, deregulated macroautophagy can result in numerous diseases[16,17].
DIVERSE PATHWAYS TRANSPORT PROTEINS TO THE YEAST VACUOLE
The yeast vacuole is homologous to the mammalian lysosome and is required for cellular processes such as osmoregulation, protein maturation and protein degradation[18,19]. The function of the yeast vacuole is dependent on the targeting of vacuole resident proteins into this organelle. For instance, the acid hydrolase carboxypeptidase Y is transported from the Golgi to the vacuole for maturation by the Vps pathway[20,21]. This process involves around 40 VPS genes[20,21]. In addition, plasma membrane proteins and other extracellular components can be internalized and targeted to the vacuole by endocytosis[18,19]. In contrast, proteins can also be transported from the cytoplasm into vacuole. Enzymes such as aminopeptidase I and α-mannosidase are synthesized in their inactive forms in the cytoplasm and are transported to the vacuole via the Cvt pathway[8,9,16]. This pathway shares many common components with the macroautophagy pathway and the pexophagy pathway[8,9,16].
Changes in nutrient conditions can influence the transport of proteins and organelles to the vacuole. For example, when Saccharomyces cerevisiae are starved of nitrogen, the macroautophagy pathway delivers proteins sequestered in autophagosomes to the vacuole for degradation[8,9,16]. Tor1p, a protein kinase and rapamycin target, is a component of the target of rapamycin complex 1 (TORC1) that regulates cell growth in response to stimuli such as nutrients and cellular stresses[8,9]. It achieves this by regulating gene transcription, protein translation, ribosomal biogenesis and macroautophagy[8,9]. Moreover, the macroautophagy pathway is inhibited by Tor1p and is induced by rapamycin even in the absence of nitrogen starvation[8,9,16]. Another example, the pexophagy pathway targets peroxisomes to the vacuole for degradation when yeast is shifted from growth in media containing oleic acid to that containing glucose[22].
INDUCTION, INACTIVATION AND DEGRADATION OF GLUCONEOGENIC ENZYMES
The pivotal regulatory enzyme in the gluoconeogenesis pathway, fructose-1,6-bisphosphatase (FBPase), is induced in Saccharomyces cerevisiae by glucose starvation. When cells are replenished with media containing fresh glucose, FBPase is inactivated and degraded[23-25]. This type of inactivation is termed catabolite inactivation[26,27]. Additionally, it has been determined that other gluconeogenic enzymes such as malate dehydrogenase (MDH2), phosphoenolpyruvate carboxykinase (Pck1p) and isocitrate lyase (Icl1p) are also inactivated by glucose[28-30]. Of these, catabolite inactivation of FBPase has been studied exhaustively for the vacuole dependent pathway[29,31,32]. In the course of catabolite inactivation, it has been ascertained that FBPase is phosphorylated and inactivated by cAMP-dependent protein kinase or protein kinase A (PKA)[29,33,34].
The site of degradation of FBPase is dependent on the duration of starvation. The Wolf lab has demonstrated that following glucose replenishment, FBPase is modified by ubiquitination and is degraded in the proteasome[35-38]. However, our lab has shown that FBPase is degraded in the vacuole in cells that have been starved of glucose for 3 d[23,27,31]. We examined the degradation of FBPase using a Δpep4Δprb1Δprc1 vacuole mutant containing deletions of proteinases A, B and C[29]. In this strain, it was observed that when glucose was added to cells that have been starved for 1 d, FBPase was degraded normally. In contrast, when glucose was added to cells that were starved for 3 d, FBPase degradation was impaired. This indicates that while vacuole proteinases are not essential for FBPase degradation in 1 d starved cells, they are essential for cells starved for 3 d. More significantly, our lab has recently demonstrated that other gluconeogenesis enzymes such as MDH2, Pck1p and Icl1p also share the same degradation characteristics as FBPase[29,30].
THE VACUOLE IMPORT AND DEGRADATION PATHWAY
The vacuole import and degradation (Vid) pathway is a selective autophagy pathway that mediates degradation of FBPase, MDH2, Pck1p and Icl1p in the vacuole after glucose starvation for 3 d[23,27,29-31]. The genes involved in this pathway are cumulatively called VID genes[32,39,40]. Upon characterizing the wild-type and Δpep4 strains by fractionation of lysates in a size column, it was ascertained that four peaks were found to contain FBPase[32]. The first peak contained the plasma membrane protein Pma1p and the fourth peak was enriched for Vid vesicles[32]. By kinetic analysis, it was determined that Vid vesicles are transitional carriers in the Vid pathway[41]. Thus, gluconeogenic enzymes destined for degradation by the Vid pathway associate with these vesicles prior to their delivery to the vacuole. Through in vitro assays, it was discerned that FBPase was imported into purified Vid vesicles[42]. In addition, the plasma membrane protein Vid22p, the cytosolic heat shock proteins Ssa1p and Ssa2p, and the peptidylprolyl isomerase Cyclophilin A are required for the sequestration of FBPase into Vid vesicles[42,43].
Cells lacking the ubiquitin conjugating enzyme 1 gene were observed to block the formation of Vid vesicles, thereby suggestive of a role in Vid vesicle biogenesis[44]. Vid24p has been characterized as a peripheral protein on Vid vesicles and is extensively used to study the trafficking of Vid vesicles in response to glucose[45]. Data from our recent studies suggest that the Vid pathway converges with the endocytic pathway[40]. COPI coatomer proteins have also been identified as peripheral proteins on Vid vesicles and they recruit Vid24p to Vid vesicles[40]. Furthermore, the coatomer subunit Sec28p traffics to endosomes and is distributed on retrograde vesicles forming on the vacuole membrane in response to glucose re-feeding[40]. Following the merging of the Vid vesicles with endosomes, the Vid-endosome clusters transport their cargo to the vacuole, a step which requires Vph1p[40]. This was determined in the Δvph1 strain where FBPase is distributed in the lumen of FM-containing endosomes[40]. FM is an endocytic dye that stains the endosomes and reaches the vacuole[40].
ENDOCYTOSIS AND ACTIN POLYMERIZATION ARE REQUIRED FOR DELIVERY OF CARGO PROTEINS TO THE VACUOLE FOR DEGRADATION
Having previously demonstrated that the Vid pathway merges with the endocytic pathway, it would be significant to elaborate on this finding. One possible explanation is that the Vid vesicles may merge with the endocytic vesicles that are forming on the plasma membrane. Hence, it follows that FBPase may also be targeted to the plasma membrane. In order to investigate this, the distribution of FBPase was visualized at the ultra-structural level[46]. This revealed that in wild type and in Δpep4 strains, FBPase is distributed near the plasma membrane and in irregularly shaped intracellular structures at 15 min following glucose replenishment. Based on the FBPase distribution, it can be inferred that the Vid pathway utilizes the early steps of the endocytic pathway to deliver FBPase to the vacuole for degradation. Upon purifying the FBPase containing intracellular structures, it was determined that these were enriched for the endosomal marker Pep12p and the Vid vesicle marker Vid24p. This suggests that following glucose replenishment, Vid vesicles may cluster or aggregate with endosomes to form large FBPase-containing structures.
The distribution of FBPase near the plasma membrane indicates that the early steps of endocytosis are required for the Vid pathway. Moreover, it has been previously reported that the early steps of endocytosis are mediated by actin polymerization in yeast[47-52]. Actin polymerization comprises recruitment and interplay of different actin related proteins at the site of internalization. Proteins such as End3p, Sla1p and Pan1p are recruited at the early steps and promote initiation of actin polymerization assembly. Subsequently, Myo3p and Myo5p are mobilized during the later stages of actin polymerization. Finally, Rvs161p and Rvs167p facilitate scission of endocytic vesicles[47-52]. Initially, the distribution of FBPase was studied in a yeast strain where the END3 gene had been deleted[46]. It was ascertained that FBPase distribution in the plasma membrane, endosome and Vid vesicle fractions were reduced in the Δend3 mutant in comparison to that observed in the positive control Δvph1 strain. These results highlight a requirement for the early steps of endocytosis in mediating association of FBPase with Vid vesicles. Owing to a requirement for actin polymerization in mediating pinching off of endocytic vesicles from the plasma membrane, FBPase degradation was studied in mutants that impaired actin polymerization at different steps[46]. As such, FBPase degradation was inhibited in mutant strains such as Δend3 and Δsla1. In summation, association of FBPase with Vid vesicles required the actin polymerization genes.
It was determined that the gluconeogenesis enzymes FBPase and MDH2 displayed a low distribution to actin patches (sites of actin polymerization) in the wild type yeast strain during glucose starvation. However, there was an increased distribution of FBPase and MDH2 to actin patches by 30 min following glucose replenishment. By 60 min of glucose addition, less co-localization of FBPase and MDH2 with actin patches was observed. As such, the cargo proteins were targeted to actin patches on the plasma membrane[46]. As means for determining whether Vid vesicles are targeted to the actin patches, Vid vesicle markers Vid24p and Sec28p distribution was examined in wild type cells[46]. Interestingly, Sec28p and Vid24p were observed to be distributed to the actin patches during glucose starvation and for up to 30 min following glucose replenishment. Sec28p and Vid24p demonstrated less co-localization to the actin patches by 60 min of glucose addition. Hence, it can be inferred that Vid vesicles are targeted to actin patches during glucose starvation and for up to 30 min following glucose replenishment. Moreover, Sec28p and Vid24p association with actin patches was prolonged in cells lacking the RVS167 gene (involved in scission of endocytic vesicles from the plasma membrane)[46]. This demonstrates that actin polymerization is required for Vid-endocytic vesicles to pinch off from the plasma membrane.
Based on these studies, we contend that Sec28p and Vid24p are present at actin patches during glucose starvation. These are also sites of endocytic vesicle formation from the plasma membrane. During glucose replenishment, FBPase and MDH2 are imported into free Vid vesicles and into Vid vesicles at the site of the actin patches. Endocytic vesicles are then released into the cytoplasm to become small endosomes with Vid vesicles clustering or aggregating to form irregularly shaped structures. In this manner, the Vid-endosome clusters serve to deliver their cargo to the vacuole for degradation.
THE TOR COMPLEX 1 INTERACTS WITH MULTIPLE CARGO PROTEINS TARGETED FOR DEGRADATION IN THE VID PATHWAY
In order to garner a more cumulative understanding of the Vid pathway, it is imperative to know how cells recognize cargo proteins that are destined for degradation. To address these goals, we sought to identify cellular proteins that interacted with FBPase. Under previously characterized growth conditions, putative FBPase interacting proteins were purified by affinity chromatography. The bound material was subjected to MALDI analysis. This facilitated in identifying Tco89p among other cellular protein candidates[30]. Tco89p is a component of the TORC1 which is also comprised of Tor1p, Kog1p and Lst8p[53,54].
The role of Tco89p in the Vid pathway was characterized by examining the degradation of FBPase, MDH2, Icl1p and Pck1p in the Δtco89 mutant[30]. It was determined that while FBPase, MDH2, Iclp and Pck1p were degraded in wild-type cells, the degradation of these proteins was impaired in Δtco89 cells. This indicates that Tco89p is required for the vacuolar dependent degradation of multiple proteins selected for the Vid pathway. It was also ascertained that components of TORC1 interacted with FBPase, MDH2, Icl1p and Pck1p during glucose starvation[30]. Through kinetic studies, it was established that Tor1p was dissociated from cargo proteins after the addition of glucose. Interestingly, Tco89p remained associated with FBPase after the addition of glucose. As such, Tor1p and Tco89p may dictate different functions in the Vid pathway. This also alludes to an inhibitory function of Tor1p association in mediating cargo protein degradation. This was verified by observing that cells overexpressing the TOR1 gene exhibited a delay in FBPase degradation. As it has been demonstrated that Tor1p is inhibitory to FBPase degradation, it was hypothesized that treatment of wild-type cells with rapamycin would promote degradation of cargo protein. However, it was determined that the addition of rapamycin impaired FBPase degradation in wild-type cells following glucose replenishment. Furthermore, TOR1 overexpressing cells inhibited the sequestering of cargo proteins into Vid vesicles. Similar results were also observed in cells lacking the TCO89 gene. Surprisingly, TOR1 deletion has little effect on FBPase degradation.
It was next determined whether Vid vesicle biogenesis was affected in cells overexpressing TOR1 or in cells lacking this gene[30]. Vid24p, a peripheral protein on Vid vesicles, was used to study the biogenesis of Vid vesicles. From differential centrifugation, it was ascertained that a fraction of Vid24p was detected in the Vid vesicle-enriched fraction in wild-type cells. Similarly, in Δtor1 cells, a fraction of Vid24p was also detected in the Vid vesicle-enriched fraction. However, low levels of Vid24p were present in the Vid vesicle-enriched fraction in cells overexpressing TOR1. In support, low levels of Vid24p were also found in the Vid vesicle-enriched fraction in Δtco89 cells. These results suggest that TOR1 and TCO89 are involved in the biogenesis of Vid vesicles.
From localization studies, it was determined that Tor1p and Tco89p were both distributed on endosomes emerging from the plasma membrane[30]. Moreover, these proteins were also detected on vesicles forming from the vacuole membrane. Such vesicles have been termed retrograde vesicles and these findings furnish support for previous observations where retrograde vesicles containing Sec28p could form on the vacuole membrane. Based on these results, it is proposed that TORC1 cycles between the plasma membrane and the vacuole. This facilitates in maintaining the size of the vacuole via the anterograde and retrograde transport pathways.
CURENT MODEL FOR THE VID PATHWAY
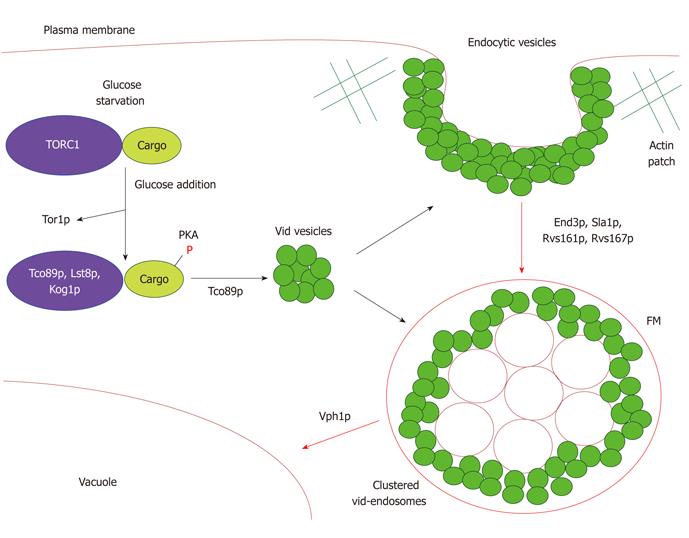
Based on the aforementioned studies, we propose the following model for the Vid pathway (Figure 1). Growth of yeast cells under glucose starvation induces synthesis of gluconeogenic enzymes such as FBPase, MDH2, Icl1p and Pck1p. During glucose starvation, Vid24p and Sec28p are distributed as peripheral proteins on free Vid vesicles and on Vid vesicles clustering around endocytic vesicles at the sites of actin polymerization. Tor1p, Tco89p and proteins involved in the early steps of actin polymerization, such as End3p and Sla1p, are believed to play a role in Vid vesicle biogenesis. Additionally, members of TORC1 interact with FBPase and other cargo proteins during glucose starvation. Following glucose replenishment, Tor1p dissociates from cargo proteins, thereby enabling cargo proteins to be phosphorylated by PKA. It should be noted that both the TORC1 and PKA pathways regulate cell growth with respect to nutrient availability[55]. However, there are conflicting reports concerning the order of involvement of these two pathways. As such, dissociation of Tor1p from cargo proteins may precede phosphorylation of cargo proteins by PKA and vice versa. Moreover, the TORC1 and PKA pathways could also be acting in concert via parallel pathways[55]. Further studies will be required to clarify this relationship. Following inactivation of cargo proteins, Tco89p is required to sequester cargo proteins into the Vid vesicles aggregating around endocytic vesicles forming from the plasma membrane. Thereafter, proteins involved in the later steps of actin polymerization, namely Rvs161p and Rvs167p, mediate scission of endocytic vesicles and these are released into the cytoplasm as small endosomes. The Vid vesicles accrue around these endosomes to form irregularly shaped structures. The transport of the Vid-endosome clusters to the vacuole for degradation of cargo proteins requires the VPH1 gene. We further postulate that the Vid pathway uses a specialized endocytic pathway. As such, we surmise that our model delineates multiple events ranging from cargo protein interaction with TORC1 to merging of the Vid pathway with the endocytic pathway at the actin patches as vital steps in the vacuole dependent degradation of gluconeogenic enzymes.
Figure 1 A model for the vacuole import and degradation pathway.
Gluconeogenic enzymes (cargo) targeted for degradation by the Vid pathway associate with the TORC1 complex during glucose starvation. Tor1p dissociates from this complex following glucose replenishment. Thereafter, the cargo proteins are phosphorylated by PKA. Tco89p mediates sequestration of the cargo proteins into free Vid vesicles and into Vid vesicles clustered around endocytic vesicles forming on the plasma membrane. Proteins involved in the later steps of actin polymerization, namely Rvs161p and Rvs167p, mediate scission of the endocytic vesicles and these are released into the cytoplasm as small endosomes. The Vid vesicles aggregate around these endosomes to form irregularly shaped structures. Vph1p is required for the transport of the Vid-endosome clusters to the vacuole.
FUTURE DIRECTIONS
Despite having elucidated novel components of the Vid pathway such as TORC1 and actin polymerization, innumerable questions remain to be answered. For instance, what is the origin of Vid vesicles? Are they derived from retrograde vesicles that emerge from the vacuole membrane? Or do Vid vesicles originate from the site of plasma membrane internalization? We have demonstrated that endocytic mutants, such as Δrvs161 and Δrvs167, that impair plasma membrane internalization, result in a prolonged distribution of peripheral Vid vesicle proteins such as Sec28p and Vid24p at the site of actin patches. Moreover, these endocytic mutants impede FBPase degradation following glucose replenishment. From these observations, one could argue that Vid vesicles are distributed at the site of plasma membrane internalization. Perhaps Vid vesicles are a specialized type of endosome? Interestingly, in support of this hypothesis, it should be noted that the Vid-endosome clusters observed following glucose re-feeding are reminiscent of multivesicular bodies. Furthermore, the significance of the requirement of internalization for FBPase degradation warrants further investigation. Are cargo proteins secreted out into the periplasmic space and internalized at the site of actin polymerization? Perhaps this may facilitate in sequestration of cargo proteins into Vid vesicles.
Another intriguing aspect of the regulation of gluconeogenic enzymes is what mechanisms determine degradation of proteins via the ubiquitin-proteasome pathway vs the vacuole dependent Vid pathway. It has been demonstrated that the site of degradation is governed by disparate modifications of cargo proteins following glucose replenishment. Cargo proteins are ubiquitinated before degradation in the proteasome[35-38]. On the other hand, cargo proteins are phosphorylated via PKA prior to their targeting to the vacuole[56]. However, the signaling cascade that mediates the transition from the ubiquitin-proteasome pathway to the Vid pathway requires elucidation.
FBPase has been described among components of excretory secretory products from Clonorchis sinensis adult worms and indicate that FBPase may serve as a marker in diagnosing clonorchiasis-associated hepatic fibrosis[57]. In addition, FBPase has also been identified as a bio-marker for assessing damage to the proximal renal tubules[58]. Furthermore, it has been determined that gluconeogenesis is upregulated in patients suffering from Type II diabetes[59]. Managlinat dialanetil (an FBPase inhibitor) has been demonstrated to show promise in the treatment of Type II diabetes[59]. As such, an understanding of how to activate or inactivate the Vid pathway could prove invaluable in identifying targets for developing therapies against diseases caused by aberrant gluconeogenesis in humans. Thus, addressing the abovementioned questions will form the core of our future investigations in our quest to better characterize this unique autophagy pathway.
Peer reviewers: Hong-Gang Wang, PhD, Professor, Lois High Berstler Professor of Pharmacology, Penn State College of Medicine, Penn State Hershey Cancer Institute, CH74, 500 University Drive, PO Box 850, Hershey, PA 17033-0850, United States; Hoyun Lee, Senior Scientist and Professor, Tumor Biology Group, Regional Cancer Program, Sudbury Regional Hospital, 41 Ramsey Lake Road, Sudbury, Ontario P3E 5J1, Canada; Michiaki Yamashita, PhD, Chief, Food Biotechnology Section, National Research Institute of Fisheries Science, 2-12-4 Fukuura, Yokohama 236-8648, Japan; Bin Shan, MD, PhD, Assistant Professor, Department of Medicine, Tulane University School of Medicine, 1430 Tulane Ave. SL-9, New Orleans, LA 70112, United States
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM